Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates
Abstract
:1. Introduction
2. Porifera
3. Cnidaria
4. Mollusca
5. Echinodermata
6. Tunicata
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; He, Y.; Zhao, M.; Jiang, J. Collective cell migration: Implications for wound healing and cancer invasion. Burns Trauma 2013, 1, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Camley, B.A.; Rappel, W.-J. Physical models of collective cell motility: From cell to tissue. J. Phys. D Appl. Phys. 2017, 50, 113002. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Mège, R.-M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial Cell Metabolism in Normal and Diseased Vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Translat. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Arwert, E.N.; Hoste, E.; Watt, F.M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 2012, 12, 170–180. [Google Scholar] [CrossRef]
- Plikus, M.V.; Gay, D.L.; Treffeisen, E.; Wang, A.; Supapannachart, R.J.; Cotsarelis, G. Epithelial stem cells and implications for wound repair. Semin. Cell Dev. Biol. 2012, 23, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Stefonek-Puccinelli, T.J.; Masters, K.S. Regulation of cell signaling and function via changes in growth factor presentation. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 1167–1171. [Google Scholar]
- Hiroyasu, S.; Colburn, Z.T.; Jones, J.C.R. A hemidesmosomal protein regulates actin dynamics and traction forces in motile keratinocytes. FASEB J. 2016, 30, 2298–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiwanuka, E.; Junker, J.P.; Eriksson, E. Transforming growth factor β1 regulates the expression of CCN2 in human keratinocytes via Smad-ERK signalling: TGF-β1-induced CCN2 expression in keratinocytes. Int. Wound J. 2017, 14, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Boudra, R.; Ramsey, M.R. Understanding Transcriptional Networks Regulating Initiation of Cutaneous Wound Healing. Yale J. Biol. Med. 2020, 93, 161–173. [Google Scholar]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migrat. 2014, 8, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Albini, A.; Iwamoto, Y.; Kleinman, H.K.; Martin, G.R.; Aaronson, S.A.; Kozlowski, J.M.; McEwan, R.N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987, 47, 3239–3245. [Google Scholar] [PubMed]
- Luparello, C. PTHrP regulates the expression of stress proteins in breast cancer cells inducing modifications in urokinase-plasminogen activator and MMP-1 expression. J. Cell Sci. 2003, 116, 2421–2430. [Google Scholar] [CrossRef] [Green Version]
- Luparello, C. Effect of Manganese Chloride and of Cotreatment with Cadmium Chloride on the In vitro Proliferative, Motile and Invasive Behavior of MDA-MB231 Breast Cancer Cells. Molecules 2019, 24, 1205. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Naik, M.; Brahma, P.; Dixit, M. A Cost-Effective and Efficient Chick Ex-Ovo CAM Assay Protocol to Assess Angiogenesis. Methods Protoc. 2018, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- João De Masi, E.C.D.; Campos, A.C.L.; João De Masi, F.D.; Ratti, M.A.S.; Ike, I.S.; João De Masi, R.D. The influence of growth factors on skin wound healing in rats. Braz. J. Otorhinolaryngol. 2016, 82, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Cai, E.Z.; Ang, C.H.; Raju, A.; Tan, K.B.; Hing, E.C.H.; Loo, Y.; Wong, Y.C.; Lee, H.; Lim, J.; Moochhala, S.M.; et al. Creation of Consistent Burn Wounds: A Rat Model. Arch. Plast. Surg. 2014, 41, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, B.; Lackner, I.; Haffner-Luntzer, M.; Palmer, A.; Pressmar, J.; Scharffetter-Kochanek, K.; Knöll, B.; Schrezenemeier, H.; Relja, B.; Kalbitz, M. Modeling trauma in rats: Similarities to humans and potential pitfalls to consider. J. Transl. Med. 2019, 17, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnell, L.K.S.; Volk, S.W. The Evolution of Animal Models in Wound Healing Research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Lindequist, U. Marine-Derived Pharmaceuticals-Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Simpson, T.L. The Cell Biology of Sponges; Springer New York: New York, NY, USA, 1984; ISBN 978-1-4612-9740-6. [Google Scholar]
- Pozzolini, M.; Gallus, L.; Ghignone, S.; Ferrando, S.; Candiani, S.; Bozzo, M.; Bertolino, M.; Costa, G.; Bavestrello, G.; Scarfì, S. Insights into the evolution of metazoan regenerative mechanisms: Roles of TGF superfamily members in tissue regeneration of the marine sponge Chondrosia reniformis. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [Green Version]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Kreuter, J.; Muller, W.; Swatschek, D.; Schatton, W.; Schatton, M.; Wolfgang S. Inc. Method for Isolating Sponge Collagen and Producing Nanoparticulate Collagen, and the Use. U.S. Patent 20030032601A1, 13 February 2003. [Google Scholar]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef] [Green Version]
- Fassini, D.; Duarte, A.R.; Reis, R.; Silva, T. Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material. Mar. Drugs 2017, 15, 380. [Google Scholar] [CrossRef] [Green Version]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [Green Version]
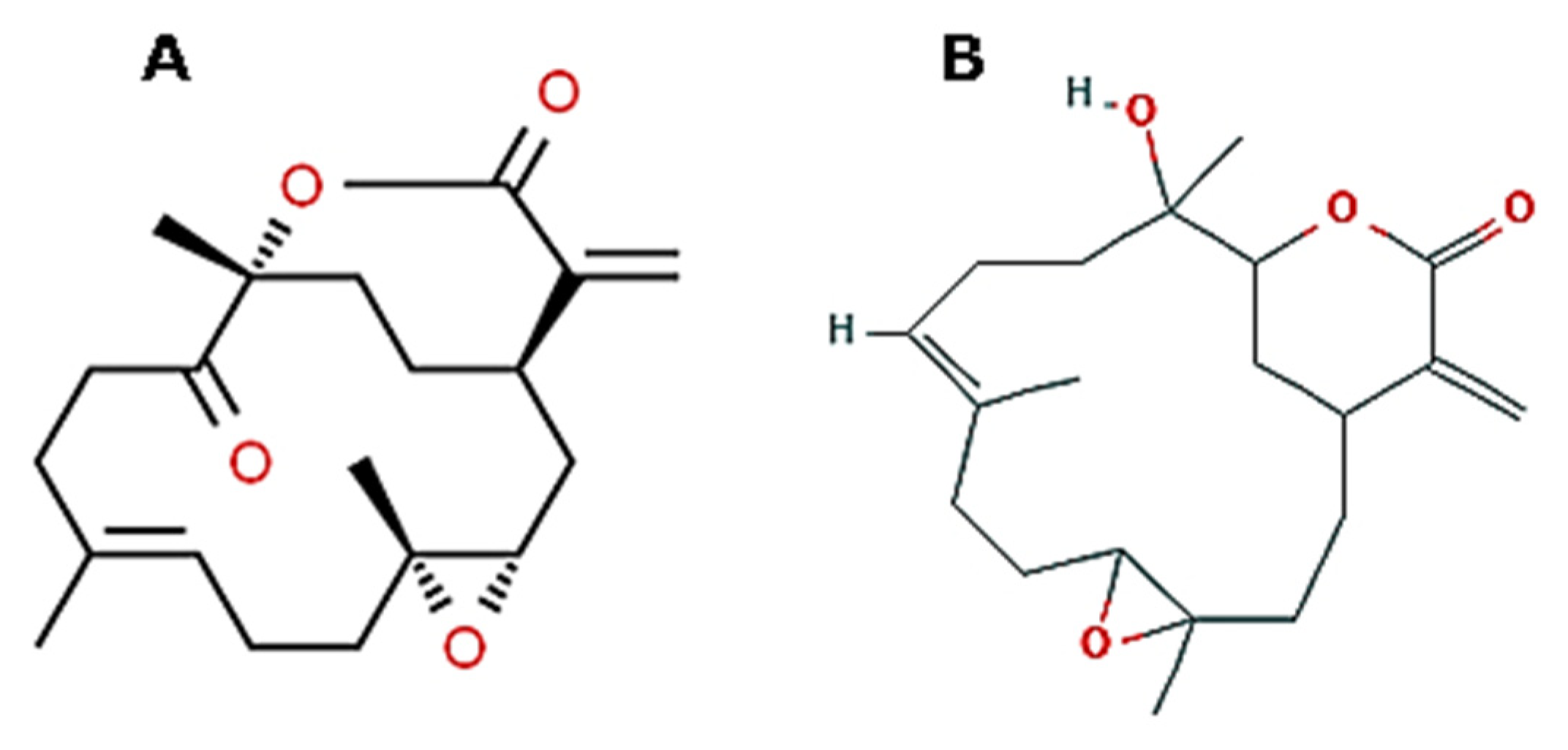
- Tsuda, M.; Ishibashi, M.; Agemi, K.; Sasaki, T.; Kobayashi, J. Stelliferins A–F, new antineoplastic isomalabaricane triterpenes from the Okinawan marine sponge Jaspis stellifera. Tetrahedron 1991, 47, 2181–2194. [Google Scholar] [CrossRef]
- Kobayashi, J.; Yuasa, K.; Kobayashi, T.; Sasaki, T.; Tsuda, M. Jaspiferals A ∼ G, new cytotoxic isomalabaricane-type nortriterpenoids from Okinawan marine sponge Jaspis stellifera. Tetrahedron 1996, 52, 5745–5750. [Google Scholar] [CrossRef]
