Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Vesicle Characterization
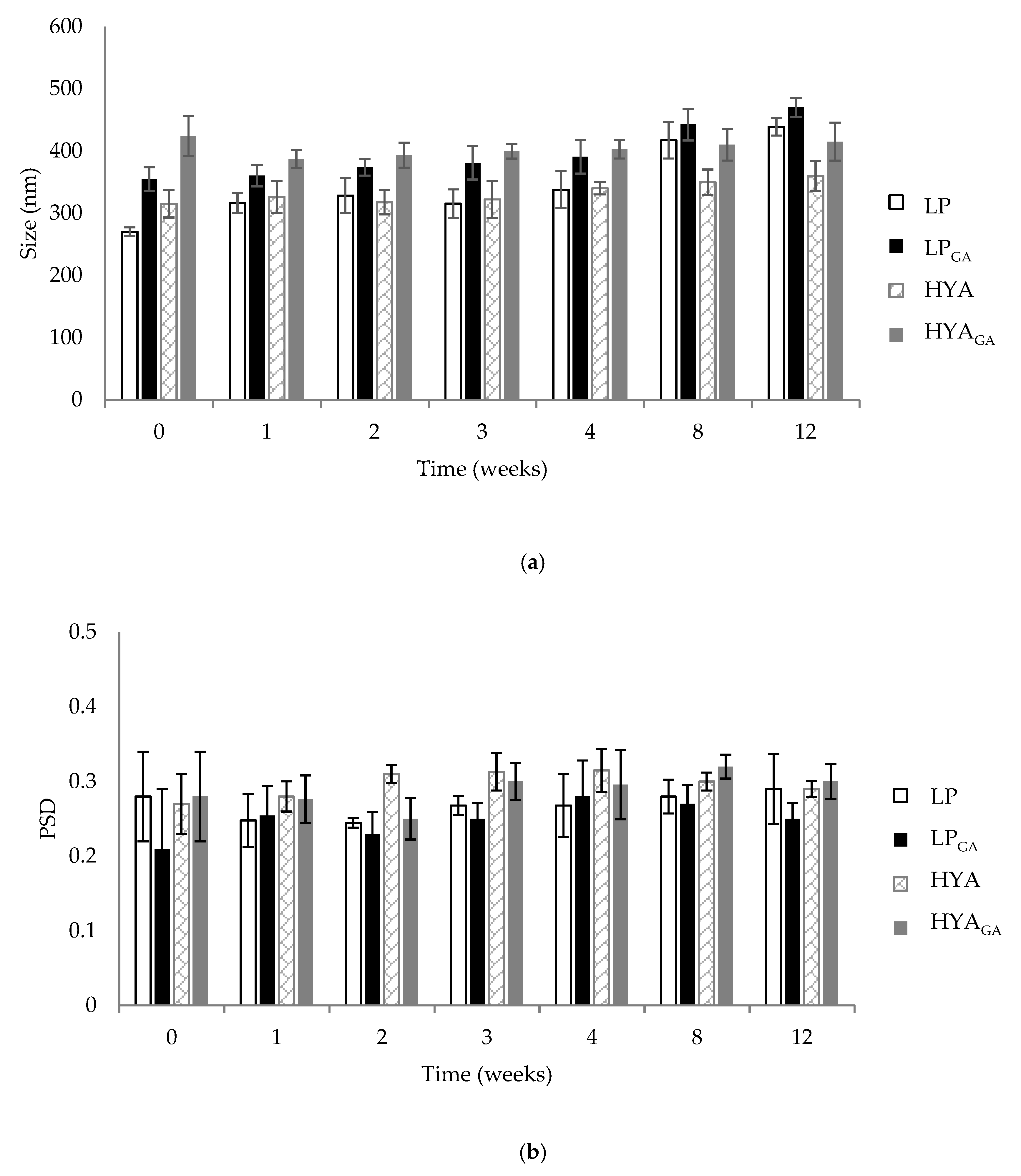
2.2. Vesicle Physical Stability
2.3. Determination of Encapsulation Efficiency
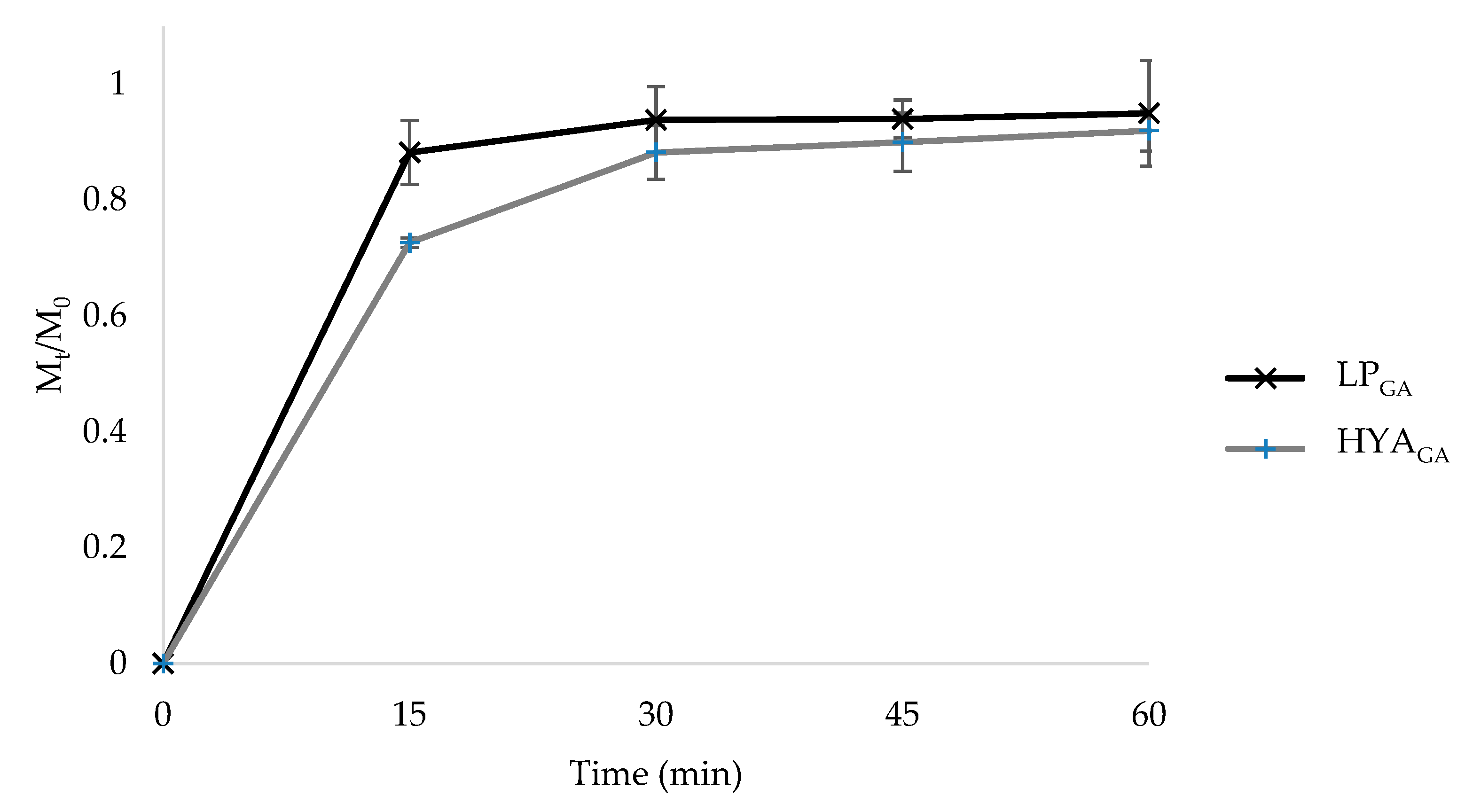
2.4. In Vitro Release Studies from Liposomes and Hyalurosomes
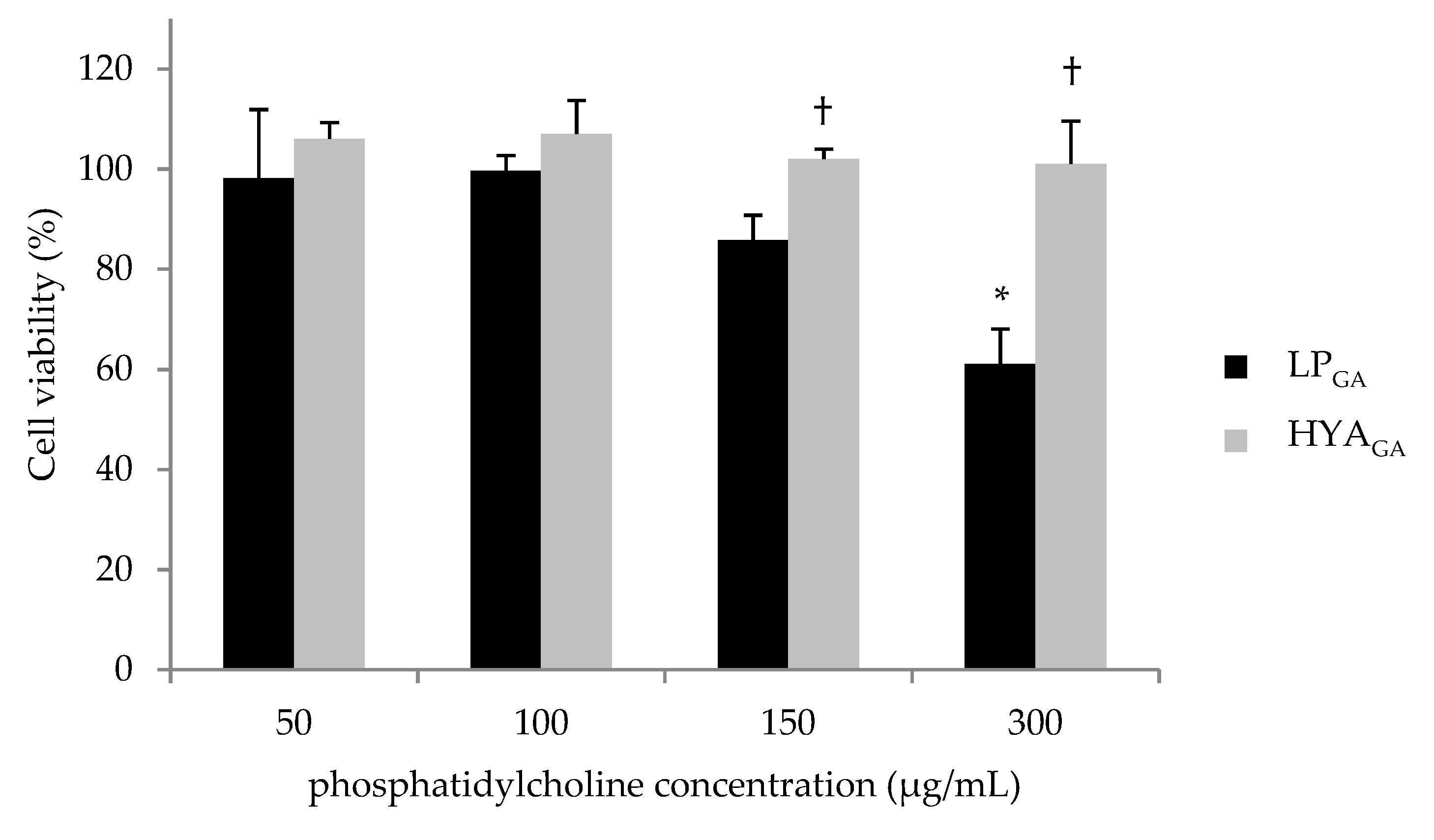
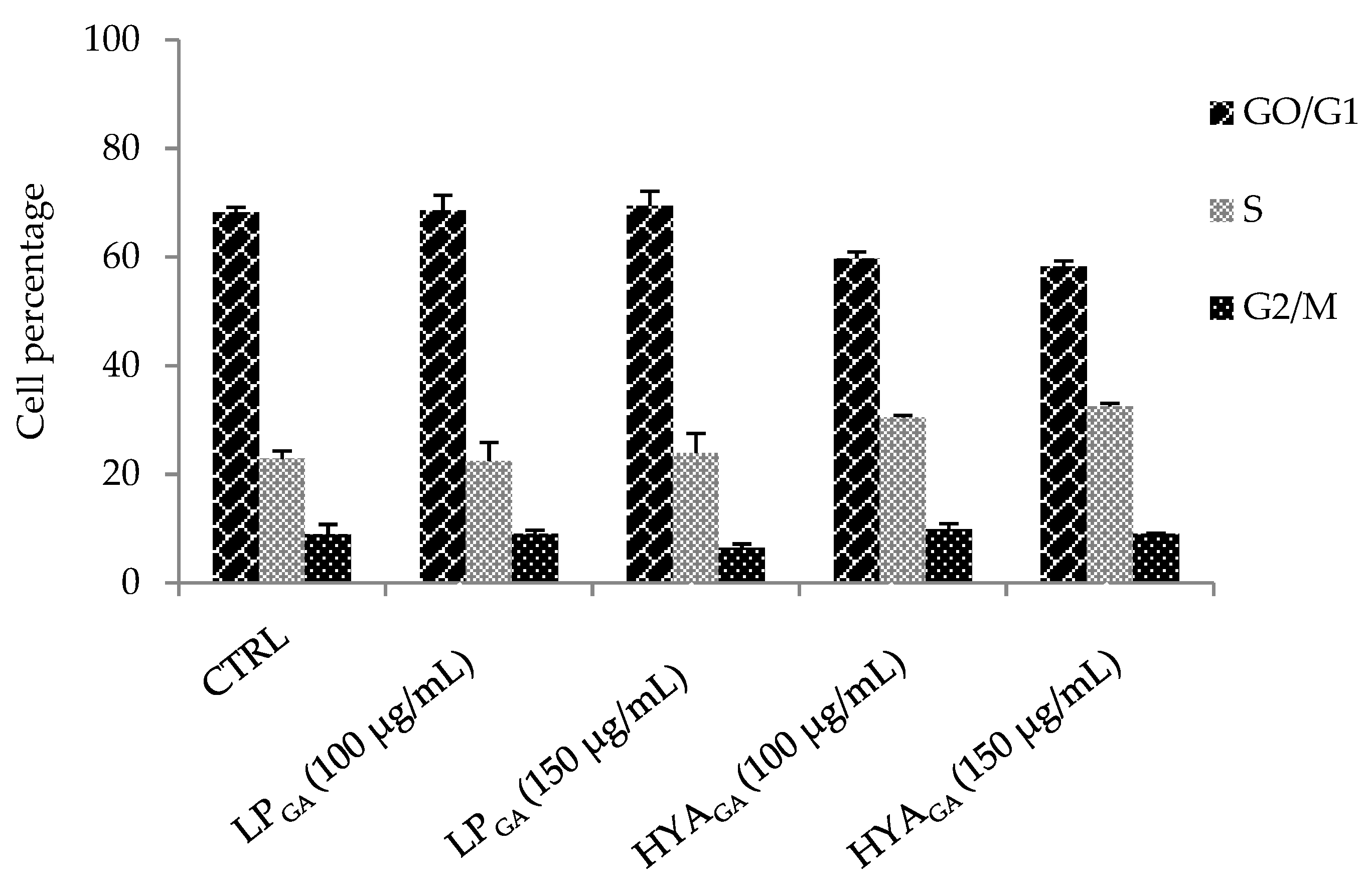
2.5. Biocompatibility of LPGA and HYAGA
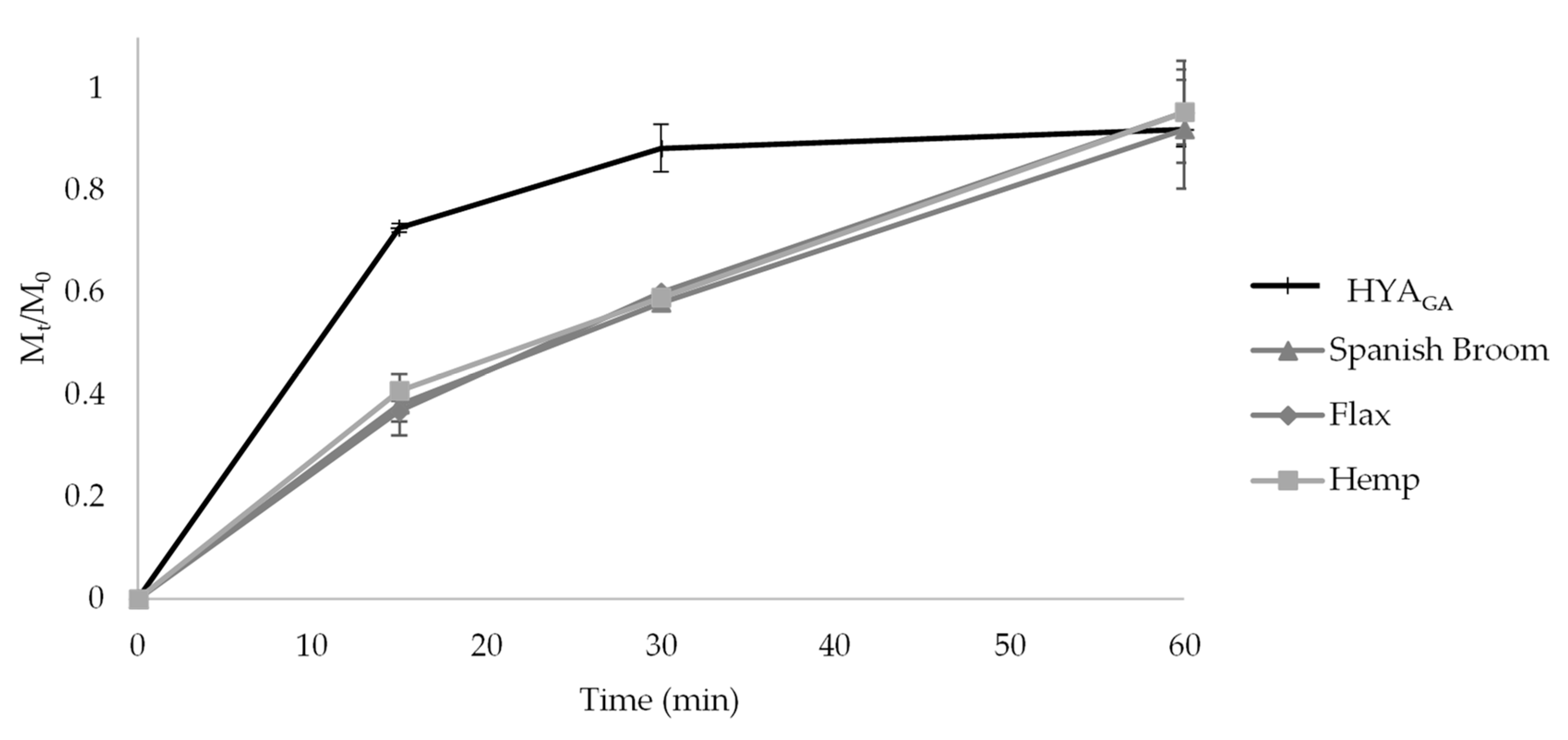
2.6. In Vitro Release Studies from the Final Dressings
3. Materials and Methods
3.1. Materials
3.2. Liposome and Hyalurosome Preparation
3.3. Vesicle Characterization
3.4. Vesicle Physical Stability
3.5. Determination of Encapsulation Efficiency
3.6. In Vitro Release Studies from Liposomes and Hyalurosomes
3.7. Cell Culture and Treatment
3.7.1. Cell Viability Assay (MTT)
3.7.2. Cell Cycle Analysis
3.8. Preparation of Wound Dressings
3.8.1. Impregnation of Spanish Broom, Flax, and Hemp Dressings with Vesicle Suspensions
3.8.2. In Vitro Release Studies from Wound Dressings
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Bonafonte, D.C.; Moreno, B.; Aguirre, J.J.; Gutierrez, F.B.; Villullas, S.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J. Control. Release. 