Five New Pregnane Glycosides from Gymnema sylvestre and Their α-Glucosidase and α-Amylase Inhibitory Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Compounds
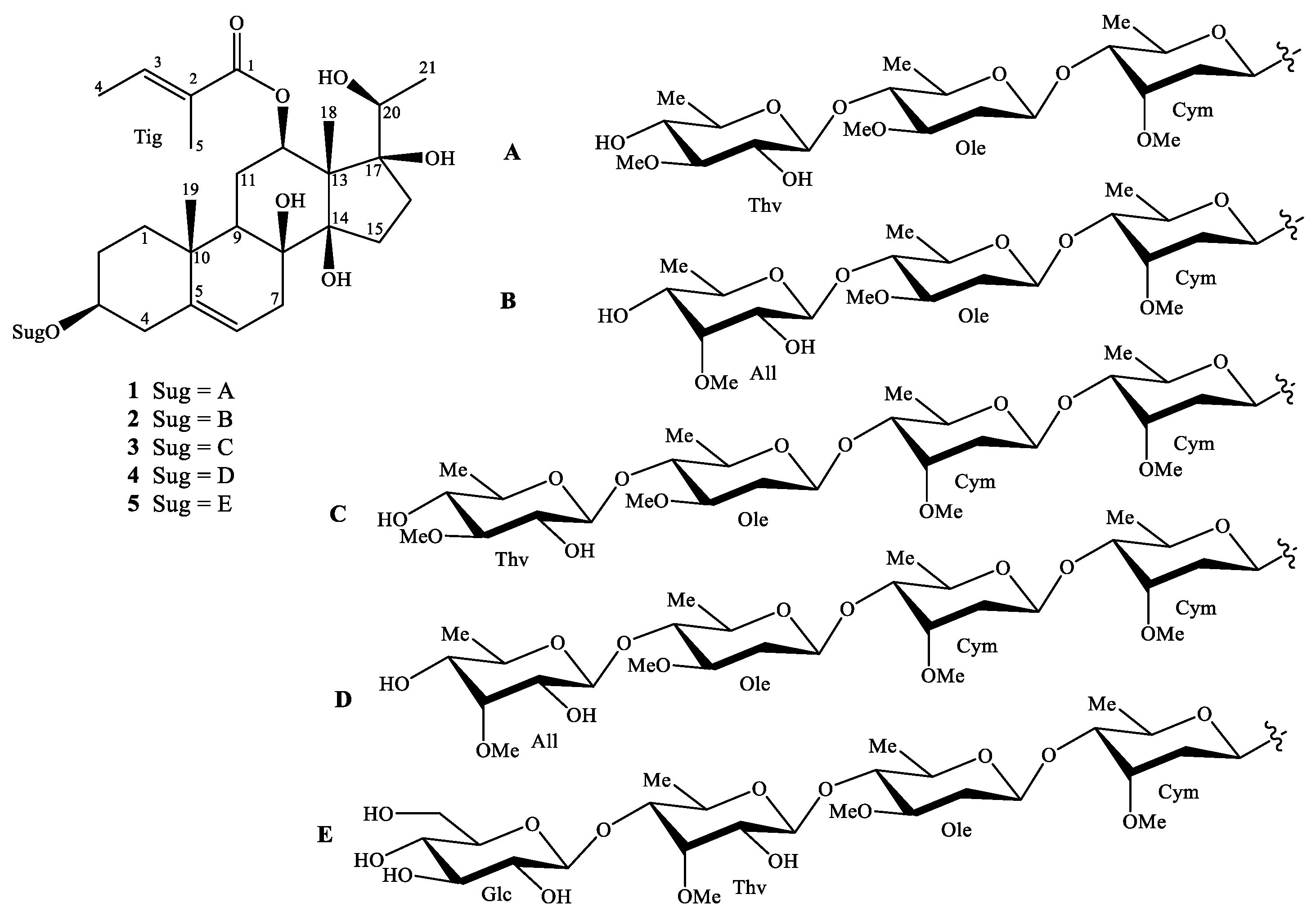
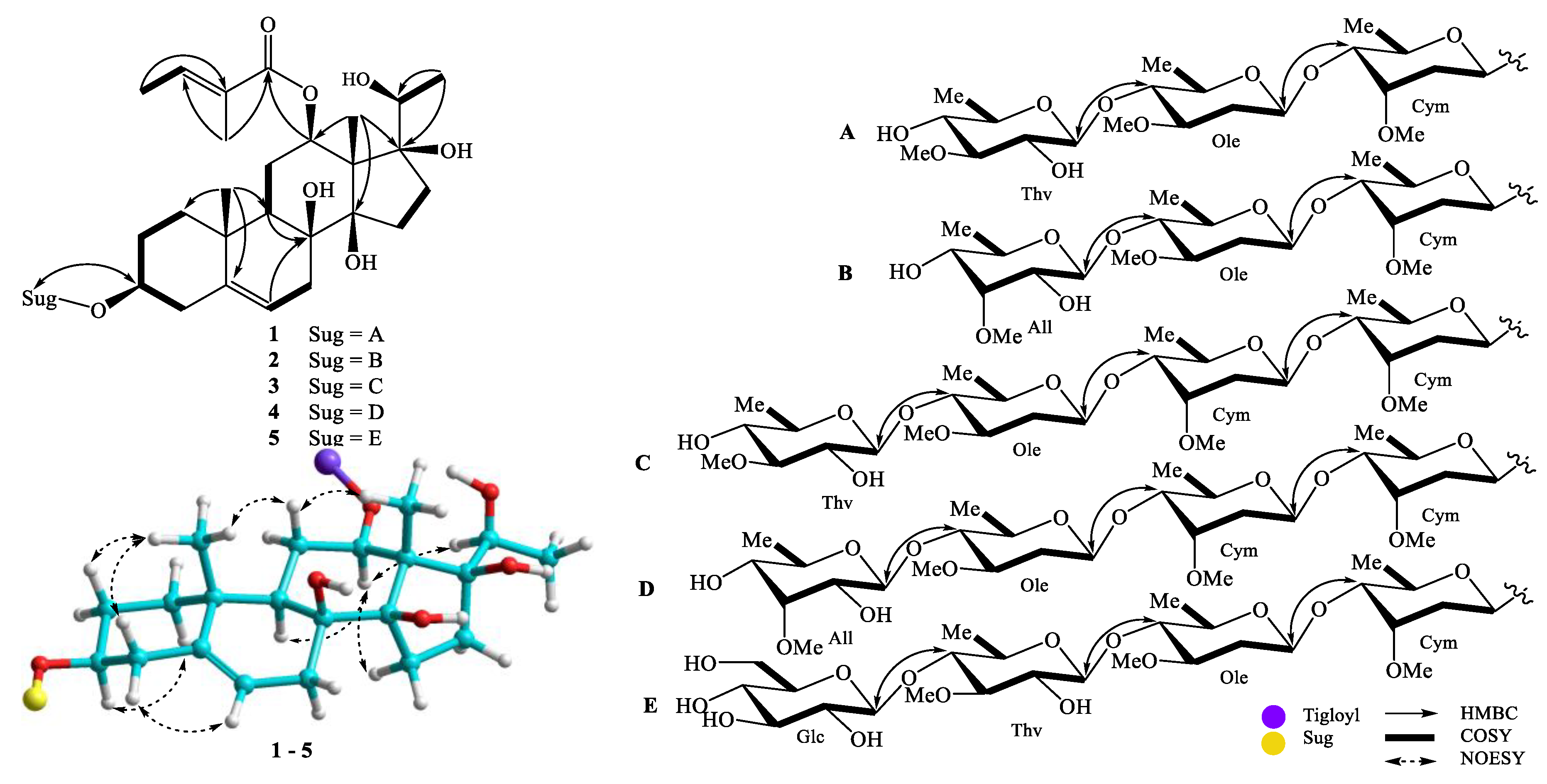
2.2. Compound Identification
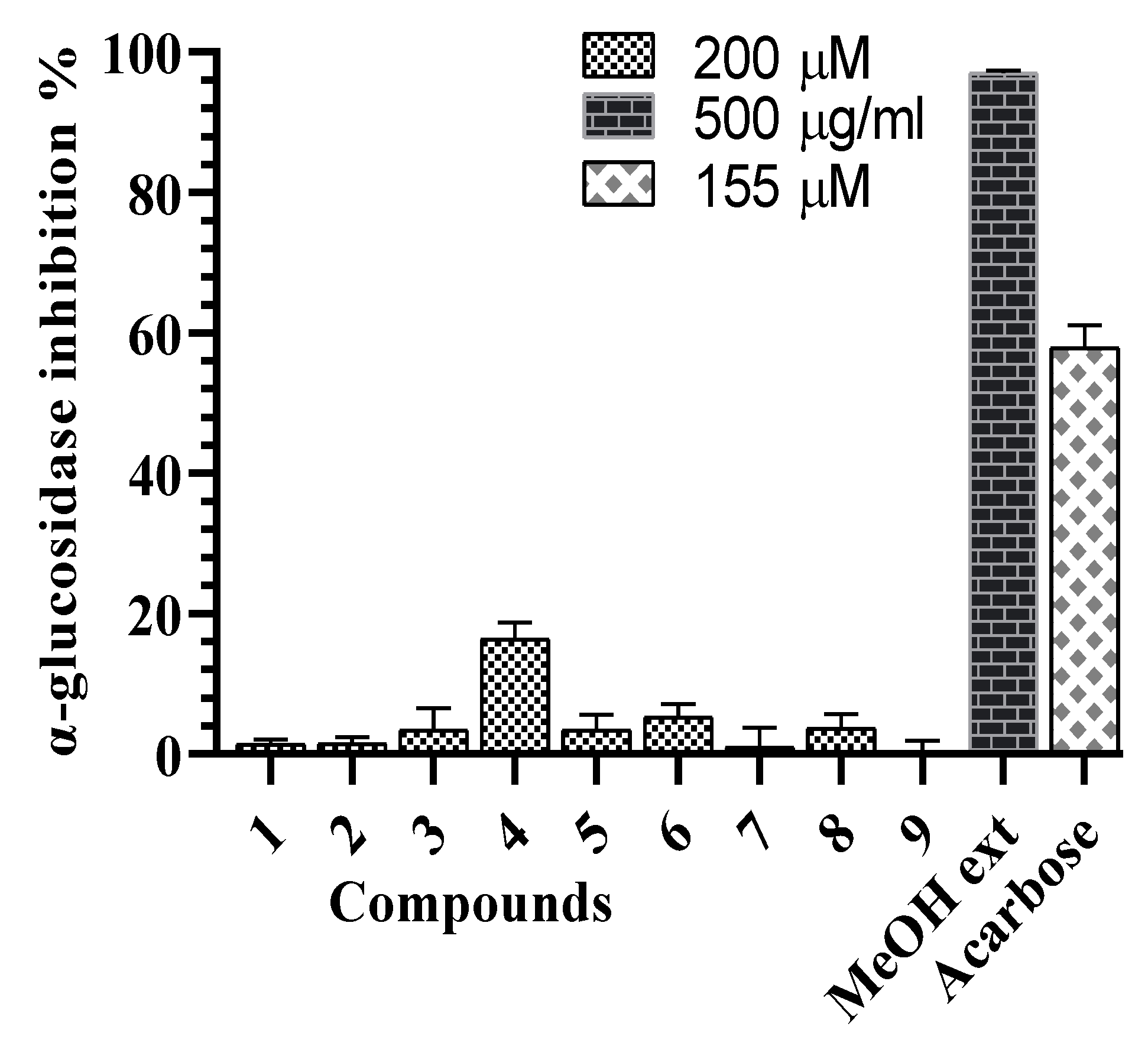
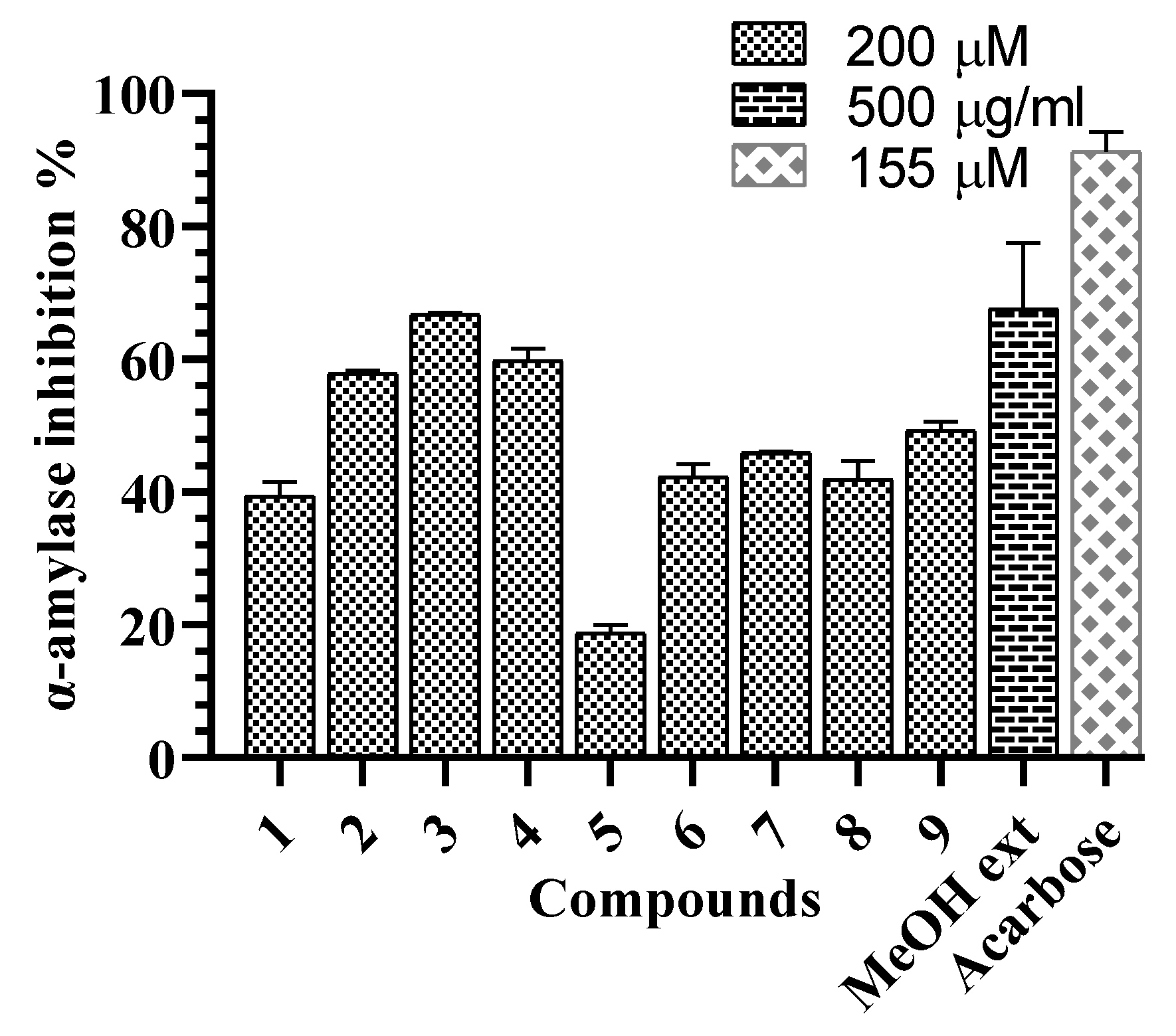
2.3. α-Glucosidase and α-Amylase Inhibitory Activities
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Gymsyloside A (1)
3.3.2. Gymsyloside B (2)
3.3.3. Gymsyloside C (3)
3.3.4. Gymsyloside D (4)
3.3.5. Gymsyloside E (5)
3.4. Acid Hydrolysis
3.5. Alkaline Hydrolysis
3.6. α-Glucosidase Inhibitory Assay
3.7. α-Amylase Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thangavelu, D.; Thangavelu, T.; Vembu, T.; Venkatachalam, K.; Selladurai, E.; Jegadeesan, M. Pharmacognostic and phytochemical studies on Gymnema sylvestre R. Br. hairy variant. Int. J. Pharm. Phytopharm. Res. 2012, 2, 143–147. [Google Scholar]
- Bala Krishna, M. Review on: Gymnema sylvestre. J. Pharm. Res. 2011, 4, 3963–3965. [Google Scholar]
- Ahmed, A.B.A.; Rao, A.S.; Rao, M.V. Role of in vivo leaf and in vitro callus of Gymnema sylvestre in maintaining the normal levels of blood glucose and lipid profile in diabetic Wistar rats. Biomedicine 2008, 28, 134–138. [Google Scholar]
- Al-Romaiyan, A.; Liu, B.; Asare-Anane, H.; Maity, C.R.; Chatterjee, S.K.; Koley, N.; Biswas, T.; Chatterji, A.K.; Huang, G.C.; Amiel, S.A.; et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother. Res. 2010, 24, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Y.; Zhang, Y.; Ren, F.; Xu, J.; Yu, N.; Zhao, Y. New pregnane glycosides from Gymnema sylvestre. Molecules 2015, 20, 3050–3066. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.P.; Mahato, S.B.; Sarkar, S.K.; Poddar, G. Triterpenoid saponins from Gymnema sylvestre. Phytochemistry 1996, 41, 1181–1185. [Google Scholar] [CrossRef]
- Ye, W.-C.; Zhang, Q.-W.; Liu, X.; Che, C.-T.; Zhao, S.-X. Oleanane saponins from Gymnema sylvestre. Phytochemistry 2000, 53, 893–899. [Google Scholar] [CrossRef]
- Vats, S.; Kamal, R. Identification of flavonoids from plant parts and callus culture of Gymnema sylvestre R.Br.: An antidiabetic plant. Curr. Bioact. Compd. 2016, 12, 264–268. [Google Scholar] [CrossRef]
- Trang, D.T.; Yen, D.T.H.; Cuong, N.T.; Anh, L.T.; Hoai, N.T.; Tai, B.H.; Doan, V.V.; Yen, P.H.; Quang, T.H.; Nhiem, N.X.; et al. Pregnane glycosides from Gymnema inodorum and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2019, 1–7, in press. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Kiem, P.V.; Minh, C.V.; Ban, N.K.; Cuong, N.X.; Tung, N.H.; Minh, H.L.; Ha, D.T.; Tai, B.H.; Quang, T.H.; et al. α-Glucosidase inhibition properties of cucurbitane-type triterpene glycosides from the fruits of Momordica charantia. Chem. Pharm. Bull. 2010, 58, 720–724. [Google Scholar] [CrossRef]
- Yen, D.T.H.; Trang, D.T.; Tai, B.H.; Doan, V.V.; Yen, P.H.; Nhiem, N.X.; Van Minh, C.; Hoang Duc, M.; Park, S.; Hyuk Lee, J.; et al. Four new pregnane glycosides from Gymnema latifolium and their α-glucosidase and α-amylase inhibitory activities. Nat. Prod. Res. 2020, 1–8, in press. [Google Scholar] [CrossRef] [PubMed]