- Tang, S.-A.; Zhou, Q.; Guo, W.-Z.; Qiu, Y.; Wang, R.; Jin, M.; Zhang, W.; Li, K.; Yamori, T.; Dan, S.; et al. In vitro Antitumor Activity of Stellettin B, a Triterpene from Marine Sponge Jaspis stellifera, on Human Glioblastoma Cancer SF295 Cells. Mar. Drugs 2014, 12, 4200–4213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, D.-J.; Tang, S.-A.; Xing, G.-S.; Zhao, W.-J.; Zhao, C.; Duan, H.-Q.; Lin, W.-H. Jaspiferins C–F, four new isomalabaricane-type triterpenoids from the South China Sea sponge Jaspis stellifera. J. Asian Nat. Prod. Res. 2014, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/mTOR Pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.-G.; Wang, J.; Xing, G.-S.; Xu, J.-J.; Qiao, W.; Zhao, C.; Tang, S.-A. Jaspiferin G, a new isomalabaricane-type triterpenoid from the sponge Jaspis stellifera. Z. Nat. C 2016, 71, 111–114. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Qiao, W.; Zhao, C.; Tang, S. Jaspiferins H–J, New Isomalabaricane-Type Terpenoids from the South China Sea Marine Sponge Jaspis stellifera. Chem. Nat. Compd. 2018, 54, 84–87. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Zhang, L.; Zhong, Y.; Fan, G.; Zhang, Z.; Wang, R.; Jin, M.; Qiu, Y.; Kong, D. Stellettin B induces apoptosis in human chronic myeloid leukemia cells via targeting PI3K and Stat5. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-Y.; Chen, N.-F.; Lin, P.-Y.; Su, J.-H.; Chen, B.-H.; Kuo, H.-M.; Sung, C.-S.; Sung, P.-J.; Wen, Z.-H.; Chen, W.-F. Anti-Invasion and Antiangiogenic Effects of Stellettin B through Inhibition of the Akt/Girdin Signaling Pathway and VEGF in Glioblastoma Cells. Cancers 2019, 11, 220. [Google Scholar] [CrossRef] [Green Version]
- Gu, F.; Wang, L.; He, J.; Liu, X.; Zhang, H.; Li, W.; Fu, L.; Ma, Y. Girdin, an actin-binding protein, is critical for migration, adhesion, and invasion of human glioblastoma cells. J. Neurochem. 2014, 131, 457–469. [Google Scholar] [CrossRef]
- Kashman, Y.; Groweiss, A.; Shmueli, U. Latrunculin, a new 2-thiazolidinone macrolide from the marine sponge. Tetrahedron Lett. 1980, 21, 3629–3632. [Google Scholar] [CrossRef]
- Sabanay, I.; Tian, B.; Gabelt, B.T.; Geiger, B.; Kaufman, P.L. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp. Eye Res. 2006, 82, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Lin, Y.-K.; Wen, Z.-H.; Chen, Y.-C.; Chen, S.-A.; Chen, Y.-J. Latrunculin B modulates electrophysiological characteristics and arrhythmogenesis in pulmonary vein cardiomyocytes. Clin. Sci. 2016, 130, 721–732. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Youssef, D.T.A.; Marchetti, D. Bioactive Natural and Semisynthetic Latrunculins. J. Nat. Prod. 2006, 69, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Sayed, K.A.E.; Khanfar, M.A.; Shallal, H.M.; Muralidharan, A.; Awate, B.; Youssef, D.T.A.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G.; Shah, G. Latrunculin A and Its C-17-O-Carbamates Inhibit Prostate Tumor Cell Invasion and HIF-1 Activation in Breast Tumor Cells. J. Nat. Prod. 2008, 71, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, Y.X.; Luo, Y.; Zhao, J.; Li, Q.; Zhang, J.; Jiang, Y. Hypoxia inducible factor-1α-dependent epithelial to mesenchymal transition under hypoxic conditions in prostate cancer cells. Oncol. Rep. 2016, 36, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Khanfar, M.A.; Youssef, D.T.A.; El Sayed, K.A. Semisynthetic Latrunculin Derivatives as Inhibitors of Metastatic Breast Cancer: Biological Evaluations, Preliminary Structure-Activity Relationship and Molecular Modeling Studies. ChemMedChem 2010, 5, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Badr, J.M.; Shaala, L.A.; Abou-Shoer, M.I.; Tawfik, M.K.; Habib, A.-A.M. Bioactive Brominated Metabolites from the Red Sea Sponge Pseudoceratina arabica. J. Nat. Prod. 2008, 71, 1472–1474. [Google Scholar] [CrossRef]
- Shaala, L.; Asfour, H.; Youssef, D.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New Source of 3D Chitin Scaffolds: The Red Sea Demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Kao, Y.-C.; Yang, P.-H.; Sung, P.-J.; Wen, Z.-H.; Chen, J.-J.; Huang, Y.-B.; Chen, P.-Y. A Small Dibromotyrosine Derivative Purified from Pseudoceratina Sp. Suppresses TGF-β Responsiveness by Inhibiting TGF-β Type I Receptor Serine/Threonine Kinase Activity: S PONGE -D ERIVED D IBROMOTYROSINE M ETABOLITE I NHIBITS TGF-β R ESPONSIVENESS. J. Cell. Biochem. 2016, 117, 2800–2814. [Google Scholar] [CrossRef]
- Shaala, L.; Youssef, D.; Sulaiman, M.; Behery, F.; Foudah, A.; Sayed, K. Subereamolline A as a Potent Breast Cancer Migration, Invasion and Proliferation Inhibitor and Bioactive Dibrominated Alkaloids from the Red Sea Sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2492–2508. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shoer, M.I.; Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Habib, A.-A.M. Bioactive Brominated Metabolites from the Red Sea Sponge Suberea mollis. J. Nat. Prod. 2008, 71, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Bamane, F.H.; Badr, J.M.; Youssef, D.T.A. Brominated Arginine-Derived Alkaloids from the Red Sea Sponge Suberea mollis. J. Nat. Prod. 2011, 74, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.T.; El-Shitany, N.A.; Shaala, L.A.; Ali, S.S.; Azhar, E.I.; Abdel-dayem, U.A.; Youssef, D.T.A. Red Sea Suberea mollis Sponge Extract Protects against CCl 4 -Induced Acute Liver Injury in Rats via an Antioxidant Mechanism. Evid.-Based Compl. Altern. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Ibrahim, A.K.; Khalifa, S.I.; Mesbah, M.K.; Mayer, A.M.S.; van Soest, R.W.M. New anti-inflammatory sterols from the Red Sea sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat. Prod. Commun. 2010, 5, 27–31. [Google Scholar]
- Sobahi, T.R.A.; Ayyad, S.-E.N.; Abdel-Lateff, A.; Algandaby, M.M.; Alorfi, H.S.; Abdel-Naim, A.B. Cytotoxic metabolites from Callyspongia siphonella display antiproliferative activity by inducing apoptosis in HCT-116 cells. Phcog. Mag. 2017, 13, 37. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A.; et al. Bioactive Brominated Oxindole Alkaloids from the Red Sea Sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef] [Green Version]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Foudah, A.I.; Jain, S.; Busnena, B.A.; El Sayed, K.A. Optimization of Marine Triterpene Sipholenols as Inhibitors of Breast Cancer Migration and Invasion. ChemMedChem 2013, 8, 497–510. [Google Scholar] [CrossRef]