2015, 197, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Boselli, C.; Di Lorenzo, A.; Daglia, M.; Icaro Cornaglia, A.; Gioglio, L.; Perotti, C.; et al. Particulate systems based on pectin/chitosan association for the delivery of manuka honey components and platelet lysate in chronic skin ulcers. Int. J. Pharm. 2015, 509, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Uzun, M. A review of wound management materials. J. Text. Eng. Fash. Tech. 2018, 4, 53–59. [Google Scholar] [CrossRef]
- Rajendran, S. Advanced Textiles for Wound Care, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2018; p. 26. [Google Scholar]
- Cerchiara, T.; Chidichimo, G.; Rondi, G.; Gallucci, M.C.; Gattuso, C.; Luppi, B.; Bigucci, F. Chemical composition, morphology and tensile properties of Spanish Broom (Spartium junceum L.) fibres in comparison with flax (Linum usitatissimum L.). Fibres Text. East. Eur. 2014, 22, 25–28. [Google Scholar]
- Cerchiara, T.; Abruzzo, A.; Ñahui Palomino, R.A.; Vitali, B.; De Rose, R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F.; et al. Spanish Broom (Spartium junceum L.) fibers impregnated with vancomycin- loaded chitosan nanoparticles as new antibacterial wound dressing: Preparation, characterization and antibacterial activity. Eur. J. Pharm. Sci. 2017, 99, 105–112. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef]