- Yen, D.T.H.; Thu, V.K.; Tai, B.H.; Yen, P.H.; Nhiem, N.X.; Kiem, P.V. Pregnane glycosides from Gymnema sylvestre. Vietnam, J. Chem. 2019, 57, 208–212. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Ye, Y.; Chen, F.; Tu, J.; Pan, Y. Three new immunomodulating C21-steroidal glycosides from the stems of Stephanotis mucronata. Chem. Biodivers 2005, 2, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Warashina, T.; Noro, T. Steroidal glycosides from the aerial part of Asclepias incarnata. Phytochemistry 2000, 53, 485–498. [Google Scholar] [CrossRef]
- Srisurichan, S.; Puthong, S.; Pornpakakul, S. Pregnane-type steroidal glycosides from Gymnema griffithii Craib. Phytochemistry 2014, 106, 197–206. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Pregnane glycosides from the roots of Asclepias tuberosa. Chem. Pharm. Bull. 2000, 48, 1017–1022. [Google Scholar] [CrossRef]
- Abe, F.; Okabe, H.; Yamauchi, T.; Honda, K.; Hayashi, N. Pregnane glycosides from Marsdenia tomentosa. Chem. Pharm. Bull. 1999, 47, 869–875. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Xie, P.; Di, Y.-T.; Peng, S.-L.; Ding, L.-S.; Wang, M.-K. Two new triterpenoid saponins from Gymnema sylvestre. J. Integr. Plant Biol. 2008, 50, 589–592. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Matsuchika, K.; Arihara, S.; Chang, H.-C.; Wang, J.-D. Pregnane glycosides, gymnepregosides A-F from the roots of Gymnema alternifolium. Chem. Pharm. Bull. 1998, 46, 1239–1243. [Google Scholar] [CrossRef]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; El-Maraghy, S.A.; El-Dine, R.S.; Rizk, S.M. Russelioside B, a pregnane glycoside ameliorates hyperglycemia in streptozotocin induced diabetic rats by regulating key enzymes of glucose metabolism. Chemico-Biol. Interact. 