- Ito, T.; Aimaiti, S.; Win, N.N.; Kodama, T.; Morita, H. New sesquiterpene lactones, vernonilides A and B, from the seeds of Vernonia anthelmintica in Uyghur and their antiproliferative activities. Bioorganic Med. Chem. Lett. 2016, 26, 3608–3611. [Google Scholar] [CrossRef]
- Akl, M.; Foudah, A.; Ebrahim, H.; Meyer, S.; Sayed, K. The Marine-Derived Sipholenol A-4-O-3′,4′-Dichlorobenzoate Inhibits Breast Cancer Growth and Motility in vitro and in vivo through the Suppression of Brk and FAK Signaling. Mar. Drugs 2014, 12, 2282–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Kawazoe, K.; Kitagawa, I. Araguspongines B, C, D, E, F, G, H, and J, new vasodilative bis-1-oxaquinolizidine alkaloids from an Okinawan marine sponge, Xestospongia sp. Chem. Pharm. Bull. 1989, 37, 1676–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Kelly, M.; Williams, P.G. Xestosaprols from the Indonesian Marine Sponge Xestospongia sp. J. Nat. Prod. 2010, 73, 1188–1191. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Lu, Y.; Lin, X.; Yang, B.; Yang, X.; Liu, Y. Brominated aliphatic hydrocarbons and sterols from the sponge Xestospongia testudinaria with their bioactivities. Chem. Phys. Lipids 2011, 164, 703–706. [Google Scholar] [CrossRef]
- Ankisetty, S.; Slattery, M. Antibacterial Secondary Metabolites from the Cave Sponge Xestospongia sp. Mar. Drugs 2012, 10, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecoy, G.A.U.; Chamni, S.; Suwanborirux, K.; Chanvorachote, P.; Chaotham, C. Jorunnamycin A from Xestospongia sp. Suppresses Epithelial to Mesenchymal Transition and Sensitizes Anoikis in Human Lung Cancer Cells. J. Nat. Prod. 2019, 82, 1861–1873. [Google Scholar] [CrossRef]
- Murtihapsari, M.; Salam, S.; Kurnia, D.; Darwati, D.; Kadarusman, K.; Abdullah, F.F.; Herlina, T.; Husna, M.H.; Awang, K.; Shiono, Y.; et al. A new antiplasmodial sterol from Indonesian marine sponge, Xestospongia sp. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Halim, H.; Chunhacha, P.; Suwanborirux, K.; Chanvorachote, P. Anticancer and antimetastatic activities of Renieramycin M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anticancer Res. 2011, 31, 193–201. [Google Scholar]
- Satyanarayana, S.; Satyavati, D.; Rao, D.V. Hypoglycaemic activity of extracts from soft corals of Andaman and Nicobar coasts in rats. Indian J. Exp. Biol. 2000, 38, 180–181. [Google Scholar]
- Liang, C.-H.; Wang, G.-H.; Liaw, C.-C.; Lee, M.-F.; Wang, S.-H.; Cheng, D.-L.; Chou, T.-H. Extracts from Cladiella australis, Clavularia viridis and Klyxum simplex (Soft Corals) are Capable of Inhibiting the Growth of Human Oral Squamous Cell Carcinoma Cells. Mar. Drugs 2008, 6, 595–606. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Wang, H.-M.; Wen, Y.-S.; Liu, W.; Li, P.-H.; Chiu, C.-C.; Chen, P.-C.; Huang, C.-Y.; Sheu, J.-H.; Wen, Z.-H. 4-(Phenylsulfanyl)butan-2-One Suppresses Melanin Synthesis and Melanosome Maturation In vitro and In vivo. IJMS 2015, 16, 20240–20257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.-H.; Chao, C.-H.; Wu, M.-H.; Sheu, J.-H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Chien, Y.-C.; Pan, C.-H.; Sheu, J.-H.; Chen, C.-Y.; Wu, C.-H. Inhibitory Effect of Dihydroaustrasulfone Alcohol on the Migration of Human Non-Small Cell Lung Carcinoma A549 Cells and the Antitumor Effect on a Lewis Lung Carcinoma-Bearing Tumor Model in C57BL/6J Mice. Mar. Drugs 2014, 12, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wen, Z.-H.; Lee, Y.-H.; Chen, C.-L.; Hung, H.-C.; Chen, C.-H.; Chen, W.-F.; Tsai, M.-C. Dihydroaustrasulfone Alcohol Inhibits PDGF-Induced Proliferation and Migration of Human Aortic Smooth Muscle Cells through Inhibition of the Cell Cycle. Mar. Drugs 2015, 13, 2390–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.-C.; Sheu, M.-J.; Ma, W.-F.; Pan, C.-H.; Sheu, J.-H.; Wu, C.-H. Anti-Restenotic Roles of Dihydroaustrasulfone Alcohol Involved in Inhibiting PDGF-BB-Stimulated Proliferation and Migration of Vascular Smooth Muscle Cells. Mar. Drugs 2015, 13, 3046–3060. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.M.; Khanfar, M.A.; Elnagar, A.Y.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Pachycladins A−E, Prostate Cancer Invasion and Migration Inhibitory Eunicellin-Based Diterpenoids from the Red Sea Soft Coral Cladiella pachyclados. J. Nat. Prod. 2010, 73, 848–853. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Sung, P.-J.; Su, J.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive Cembranoids, Sarcocrassocolides P–R, from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Mar. Drugs 2014, 12, 840–850. [Google Scholar] [CrossRef]
- Su, C.-C.; Su, J.-H.; Lin, J.-J.; Chen, C.-C.; Hwang, W.-I.; Huang, H.H.; Wu, Y.-J. An Investigation into the Cytotoxic Effects of 13-Acetoxysarcocrassolide from the Soft Coral Sarcophyton crassocaule on Bladder Cancer Cells. Mar. Drugs 2011, 9, 2622–2642. [Google Scholar] [CrossRef]
- Su, C.-C.; Chen, J.; Din, Z.-H.; Su, J.-H.; Yang, Z.-Y.; Chen, Y.-J.; Wang, R.; Wu, Y.-J. 13-Acetoxysarcocrassolide Induces Apoptosis on Human Gastric Carcinoma Cells Through Mitochondria-Related Apoptotic Pathways: p38/JNK Activation and PI3K/AKT Suppression. Mar. Drugs 2014, 12, 5295–5315. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-I.; Chen, C.-C.; Chen, J.-C.; Su, J.-H.; Huang, H.H.; Chen, J.Y.-F.; Wu, Y.-J. Proteomic Analysis of Anti-Tumor Effects of 11-Dehydrosinulariolide on CAL-27 Cells. Mar. Drugs 2011, 9, 1254–1272. [Google Scholar] [CrossRef] [Green Version]
- Su, T.-R.; Lin, J.-J.; Chiu, C.-C.; Chen, J.Y.-F.; Su, J.-H.; Cheng, Z.-J.; Hwang, W.-I.; Huang, H.H.; Wu, Y.-J. Proteomic investigation of anti-tumor activities exerted by sinularin against A2058 melanoma cells: Proteomics and 2DE. Electrophoresis 2012, 33, 1139–1152. [Google Scholar] [CrossRef]