- Hung, C.F.; Hsiao, C.Y.; Hsieh, W.H.; Li, H.J.; Tsai, Y.J.; Lin, C.N.; Chang, H.H.; Wu, N.L. 18β-glycyrrhetinic acid derivate promotes proliferation, migration and aquaporin-3 expression in human dermal fibroblasts. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/ GSK3beta signaling and glucocorticoid receptor activation. J. Agric. Food. Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef]
- Kalaiarasi, P.; Pugalendi, K.V. Protective effect of 18b-glycyrrhetinic acid on lipid peroxidation and antioxidant enzymes in experimental diabetes. J. Pharm. Res. 2011, 4, 107–111. [Google Scholar]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, W.; Han, C.; Sui, X.; Liu, C.; Ma, X.; Dong, Y. Preparation of glycyrrhetinic acid liposomes using lyophilization monophase solution method: Preformulation, optimization, and in vitro evaluation. Nanoscale Res. Lett. 2018, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, Y.; Zhang, S.; Gao, Y. Enhanced transdermal delivery of 18β-glycyrrhetic acid via elastic vesicles: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2012, 38, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine (Lond) 2018, 13, 2881–2899. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.B.; Dos Santos, M.; Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol Res. 2011, 303, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Lizu, M.; Stewart, M.; Kyle, Z.; Yang, L.; Rajat, C.; Xingru, Y.; Zhanhu, G.; Evan, K.W.; Suying, W. Multifunctional nanofibers towards active biomedical therapeutics. Polymers. 2015, 7, 186–219. [Google Scholar] [CrossRef]
- Zehao, L.; Meifeng, L.; Huijuan, W.; Song, D. Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: Preparation optimization, in vitro dermal permeation, and in vivo bioevaluation. Int. J. Nanomed.. 2016, 11, 2995–3007. [Google Scholar]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nacher, A.; Valenti, D.; Fernandez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their application on prevention and rapid restoring of mouse skin wound. Biomaterials. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chen, W.Y.J.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Voss, G.T.; Gularte, M.S.; Vogt, A.G.; Giongo, J.L.; Vaucher, R.A.; Echenique, J.V.Z.; Soares, M.P.; Luchese, C.; Wilhelm, E.A.; Fajardo, A.R. Polysaccharide-based film loaded with vitamin C and propolis: A promising device to accelerate diabetic wound healing. Int. J. Pharm. 2018, 552, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Giordani, B.; Melgoza, L.M.; Prata, C.; Parolin, C.; Dalena, F.; Abruzzo, A.; Bigucci, F.; Luppi, B.; Vitali, B. New spanish broom dressings based on vitamin E and Lactobacillus plantarum for superficial skin wounds. J. Drug Deliv. Sci. Tech. 2020, 56, 101499. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Giordani, B.; Parolin, C.; Vitali, B.; Protti, M.; Mercolini, L.; Cappelletti, M.; Fedi, S.; Bigucci, F.; Cerchiara, T.; et al. Novel mixed vesicles containing lactobacilli biosurfactant for vaginal delivery of an anti-Candida agent. Eur. J. Pharm. Sci. 2018, 112, 95–101. [Google Scholar] [CrossRef]
- Perttu, E.K.; Kohli, A.G.; Szoka, F.C. Inverse-phosphocholine lipids: A remix of a common phospholipid. J. Am. Chem. Soc. 2012, 134, 4485–4488. [Google Scholar] [CrossRef]
- Giordani, B.; Costantini, P.E.; Fedi, S.; Cappelletti, M.; Abruzzo, A.; Parolin, C.; Foschi, C.; Frisco, G.; Calonghi, N.; Cerchiara, T.; et al. Liposomes containing biosurfactants isolated from Lactobacillus gasseri exert antibiofilm activity against methicillin resistant Staphylococcus aureus strains. Eur J. Pharm. Biopharm. 2019, 139, 246–252. [Google Scholar] [CrossRef]
- Scherer, P.G.; Seelig, J. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry 1989, 28, 7720–7728. [Google Scholar] [CrossRef]
- Franzé, S.; Marengo, A.; Stella, B.; Minghetti, P.; Arpicco, S.; Cilurzo, F. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int. J. Pharm. 2018, 535, 333–339. [Google Scholar] [CrossRef]