2016, 252, 47–53. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |
| C | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (mult., J, in Hz) | δC | δH (mult., J, in Hz) | δC | δH (mult., J, in Hz) | |
| 1 | 39.8 | 1.09, m, α/1.80, m, β | 39.8 | 1.09, m, α/1.79, m, β | 39.8 | 1.09, m, α/1.80, m |
| 2 | 30.2 | 1.57, m, β/1.84, m, α | 30.2 | 1.56, m, β/1.84, m, α | 30.1 | 1.57, m, β/1.83, m, α |
| 3 | 79.2 | 3.50, m | 79.3 | 3.50, m | 79.3 | 3.49, m |
| 4 | 39.8 | 2.20, m, β/2.33, m, α | 39.8 | 2.20, m, β/2.33, m, α | 39.8 | 2.19, m, β/2.33, m, α |
| 5 | 140.0 | - | 140.0 | - | 140.0 | - |
| 6 | 120.0 | 5.32, br s | 120.0 | 5.32, br s | 119.9 | 5.33, br s |
| 7 | 35.2 | 2.11, m | 35.2 | 2.12, m | 35.2 | 2.12, m |
| 8 | 74.9 | - | 75.0 | - | 75.0 | - |
| 9 | 44.7 | 1.49, m | 44.7 | 1.48, m | 44.7 | 1.49, m |
| 10 | 38.0 | - | 38.0 | - | 38.0 | - |
| 11 | 26.0 | 1.63, m, α/2.00, m, β | 26.0 | 1.62, m, α/2.00, m, β | 25.9 | 1.62, m, α/2.00, m, β |
| 12 | 75.1 | 4.68, dd, 4.0, 11.6) | 75.2 | 4.68, m | 75.2 | 4.70, dd, 4.0, 9.6) |
| 13 | 57.6 | - | 57.6 | - | 57.6 | - |
| 14 | 89.3 | - | 89.3 | - | 89.2 | - |
| 15 | 34.3 | 1.83, m, β/1.88, m, α | 34.3 | 1.82, m, β/1.91, m, α | 34.3 | 1.81, m, β/1.89, m, α |
| 16 | 33.5 | 1.74, m | 33.5 | 1.75, m | 33.5 | 1.74, m |
| 17 | 89.1 | - | 89.2 | - | 89.3 | - |
| 18 | 11.2 | 1.53, s | 11.2 | 1.53, s | 11.2 | 1.52, s |