- Iguchi, K.; Fukaya, T.; Takahashi, H.; Watanabe, K. Stolonilactone, a novel terpenoid-related compound, isolated from the Okinawan soft coral Clavularia koellikeri. J. Org. Chem. 2004, 69, 4351–4355. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Fukaya, T.; Yasumoto, A.; Watanabe, K. New Marine Sesquiterpenoids and Diterpenoids from the Okinawan Soft Coral Clavularia koellikeri. J. Nat. Prod. 2004, 67, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, Z.; Luo, X.; Liu, J.; Li, G.; Cao, S.; Liu, Q. Clavukoellians A–F, Highly Rearranged Nardosinane Sesquiterpenoids with Antiangiogenic Activity from Clavularia koellikeri. J. Nat. Prod. 2019, 82, 1331–1337. [Google Scholar] [CrossRef]
- Båmstedt, U. Trophodynamics of the two scyphozoan jellyfishes, Aurelia aurita and Cyanea capillata, in western Norway. ICES J. Mar. Sci. 1994, 51, 369–382. [Google Scholar] [CrossRef]
- Holst, S.; Jarms, G. Effects of low salinity on settlement and strobilation of scyphozoa (Cnidaria): Is the lion’s mane Cyanea capillata (L.) able to reproduce in the brackish Baltic Sea? Hydrobiologia 2010, 645, 53–68. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, J.; Wang, Q.; He, Q.; Liu, S.; Li, Y.; Zhang, L. In vitro and in vivo haemolytic studies of tentacle-only extract from jellyfish Cyanea capillata. Toxicol. In Vitro 2010, 24, 1203–1207. [Google Scholar] [CrossRef]
- Doyle, T.; Headlam, J.; Wilcox, C.; MacLoughlin, E.; Yanagihara, A. Evaluation of Cyanea capillata Sting Management Protocols Using Ex Vivo and In vitro Envenomation Models. Toxins 2017, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Z.; Liu, G.; Guo, Y.; Zhou, Y.; Wang, Q.; Chang, Y.; Wang, B.; Zheng, J.; Zhang, L. First Report of a Thioredoxin Homologue in Jellyfish: Molecular Cloning, Expression and Antioxidant Activity of CcTrx1 from Cyanea capillata. PLoS ONE 2014, 9, e97509. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Z.; Liu, G.; Wang, B.; Zhou, Y.; Lu, J.; Wang, Q.; Zhao, J.; Zhang, L. First Report of a Peroxiredoxin Homologue in Jellyfish: Molecular Cloning, Expression and Functional Characterization of CcPrx4 from Cyanea capillata. Mar. Drugs 2014, 12, 214–231. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.; Wang, C.; Ruan, Z.; Wang, Q.; Wang, B.; Qiu, L.; Zou, S.; Zhang, X.; Zhang, L. Molecular cloning and functional characterization of a Cu/Zn superoxide dismutase from jellyfish Cyanea capillata. Int. J. Biol. Macromol. 2020, 144, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, G.; Cheng, X.; Wang, Q.; Wang, B.; Wang, B.; Zhang, H.; He, Q.; Zhang, L. Antimicrobial activity of a newly identified Kazal-type serine proteinase inhibitor, CcKPI1, from the jellyfish Cyanea capillata. Int. J. Biol. Macromol. 2018, 107, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, D.; Wang, C.; Wang, Q.; Zhang, H.; Liu, G.; He, Q.; Zhang, L. Tentacle extract from the jellyfish Cyanea capillata increases proliferation and migration of human umbilical vein endothelial cells through the ERK1/2 signaling pathway. PLoS ONE 2017, 12, e0189920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, B.; Wang, B.; Wang, Q.; Liu, G.; Fan, C.; Zhang, L. A novel granulin homologue isolated from the jellyfish Cyanea capillata promotes proliferation and migration of human umbilical vein endothelial cells through the ERK1/2-signaling pathway. Int. J. Biol. Macromol. 2019, 135, 212–225. [Google Scholar] [CrossRef]
- Liu, C.; Chen, S.; Zhuang, Z.; Yan, J.; Liu, C.; Cui, H. Potential of utilizing jellyfish as food in culturing Pampus argenteus juveniles. Hydrobiologia 2015, 754, 189–200. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, M.; Xu, J.; Guo, C.; Zheng, H.; Hu, J.; Chen, J.; Wang, Y.; Xu, S.; Yan, X. Lipid Profile in Different Parts of Edible Jellyfish Rhopilema esculentum. J. Agric. Food Chem. 2015, 63, 8283–8291. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Dong, X.; Li, C.; Xing, R.; Liu, S.; Li, P. Insecticidal activity of proteinous venom from tentacle of jellyfish Rhopilema esculentum Kishinouye. Bioorganic Med. Chem. Lett. 2005, 15, 4949–4952. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Xing, R.; Liu, S.; Li, C.; Li, P. Radical scavenging activity of protein from tentacles of jellyfish Rhopilema esculentum. Bioorganic Med. Chem. Lett. 2005, 15, 2659–2664. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Xing, R.; Liu, S.; Guo, Z.; Wang, P.; Li, C.; Li, P. In vitro determination of antioxidant activity of proteins from jellyfish Rhopilema esculentum. Food Chem. 2006, 95, 123–130. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Pustlauk, W.; Paul, B.; Brueggemeier, S.; Gelinsky, M.; Bernhardt, A. Modulation of chondrogenic differentiation of human mesenchymal stem cells in jellyfish collagen scaffolds by cell density and culture medium: Chondrogenic differentiation of hMSCs in jellyfish collagen scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices: Jellyfish collagen matrices conserve the chondrogenic phenotype. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef] [PubMed]
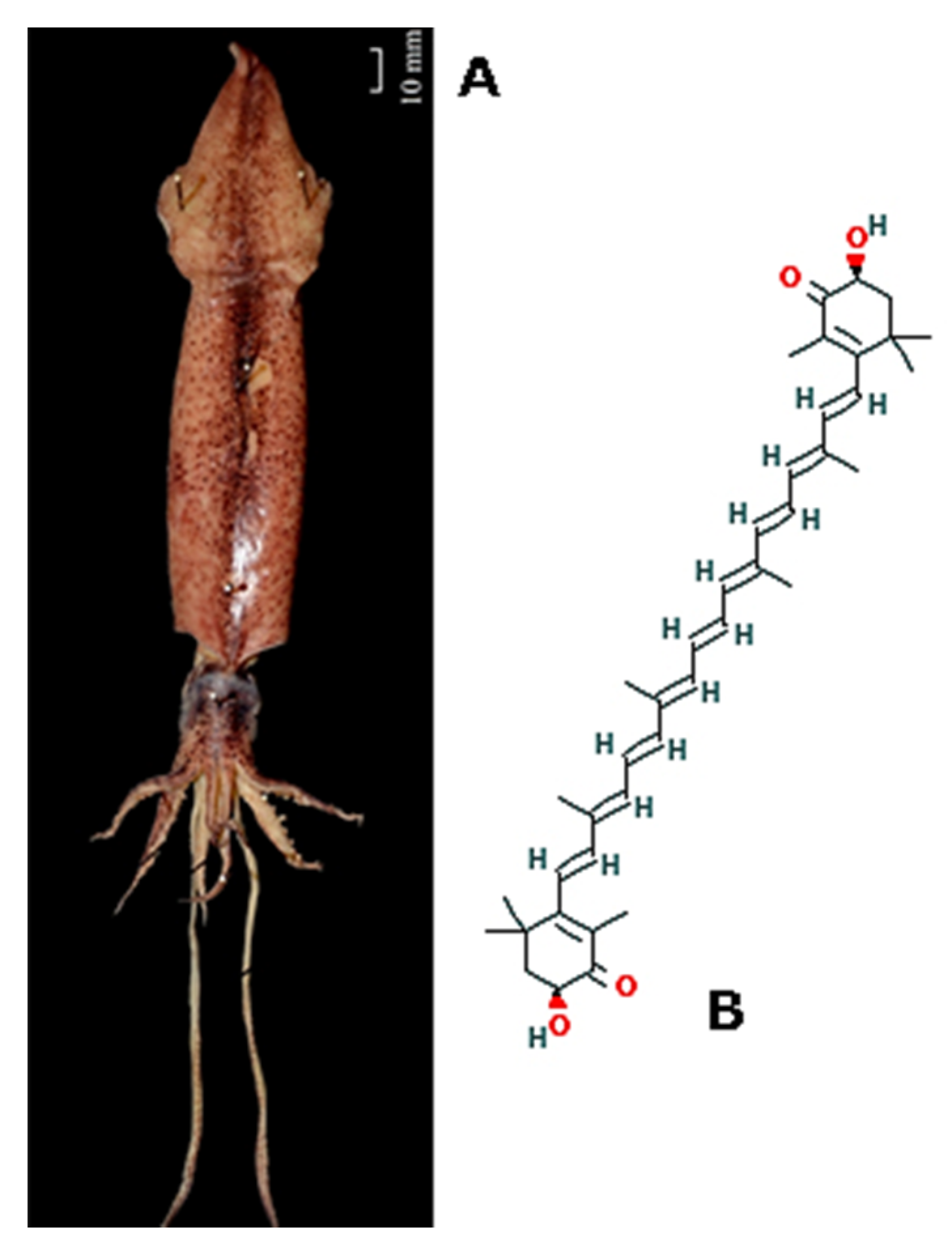
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Thinesh, T.; Selvin, J.; Selvan, K.M.; Shanmugam, V.; Shanmugam, A. Characterization of bioactive chitosan and sulfated chitosan from Doryteuthis singhalensis (Ortmann, 1891). Int. J. Biol. Macromol. 2017, 99, 682–691. [Google Scholar] [CrossRef]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Sudharsan, S.; Seedevi, P.; Shanmugam, V.; Shanmugam, A. Nutritional evaluation of the different body parts of cuttlefish Sepia kobiensis Hoyle, 1885. Afr. J. Food Sci. 2012, 6, 535–538. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Srinivasan, A.; Shanmugam, V.; Krishnamoorthy, J.; Shanmugam, A. In vitro evaluation of antimicrobial activity of methanolic extract from selected species of Cephalopods on clinical isolates. Afr. J. Microbiol. Res. 2011, 5, 3884–3889. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Protective effect of chitosan from Sepia kobiensis (Hoyle 1885) cuttlebone against CCl4 induced hepatic injury. Int. J. Biol. Macromol. 2014, 65, 559–563. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and antioxidant property of chitosan from cuttlebone Sepia kobiensis (Hoyle 1885). Int. J. Biol. Macromol. 2014, 64, 202–212. [Google Scholar] [CrossRef]