- Liang, T.; Guan, R.; Shen, H.; Xia, Q.; Liu, M. Optimization of conditions for cyanidin-3-O-Glucoside (C3G) nanoliposome production by response surface methodology and cellular uptake studies in Caco-2 cells. Molecules 2017, 22, 457. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, S.S. Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials 2004, 25, 2843–2849. [Google Scholar] [CrossRef]
- Marin, Ş.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pîrvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-polyvinyl alcohol-indomethacin biohybrid matrices as wound dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Iranshahy, M.; Iranshahi, M. Glycyrrhetinic acid and its derivatives: Anti-cancer and cancer chemopreventive properties, mechanisms of action and structure- cytotoxic activity relationship. Curr. Med. Chem. 2016, 23, 98–517. [Google Scholar] [CrossRef] [PubMed]
- Manuskiatti, W.; Maibach, H.I. Hyaluronic acid and skin: Wound healing and aging. Int. J. Dermatol. 1996, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Dealey, C. The care of wounds: A Guide for Nurses, 4th ed.; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Uchino, T.; Lefeber, F.; Gooris, G.; Bouwstra, J. Physicochemical characterization of drug-loaded rigid and elastic vesicles. Int. J. Pharm. 2011, 412, 142–147. [Google Scholar] [CrossRef]