| 19 | 18.5 | 1.13, s | 18.5 | 1.14, s | 18.5 | 1.14, s |
| 20 | 71.5 | 3.45, m | 71.5 | 3.44, m | 71.6 | 3.46, m |
| 21 | 18.9 | 1.05, d (6.4) | 18.9 | 1.04, d (6.4) | 18.9 | 1.06, d (6.4) |
| Tig | Tig | Tig | ||||
| 1 | 169.1 | - | 169.2 | - | 169.1 | - |
| 2 | 130.0 | - | 130.1 | - | 130.1 | - |
| 3 | 139.5 | 6.98, q (7.2) | 139.6 | 6.98, q (7.2) | 139.6 | 7.00, q (6.8) |
| 4 | 14.5 | 1.80, d (7.2) | 14.5 | 1.80, d (7.2) | 14.5 | 1.81, d (6.8) |
| 5 | 12.2 | 1.85, s | 12.1 | 1.85, s | 12.1 | 1.87, s |
| Cym | Cym | Cym I | ||||
| 1 | 97.2 | 4.84, br d (9.6) | 97.2 | 4.85, br d (9.6) | 97.2 | 4.84, br d, 9.6) |
| 2 | 36.6 | 1.52, m, a/2.05, m, e | 36.7 | 1.53, m, a/2.04, m, e | 36.6 | 1.55, m, a/2.05, m, e |
| 3 | 78.5 | 3.82, m | 78.5 | 3.83, m | 78.5 | 3.82, m |
| 4 | 83.8 | 3.24, m | 83.8 | 3.24, m | 83.8 | 3.23, m |
| 5 | 69.9 | 3.79, m | 70.0 | 3.79, m | 70.1 | 3.79, m |
| 6 | 18.5 | 1.19, d (6.0) | 18.5 | 1.19, d (6.0) | 18.5 | 1.18, d (6.4) |
| 3-OMe | 58.5 | 3.42, s | 58.5 | 3.42, s | 58.5 | 3.42, s |
| Ole | Ole | Cym II | ||||
| 1 | 102.6 | 4.57, br d (9.2) | 102.6 | 4.56, br d (6.8) | 101.2 | 4.78, br d (9.6) |
| 2 | 37.6 | 1.40, m, a/2.30, m, e | 37.5 | 1.39, m, a/2.30, m, e | 36.4 | 1.55, m, a/2.10, m, e |
| 3 | 80.2 | 3.36, m | 80.4 | 3.36, m | 78.6 | 3.82, m |
| 4 | 84.1 | 3.18, m | 84.0 | 3.17, m | 83.9 | 3.23, m |
| 5 | 72.5 | 3.36, m | 72.6 | 3.35, m | 69.9 | 3.79, m |
| 6 | 18.9 | 1.35, d (6.0) | 19.0 | 1.34, d (6.0) | 18.6 | 1.21, d (6.0) |
| 3-OMe | 57.6 | 3.39, s | 57.5 | 3.39, s | 58.4 | 3.42, s |
| Thv | All | Ole | ||||