- Shanmugam, A.; Kathiresan, K.; Nayak, L. Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885). Biotechnol. Rep. 2016, 9, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, P.; Shanmugam, A. Characterization and wound healing property of collagen–chitosan film from Sepia kobiensis (Hoyle, 1885). Int. J. Biol. Macromol. 2015, 74, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Lassoued, I.; Kammoun, A.; Nasri, R.; Chaâbouni, M.; Nasri, M.; Souissi, N. Screening of factors influencing the extraction of gelatin from the skin of cuttlefish using supersaturated design. Food Bioprod. Proc. 2015, 94, 525–535. [Google Scholar] [CrossRef]
- García-Enriquez, S.; Guadarrama, H.E.R.; Reyes-González, I.; Mendizábal, E.; Jasso-Gastinel, C.F.; García-Enriquez, B.; Rembao-Bojórquez, D.; Pane-Pianese, C. Mechanical Performance and In vivo Tests of an Acrylic Bone Cement Filled with Bioactive Sepia Officinalis Cuttlebone. J. Biomater. Sci. Poly. Ed. 2010, 21, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Jridi, M.; Abdelhedi, O.; Nasreddine, B.; Karbowiak, T.; Nasri, M.; Debeaufort, F. Development and characterization of cuttlefish (Sepia officinalis) skin gelatin-protein isolate blend films. Int. J. Biol. Macromol. 2017, 105, 1491–1500. [Google Scholar] [CrossRef]
- Parisi, M.G.; Mauro, M.; Sarà, G.; Cammarata, M. Temperature increases, hypoxia, and changes in food availability affect immunological biomarkers in the marine mussel Mytilus galloprovincialis. J. Comp. Physiol. B 2017, 187, 1117–1126. [Google Scholar] [CrossRef]
- Vazzana, M.; Ceraulo, M.; Mauro, M.; Papale, E.; Dioguardi, M.; Mazzola, S.; Arizza, V.; Chiaramonte, M.; Buscaino, G. Effects of acoustic stimulation on biochemical parameters in the digestive gland of Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819). J. Acoust. Soc. Amer. 2020, 147, 2414–2422. [Google Scholar] [CrossRef]
- Pogoda, B.; Buck, B.H.; Saborowski, R.; Hagen, W. Biochemical and elemental composition of the offshore-cultivated oysters Ostrea edulis and Crassostrea gigas. Aquaculture 2013, 400–401, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Prato, E.; Biandolino, F.; Parlapiano, I.; Giandomenico, S.; Denti, G.; Calò, M.; Spada, L.; Di Leo, A. Proximate, fatty acids and metals in edible marine bivalves from Italian market: Beneficial and risk for consumers health. Sci. Total Environ. 2019, 648, 153–163. [Google Scholar] [CrossRef]
- Prato, E.; Fanelli, G.; Parlapiano, I.; Biandolino, F. Bioactive fatty acids in seafood from Ionian Sea and relation to dietary recommendations. Int. J. Food Sci. Nutr. 2020, 1–13. [Google Scholar] [CrossRef]
- Chandler, E.A.; McDOWELL, J.R.; Graves, J.E. Genetically monomorphic invasive populations of the rapa whelk, Rapana venosa. Mol. Ecol. 2008, 17, 4079–4091. [Google Scholar] [CrossRef]
- Lanfranconi, A.; Hutton, M.; Brugnoli, E.; Muniz, P. New record of the alien mollusc Rapana venosa (Valenciennes 1846) in the Uruguayan coastal zone of Río de la Plata. Pan-Am. J. Aquat. Sci. 2009, 4, 216–221. [Google Scholar]
- Dalgic, G.; Kiris, E.; Baltas, H.; Sirin, M. Determination of radiological hazard parameters in sea snails (Rapana venosa) in the East Black Sea Coast of Turkey. Mar. Pollut. Bull. 2018, 135, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Zhelyazkov, G.; Yankovska-Stefanova, T.; Mineva, E.; Stratev, D.; Vashin, I.; Dospatliev, L.; Valkova, E.; Popova, T. Risk assessment of some heavy metals in mussels (Mytilus galloprovincialis) and veined rapa whelks (Rapana venosa) for human health. Mar. Pollut. Bull. 2018, 128, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Dolashka-Angelova, P.; Lieb, B.; Velkova, L.; Heilen, N.; Sandra, K.; Nikolaeva-Glomb, L.; Dolashki, A.; Galabov, A.S.; Beeumen, J.V.; Stevanovic, S.; et al. Identification of Glycosylated Sites in Rapana Hemocyanin by Mass Spectrometry and Gene Sequence, and Their Antiviral Effect. Bioconjugate Chem. 2009, 20, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, O.; Dolashka, P.; Toncheva, D.; Rammensee, H.-G.; Stevanović, S. In vitro effect of molluscan hemocyanins on CAL-29 and T-24 bladder cancer cell lines. Biomed. Rep. 2013, 1, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial Activity of Molluscan Hemocyanins from Helix and Rapana Snails. CPB 2016, 17, 263–270. [Google Scholar] [CrossRef]
- Zhao, Q.; Liang, Y.; Liu, Q.; Zhang, Z.; Yu, Z.; Ren, L. Study on the Mechanical Properties of Bionic Coupling Layered B4C/5083Al Composite Materials. Materials 2018, 11, 680. [Google Scholar] [CrossRef] [Green Version]
- Badiu, D.L.; Balu, A.M.; Barbes, L.; Luque, R.; Nita, R.; Radu, M.; Tanase, E.; Rosoiu, N. Physico-Chemical Characterisation of Lipids from Mytilus galloprovincialis (L.) and Rapana venosa and their Healing Properties on Skin Burns. Lipids 2008, 43, 829–841. [Google Scholar] [CrossRef]
- Badiu, D.L.; Luque, R.; Dumitrescu, E.; Craciun, A.; Dinca, D. Amino Acids from Mytilus galloprovincialis (L.) and Rapana venosa Molluscs Accelerate Skin Wounds Healing via Enhancement of Dermal and Epidermal Neoformation. Protein J. 2010, 29, 81–92. [Google Scholar] [CrossRef]
- Gavrilović, A.; Piria, M.; Guo, X.-Z.; Jug-Dujaković, J.; Ljubučić, A.; Krkić, A.; Iveša, N.; Marshall, B.A.; Gardner, J.P.A. First evidence of establishment of the rayed pearl oyster, Pinctada imbricata radiata (Leach, 1814), in the eastern Adriatic Sea. Mar. Pollut. Bull. 2017, 125, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Q.; Hao, R.; Liao, Y.; Du, X.; Deng, Y. Effects of replacing microalgae with an artificial diet on pearl production traits and mineralization-related gene expression in pearl oyster Pinctada fucata martensii. Aquac. Res. 2017, 48, 5331–5337. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, H.; Zhu, J.; Chen, X.; Wang, A.; Wang, Y. Characterization of novel EST-SSR markers and their correlations with growth and nacreous secretion traits in the pearl oyster Pinctada martensii (Dunker). Aquaculture 2014, 420–421, S92–S97. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Liu, Y.-L.; Lin, J.-B.; Cheng, K.-L.; Wang, Y.-G.; Yao, H.-L.; Wei-Peng; Wu, H.-Y.; Su, W.-W.; Shaw, P.-C.; et al. Preparative expression and purification of a nacreous protein N16 and testing its effect on osteoporosis rat model. Int. J. Biol. Macromol. 2018, 111, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada fucata martensii) Meat Protein Hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Liang, H.; Zhu, J.; Fang, X. Separation, identification and gene expression analysis of PmAMP-1 from Pinctada fucata martensii. Fish. Shellfish Immunol. 2019, 92, 728–735. [Google Scholar] [CrossRef]