- Frank, K.J.; Westedt, U.; Rosenblatt, K.M.; Holig, P.; Rosenberg, J.; Magerlein, M.; Brandl, M.; Fricker, G. Impact of FaSSIF on the solubility and dissolution-/ permeation rate of a poorly water-soluble compound. Eur. J. Pharm. Sci. 2012, 47, 16–20. [Google Scholar] [CrossRef]
- Erba, E.; Ubezio, P.; Pepe, S.; Vaghi, M.; Marsoni, S.; Torri, W.; Mangioni, C.; Landoni, F.; D’Incalci, M. Flow cytometric analysis of DNA content in human ovarian cancers. Br. J. Cancer 1989, 60, 45–50. [Google Scholar] [CrossRef]
- Gabriele, B.; Cerchiara, T.; Salerno, G.; Chidichimo, G.; Vetere, M.V.; Alampi, C.; Gallucci, M.C.; Conidi, C.; Cassano, A. A new physical-chemical process for the efficient production of cellulose fibers from Spanish broom (Spartium junceum L.). Bioresour. Technol. 2010, 101, 724–729. [Google Scholar] [CrossRef]
- Quaglierini, C. Chimica Delle Fibre Tessili; Zanichelli: Bologna, Italy, 2012; p. 114. [Google Scholar]
- Ripoll, L.; Bordes, C.; Etheve, S.; Elaissari, A.; Fessi, H. Cosmeto-textile from formulation to characterization: An overview. e-Polymers 2010, 40, 1–34. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Size (nm) | PSD | ζ Potential (mV) | EE% | |
|---|---|---|---|---|
| LP | 270 ± 7 | 0.28 ± 0.06 | −47.96 ± 1.45 | / |