| 1 | 104.3 | 4.41, br d (8.0) | 102.2 | 4.70, br d (7.6) | 102.6 | 4.58, br d (8.4) |
| 2 | 75.6 | 3.18, m | 73.6 | 3.29, m | 37.6 | 1.40, m, a)/2.30, m, e) |
| 3 | 87.7 | 3.00, t (6.8) | 83.8 | 3.60, m | 80.2 | 3.36, m |
| 4 | 76.6 | 3.00, t (6.8) | 75.0 | 3.16, m | 84.1 | 3.18, m |
| 5 | 73.2 | 3.25, m | 71.2 | 3.64, m | 72.5 | 3.35, m |
| 6 | 18.1 | 1.25, d (6.0) | 18.5 | 1.21, d (6.0) | 18.9 | 1.36, d (5.6) |
| 3-OMe | 61.0 | 3.60, s | 62.5 | 3.58, s | 57.5 | 3.41, s |
| Thv | ||||||
| 1 | - | - | - | - | 104.3 | 4.42, d (8.0) |
| 2 | - | - | - | - | 75.6 | 3.18, m |
| 3 | - | - | - | - | 87.7 | 3.00, m |
| 4 | - | - | - | - | 76.6 | 3.00, m |
| 5 | - | - | - | - | 73.2 | 3.25, m |
| 6 | - | - | - | - | 18.1 | 1.27, d (6.0) |
| 3-OMe | - | - | - | - | 61.1 | 3.60, s |
| C | 4 | 5 | ||
|---|---|---|---|---|
| δC | δH (mult., J, in Hz) | δC | δH (mult., J, in Hz) | |
| 1 | 39.8 | 1.07, m, α/1.79, m, β | 39.8 | 1.08, m, α/1.80, m, β |
| 2 | 30.2 | 1.57, m, β/1.83, m, α | 30.2 | 1.58, m, β/1.84, m, α |
| 3 | 79.3 | 3.50, m | 79.3 | 3.50, m |
| 4 | 39.8 | 2.18, m, β/2.33, m, α | 39.8 | 2.20, m, β/2.33, m, α |
| 5 | 140.0 | - | 140.0 | - |
| 6 | 120.0 | 5.32, br s | 120.0 | 5.32, br s |
| 7 | 35.2 | 2.11, m | 35.2 | 2.11, m |
| 8 | 74.9 | - | 74.9 | - |
| 9 | 44.7 | 1.49, m | 44.7 | 1.49, m |
| 10 | 38.0 | - | 38.0 | - |
| 11 | 26.0 | 1.63, m, α/2.00, m, β | 25.9 | 1.63, m, α/2.00, m, β |
| 12 | 75.1 | 4.67, m | 75.1 | 4.68, m |
| 13 | 57.6 | - | 57.6 | - |
| 14 | 89.3 | - | 89.3 | - |
| 15 | 34.3 | 1.80, m, β/1.88, m, α | 34.3 | 1.83, m, β/1.90, m, α |
| 16 | 33.5 | 1.74, m | 33.5 | 1.75, m |