- Yang, F.; Qin, X.; Zhang, T.; Lin, H.; Zhang, C. Evaluation of Small Molecular Polypeptides from the Mantle of Pinctada Martensii on Promoting Skin Wound Healing in Mice. Molecules 2019, 24, 4231. [Google Scholar] [CrossRef] [Green Version]
- Apte, D. Field Guide to the Marine Life of India; Animesh Apte: Mumbai, India, 2012; ISBN 978-93-5067-144-3. [Google Scholar]
- Ponkshe, C.A.; Indap, M.M. In vivo and in vitro evaluation for immunomodulatory activity of three marine animal extracts with reference to phagocytosis. Indian J. Exp. Biol. 2002, 40, 1399–1402. [Google Scholar]
- Akerkar, A.S.; Ponkshe, C.A.; Indap, M.M. Evaluation of immunomodulatory activity of extracts from marine animals. Indian J. Mar. Sci. 2009, 38, 6. [Google Scholar]
- Balakrishnan, B.; Chiplunkar, S.V.; Indap, M.M. Methanol Extract of Euchelus asper Prevents Bone Resorption in Ovariectomised Mice Model. J. Osteoporos. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Chaugule, S.; More, S.; Rane, G.; Indap, M. Methanolic extract of Euchelus asper exhibits in-ovo anti-angiogenic and in vitro anti-proliferative activities. Biol. Res. 2017, 50, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Vacquier, V.D. Evolution and systematics in Haliotidae (Mollusca: Gastropoda): Inferences from DNA sequences of sperm lysin. Mar. Biol. 1995, 124, 267–278. [Google Scholar] [CrossRef]
- Lu, J.; Feng, J.; Cai, S.; Chen, Z. Metabolomic responses of Haliotis diversicolor to organotin compounds. Chemosphere 2017, 168, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, X.; Wang, Y.; Day, R.; Yang, H.; Zhang, Z. Immunity-related genes and signaling pathways under hypoxic stresses in Haliotis diversicolor: A transcriptome analysis. Sci. Rep. 2019, 9, 19741. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Sun, Y.; Zhang, X.; Wang, G.; Li, Y.; Wang, Y.; Zhang, Z. Responses of HSP70 Gene to Vibrio parahaemolyticus Infection and Thermal Stress and Its Transcriptional Regulation Analysis in Haliotis diversicolor. Molecules 2019, 24, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Cui, L.; Lin, Y.; Wu, Y. Protective effect of haliotidis on the oxidative damage in the human lens epithelial cells. Zhonghua Yan Ke Za Zhi 2013, 49, 817–821. [Google Scholar]
- Chen, Z.-C.; Wu, S.-Y.S.; Su, W.-Y.; Lin, Y.-C.; Lee, Y.-H.; Wu, W.-H.; Chen, C.-H.; Wen, Z.-H. Anti-inflammatory and burn injury wound healing properties of the shell of Haliotis diversicolor. BMC Complement. Altern. Med. 2016, 16, 487. [Google Scholar] [CrossRef] [Green Version]
- Nicol, S.; Constable, A.J.; Pauly, T. Estimates of Circumpolar Abundance of Antarctic Krill Based on Recent Acoustic Density Measurements. CCAMLR Sci. 2000, 7. [Google Scholar]
- Liu, Z.-J.; Xu, L.-X.; Zhu, G.-P. Behavioral and physiological ecology of Antarctic krill (Euphausia superba): A review. Ying Yong Sheng Tai Xue Bao 2019, 30, 4344–4352. [Google Scholar] [CrossRef]
- Schmidt, K.; Atkinson, A.; Steigenberger, S.; Fielding, S.; Lindsay, M.C.M.; Pond, D.W.; Tarling, G.A.; Klevjer, T.A.; Allen, C.S.; Nicol, S.; et al. Seabed foraging by Antarctic krill: Implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol. Oceanogr. 2011, 56, 1411–1428. [Google Scholar] [CrossRef]
- Murphy, E.J.; Hofmann, E.E.; Watkins, J.L.; Johnston, N.M.; Piñones, A.; Ballerini, T.; Hill, S.L.; Trathan, P.N.; Tarling, G.A.; Cavanagh, R.A.; et al. Comparison of the structure and function of Southern Ocean regional ecosystems: The Antarctic Peninsula and South Georgia. J. Mar. Syst. 2013, 109–110, 22–42. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Yan, J.; Li, Y. Gene analysis and structure prediction for the cold-adaption mechanism of trypsin from the krill Euphausia superba (Dana, 1852): Gene analysis and structure prediction for the cold-adaption mechanism. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Winther, B.; Hoem, N.; Berge, K.; Reubsaet, L. Elucidation of Phosphatidylcholine Composition in Krill Oil Extracted from Euphausia superba. Lipids 2011, 46, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Backes, J.M.; Howard, P.A. Krill Oil for Cardiovascular Risk Prevention: Is it for Real? Hosp. Pharm. 2014, 49, 907–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwantes, J.M.; Grundmann, O. A Brief Review of Krill Oil History, Research, and the Commercial Market. J. Diet. Suppl. 2015, 12, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jang, J.; Son, D.; Im, H.-S.; Kim, J.; Park, J.; Choi, W.; Han, S.-B.; Hong, J. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. IJMS 2017, 18, 2554. [Google Scholar] [CrossRef] [Green Version]
- Ursoniu, S.; Sahebkar, A.; Serban, M.-C.; Antal, D.; Mikhailidis, D.P.; Cicero, A.; Athyros, V.; Rizzo, M.; Rysz, J.; Banach, M. Lipid-modifying effects of krill oil in humans: Systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2017, 75, 361–373. [Google Scholar] [CrossRef]
- Mao, L.; Wang, F.; Li, Y.; Dai, Y.; Liu, Y.; Wang, J.; Xue, C. Oil from Antarctic krill (Euphausia superba) facilitates bone formation in dexamethasone-treated mice. Food Sci. Biotechnol. 2019, 28, 539–545. [Google Scholar] [CrossRef]
- Costanzo, M.; Cesi, V.; Prete, E.; Negroni, A.; Palone, F.; Cucchiara, S.; Oliva, S.; Leter, B.; Stronati, L. Krill oil reduces intestinal inflammation by improving epithelial integrity and impairing adherent-invasive Escherichia coli pathogenicity. Dig. Liver Dis. 2016, 48, 34–42. [Google Scholar] [CrossRef]