| LPGA | 355 ± 19 | 0.21 ± 0.08 | −46.72 ± 1.91 | 58.62 ± 3.25 |
| HYA | 315 ± 22 | 0.27 ± 0.04 | −55.30 ± 1.43 | / |
| HYAGA | 424 ± 32 | 0.28 ± 0.06 | −56.90 ± 0.48 | 59.22 ± 8.18 |
| GA Concentration (μg/mL) | ||||
|---|---|---|---|---|
| 3.4 | 6.7 | 10 | 20 | |
| Viable cells (%) | 91.9 ± 7.6 | 88.0 ± 8.5 | 88.6 ± 6.7 | 95.4 ± 6.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abruzzo, A.; Cappadone, C.; Farruggia, G.; Luppi, B.; Bigucci, F.; Cerchiara, T. Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds. Molecules 2020, 25, 2558. https://doi.org/10.3390/molecules25112558
Abruzzo A, Cappadone C, Farruggia G, Luppi B, Bigucci F, Cerchiara T. Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds. Molecules. 2020; 25(11):2558. https://doi.org/10.3390/molecules25112558
Chicago/Turabian StyleAbruzzo, Angela, Concettina Cappadone, Giovanna Farruggia, Barbara Luppi, Federica Bigucci, and Teresa Cerchiara. 2020. "Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds" Molecules 25, no. 11: 2558. https://doi.org/10.3390/molecules25112558
APA StyleAbruzzo, A., Cappadone, C., Farruggia, G., Luppi, B., Bigucci, F., & Cerchiara, T. (2020). Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds. Molecules, 25(11), 2558. https://doi.org/10.3390/molecules25112558