| 17 | 89.1 | - | 89.1 | - |
| 18 | 11.2 | 1.53, s | 11.2 | 1.53, s |
| 19 | 18.6 | 1.13, s | 18.6 | 1.12, s |
| 20 | 71.5 | 3.46, m | 71.5 | 3.45, m |
| 21 | 19.0 | 1.05, d (6.0) | 18.9 | 1.05, d (6.4) |
| Tig | Tig | |||
| 1 | 169.1 | - | 169.1 | - |
| 2 | 130.1 | - | 130.1 | - |
| 3 | 139.5 | 6.98, q (7.2) | 139.5 | 6.98, q (7.2) |
| 4 | 14.6 | 1.81, d (7.2) | 14.5 | 1.80, d (7.2) |
| 5 | 12.2 | 1.85, s | 12.2 | 1.85, s |
| Cym I | Cym | |||
| 1 | 97.2 | 4.83, br d (9.6) | 97.2 | 4.84, br d (9.6) |
| 2 | 36.6 | 1.54, m, a/2.04, m, e | 36.7 | 1.53, m, a/2.05, m, e |
| 3 | 78.5 | 3.82, m | 78.5 | 3.81, m |
| 4 | 83.8 | 3.24, m | 83.8 | 3.24, m |
| 5 | 69.8 | 3.78, m | 69.9 | 3.80, m |
| 6 | 18.3 | 1.17, d (6.4) | 18.4 | 1.19, d (6.4) |
| 3-OMe | 58.4 | 3.41, s | 58.5 | 3.42, s |
| Cym II | Ole | |||
| 1 | 101.2 | 4.77, br d (9.6) | 102.6 | 4.57, br d (9.2) |
| 2 | 36.4 | 1.54, m, a/2.04, m, e | 37.6 | 1.40, m, a/2.30, m, e |
| 3 | 78.4 | 3.82, m | 80.3 | 3.35, m |
| 4 | 83.8 | 3.24, m | 84.1 | 3.18, m |
| 5 | 69.9 | 3.78, m | 72.6 | 3.38, m |
| 6 | 18.6 | 1.19, d (5.6) | 18.8 | 1.36, d (6.0) |
| 3-OMe | 58.5 | 3.40, s | 57.6 | 3.39, s |
| Ole | Thv | |||
| 1 | 102.6 | 4.56, br d (9.2) | 104.2 | 4.43 (d (7.6) |
| 2 | 37.5 | 1.40, m/2.30, m | 75.2 | 3.23, m |
| 3 | 80.3 | 3.35, m | 86.3 | 3.17, m |
| 4 | 83.7 | 3.18, m | 82.9 | 3.30, m |
| 5 | 72.5 | 3.35, m | 72.5 | 3.38, m |
| 6 | 18.9 | 1.35, d (6.0) | 18.5 | 1.36, d (7.2) |
| 3-OMe | 57.4 | 3.39, s | 61.2 | 3.60, s |
| All | Glc | |||
| 1 | 102.2 | 4.70, d (8.0) | 104.3 | 4.41, d (7.6) |
| 2 | 73.5 | 3.29, m | 75.6 | 3.15, m |