- Schillaci, D.; Cusimano, M.; Cunsolo, V.; Saletti, R.; Russo, D.; Vazzana, M.; Vitale, M.; Arizza, V. Immune mediators of sea-cucumber Holothuria tubulosa (Echinodermata) as source of novel antimicrobial and anti-staphylococcal biofilm agents. AMB Express 2013, 3, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzara, V.; Arizza, V.; Luparello, C.; Mauro, M.; Vazzana, M. Bright Spots in The Darkness of Cancer: A Review of Starfishes-Derived Compounds and Their Anti-Tumor Action. Mar. Drugs 2019, 17, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Celi, M.; Arizza, V.; Vazzana, M. Cytotoxic Potential of the Coelomic Fluid Extracted from the Sea Cucumber Holothuria tubulosa against Triple-Negative MDA-MB231 Breast Cancer Cells. Biology 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Arizza, V.; Vazzana, M. Cell-Free Coelomic Fluid Extracts of the Sea Urchin Arbacia lixula Impair Mitochondrial Potential and Cell Cycle Distribution and Stimulate Reactive Oxygen Species Production and Autophagic Activity in Triple-Negative MDA-MB231 Breast Cancer Cells. JMSE 2020, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Coteur, G.; Gosselin, P.; Wantier, P.; Chambost-Manciet, Y.; Danis, B.; Pernet, P.; Warnau, M.; Dubois, P. Echinoderms as Bioindicators, Bioassays, and Impact Assessment Tools of Sediment-Associated Metals and PCBs in the North Sea. Arch. Environ. Contam. Toxicol. 2003, 45, 190–202. [Google Scholar] [CrossRef]
- Vazzana, M.; Mauro, M.; Ceraulo, M.; Dioguardi, M.; Papale, E.; Mazzola, S.; Arizza, V.; Beltrame, F.; Inguglia, L.; Buscaino, G. Underwater high frequency noise: Biological responses in sea urchin Arbacia lixula (Linnaeus, 1758). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110650. [Google Scholar] [CrossRef]
- Baveja, M.; Sarkar, A.; Mondal, S.; Pathan, J.; Chakrabarty, D. AiP1, a protein from the coelomic fluid of sea star Astropecten indicus promotes wound healing and fibrinogenolysis in vitro. JoBAZ 2019, 80, 50. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.M.; Rowe, F.W.E. Monograph of Shallow-Water Indo-West. Pacific Echinoderms; British Museum (Natural History): London, UK, 1971; ISBN 978-0-565-00690-7. [Google Scholar]
- Candia Carnevali, M. Regeneration in Echinoderms: Repair, regrowth, cloning. ISJ 2006, 3, 64–76. [Google Scholar]
- Mozzi, D.; Dolmatov, I.; Bonasoro, F.; Carnevali, M. Visceral regeneration in the crinoid Antedon mediterranea: Basic mechanisms, tissues and cells involved in gut regrowth. Open Life Sci. 2006, 1, 609–635. [Google Scholar] [CrossRef] [Green Version]
- Gahn, F.J.; Baumiller, T.K. Evolutionary History of Regeneration in Crinoids (Echinodermata). Integr. Comp. Biol. 2010, 50, 514. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskaya, N.V.; Dolmatov, I.Y. Autotomy of the Visceral Mass in the Feather Star Himerometra robustipinna (Crinoidea, Comatulida). Biol. Bull. 2014, 226, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kalacheva, N.V.; Eliseikina, M.G.; Frolova, L.T.; Dolmatov, I.Y. Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory-like cells. PLoS ONE 2017, 12, e0182001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.-C.; Lai, Y.-C.; Kuo, T.-J.; Su, J.-H.; Sung, P.-J.; Feng, C.-W.; Lin, Y.-Y.; Chen, P.-C.; Tai, M.-H.; Cheng, S.-Y.; et al. Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells. Mar. Drugs 2019, 17, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Ren, Y.; Wang, F.; Yu, D.; Cui, G.; Chen, J. A comparative study on growth, protein turnover and energy budget of green and white color morphs of sea cucumber Apostichopus japonicus (Selenka). Aquacult. Environ. Interact. 2017, 9, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Husni, A.; Shin, I.-S.; You, S.; Chung, D. Antioxidant Properties of Water and Aqueous Ethanol Extracts and Their Crude Saponin Fractions from a Far-eastern Sea Cucumber. Food Sci. Biotechnol. 2009, 18, 419–424. [Google Scholar]
- Nguyen, T.H.; Um, B.H.; Kim, S.M. Two Unsaturated Fatty Acids with Potent α-Glucosidase Inhibitory Activity Purified from the Body Wall of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2011, 76, H208–H214. [Google Scholar] [CrossRef]
- Guan, R.; Peng, Y.; Zhou, L.; Zheng, W.; Liu, X.; Wang, P.; Yuan, Q.; Gao, N.; Zhao, L.; Zhao, J. Precise Structure and Anticoagulant Activity of Fucosylated Glycosaminoglycan from Apostichopus japonicus: Analysis of Its Depolymerized Fragments. Mar. Drugs 2019, 17, 195. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, L.; Yu, M.; Wang, Y.; Jiang, T.; Yang, S.; Lv, Z. Glycosaminoglycan from Apostichopus japonicus induces immunomodulatory activity in cyclophosphamide-treated mice and in macrophages. Int. J. Biol. Macromol. 2019, 130, 229–237. [Google Scholar] [CrossRef]
- Zhu, Z.; Dong, X.; Yan, C.; Ai, C.; Zhou, D.; Yang, J.; Zhang, H.; Liu, X.; Song, S.; Xiao, H.; et al. Structural Features and Digestive Behavior of Fucosylated Chondroitin Sulfate from Sea Cucumbers Stichopus japonicus. J. Agric. Food Chem. 2019, 67, 10534–10542. [Google Scholar] [CrossRef]
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Sun, L.; Yuan, J.; Sun, Y.; Gao, Y.; Zhang, L.; Li, S.; Dai, H.; Hamel, J.-F.; Liu, C.; et al. The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 2017, 15, e2003790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Lim, H.K.; Lee, S.; Hwang, H.C.; Cho, S.K.; Cho, M. Pepsin-solubilised collagen (PSC) from Red Sea cucumber (Stichopus japonicus) regulates cell cycle and the fibronectin synthesis in HaCaT cell migration. Food Chem. 2012, 132, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Ishida, M.; Aida, K.; Tsuduki, T.; Zhang, J.; Manabe, Y.; Hirata, T.; Sugawara, T. Dietary Cerebroside from Sea Cucumber (Stichopus japonicus): Absorption and Effects on Skin Barrier and Cecal Short-Chain Fatty Acids. J. Agric. Food Chem. 2016, 64, 7014–7021. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Li, Q.; Qi, X.; Yang, J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohyd. Poly. 2018, 185, 41–47. [Google Scholar] [CrossRef]