| 3 | 83.9 | 3.60, m | 78.3 | 3.23, m |
| 4 | 75.0 | 3.16, m | 71.8 | 3.20, m |
| 5 | 71.2 | 3.63, m | 77.9 | 3.31, m |
| 6 | 18.6 | 1.21, d (6.0) | 63.2 | 3.62, m/3.84, m |
| 3-OMe | 62.5 | 3.58, s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiem, P.V.; Yen, D.T.H.; Hung, N.V.; Nhiem, N.X.; Tai, B.H.; Trang, D.T.; Yen, P.H.; Ngoc, T.M.; Minh, C.V.; Park, S.; et al. Five New Pregnane Glycosides from Gymnema sylvestre and Their α-Glucosidase and α-Amylase Inhibitory Activities. Molecules 2020, 25, 2525. https://doi.org/10.3390/molecules25112525
Kiem PV, Yen DTH, Hung NV, Nhiem NX, Tai BH, Trang DT, Yen PH, Ngoc TM, Minh CV, Park S, et al. Five New Pregnane Glycosides from Gymnema sylvestre and Their α-Glucosidase and α-Amylase Inhibitory Activities. Molecules. 2020; 25(11):2525. https://doi.org/10.3390/molecules25112525
Chicago/Turabian StyleKiem, Phan Van, Duong Thi Hai Yen, Nguyen Van Hung, Nguyen Xuan Nhiem, Bui Huu Tai, Do Thi Trang, Pham Hai Yen, Tran Minh Ngoc, Chau Van Minh, SeonJu Park, and et al. 2020. "Five New Pregnane Glycosides from Gymnema sylvestre and Their α-Glucosidase and α-Amylase Inhibitory Activities" Molecules 25, no. 11: 2525. https://doi.org/10.3390/molecules25112525
APA StyleKiem, P. V., Yen, D. T. H., Hung, N. V., Nhiem, N. X., Tai, B. H., Trang, D. T., Yen, P. H., Ngoc, T. M., Minh, C. V., Park, S., Lee, J. H., Kim, S. Y., & Kim, S. H. (2020). Five New Pregnane Glycosides from Gymnema sylvestre and Their α-Glucosidase and α-Amylase Inhibitory Activities. Molecules, 25(11), 2525. https://doi.org/10.3390/molecules25112525