- Kwon, T.-R.; Oh, C.T.; Bak, D.-H.; Kim, J.H.; Seok, J.; Lee, J.H.; Lim, S.H.; Yoo, K.H.; Kim, B.J.; Kim, H. Effects on skin of Stichopus japonicus viscera extracts detected with saponin including Holothurin A: Down-regulation of melanin synthesis and up-regulation of neocollagenesis mediated by ERK signaling pathway. J. Ethnopharmacol. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Pastrana, O.J.; Santafé, G.G.; Torres, O.L. Perfil de Ácidos Grasos y Evaluación de las Actividades Antioxidante y Antifúngica del Holotureo Isostichopus badionotus. Inf. Tecnol. 2016, 27, 03–10. [Google Scholar] [CrossRef]
- Olivera-Castillo, L.; Davalos, A.; Grant, G.; Valadez-Gonzalez, N.; Montero, J.; Barrera-Perez, H.A.M.; Chim-Chi, Y.; Olvera-Novoa, M.A.; Ceja-Moreno, V.; Acereto-Escoffie, P.; et al. Diets Containing Sea Cucumber (Isostichopus badionotus) Meals Are Hypocholesterolemic in Young Rats. PLoS ONE 2013, 8, e79446. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Poly. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Ye, X.; Li, G.; Yu, G.; Xue, C.; Chai, W. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 989–1000. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Zhi, Z.; Yan, L.; Ye, X.; Ding, T.; Yan, L.; Linhardt, R.; Chen, S. Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments. Mar. Drugs 2016, 14, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, J.; Vilanova, E.; Mourão, P.A.S.; Fernàndez-Busquets, X. Marine organism sulfated polysaccharides exhibiting significant antimalarial activity and inhibition of red blood cell invasion by Plasmodium. Sci. Rep. 2016, 6, 24368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Li, J.; Mao, G.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. Fucosylated chondroitin sulfate oligosaccharides from Isostichopus badionotus regulates lipid disorder in C57BL/6 mice fed a high-fat diet. Carbohydr. Polym. 2018, 201, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Courtman-Stock, J. The Echinoderms of Southern Africa; British Museum (Natural History): London, UK, 1976; ISBN 978-0-565-00776-8. [Google Scholar]
- Baharara, J.; Amini, E. Phytochemical Screening, Antioxidant Effect and Down Regulation of TGF-β Induced by Ophiocoma erinaceus Brittle Star Crude Extract. Zahedan J. Res. Med. Sci. 2015, 17. [Google Scholar] [CrossRef]
- Kachooei, S.A.; Rahmani, R.; Zareh, N.; Donyadideh, F.; Kachooei, S.A.; Nabiuni, M.; Yazdansetad, S. Down-regulation of TGF-β, VEGF, and bFGF in vascular endothelial cells of chicken induced by a brittle star (Ophiocoma erinaceus) extract. Heliyon 2020, 6, e03199. [Google Scholar] [CrossRef] [Green Version]
- Baharara, J.; Amini, E.; Musavi, M. Anti-Vasculogenic Activity of a Polysaccharide Derived from Brittle Star via Inhibition of VEGF, Paxillin and MMP-9. Iran. J. Biotechnol. 2017, 15, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Abolhasani, I.; Baharara, J.; Mahdavi Shahri, N.; Amini, E. The regenerative properties of the extracted polysaccharide from Brittle star (Ophiocoma erinaceus) on cutaneous wound in male Wistar rat. nbr 2020, 7, 9–18. [Google Scholar]
- Amini, E.; Nabiuni, M.; Baharara, J.; Parivar, K.; Asili, J. Metastatic Inhibitory and Radical Scavenging Efficacies of Saponins Extracted from the Brittle Star (Ophiocoma erinaceus). Asian Pac. J. Cancer Prev. 2015, 16, 4751–4758. [Google Scholar] [CrossRef] [Green Version]
- Amini, E.; Nabiuni, M.; Baharara, J.; Parivar, K.; Asili, J. In-vitro Pro Apoptotic Effect of Crude Saponin from Ophiocoma erinaceus against Cervical Cancer. Iran. J. Pharm. Res. 2017, 16, 266–276. [Google Scholar]
- Baharara, J.; Amini, E.; Salek-Abdollahi, F. Anti-inflammatory properties of saponin fraction from Ophiocoma erinaceus. Iran. J. Fish. Sci. 2019. [Google Scholar] [CrossRef]
- Khazaali, A.; Kunzmann, A.; Bastami, K.D.; Baniamam, M. Baseline of polycyclic aromatic hydrocarbons in the surface sediment and sea cucumbers (Holothuria leucospilota and Stichopus hermanni) in the northern parts of Persian Gulf. Mar. Pollut. Bull. 2016, 110, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningtyas, E.; Sugiatno, E. Stichopus Hermanni Collagen with Local Hydroxyapatite as Bone Substitute Material Toward Osteoclast Number and Toxicity. In Proceedings of the 2018 1st International Conference on Bioinformatics, Biotechnology, and Biomedical Engineering Bioinformatics and Biomedical Engineering, Yogyakarta, Indonesia, 19–20 October 2018 ; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- Zohdi, R.M.; Zakaria, Z.A.B.; Yusof, N.; Mustapha, N.M.; Abdullah, M.N.H. Sea cucumber (Stichopus hermanii) based hydrogel to treat burn wounds in rats. J. Biomed. Mater. Res. 2011, 98B, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arundina, I.; Yuliati, Y.; Soesilawati, P.; Damaiyanti, D.W.; Maharani, D. The effects of golden sea cucumber extract (Stichopus hermanii) on the number of lymphocytes during the healing process of traumatic ulcer on wistar rat’s oral mucous. Dent. J. (Majalah Kedokteran Gigi) 2015, 48, 100. [Google Scholar] [CrossRef] [Green Version]
- Arundina, I.; Suardita, K.; Setiabudi, H.; Dwi Ariani, M. Golden Sea Cucumbers (Stichopus Hermanii) as Growth Factors of Stem Cells. J. Int. Dental Med. Res. 2016, 9, 242–248. [Google Scholar]
- Kimura, S.; Ohshima, C.; Hirose, E.; Nishikawa, J.; Itoh, T. Cellulose in the house of the appendicularian Oikopleura rufescens. Protoplasma 2001, 216, 71–74. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-Responsive Polymer Nanocomposites Inspired by the Sea Cucumber Dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef]
- Kime-Hunt, E.; Spartalian, K.; Holmes, S.; Mohan, M.; Carrano, C.J. Vanadium metabolism in tunicates: The coordination chemistry of V(III), V(IV), and V(V) with models for the tunichromes. J. Inorg. Biochem. 1991, 41, 125–141. [Google Scholar] [CrossRef]
- Taylor, S.W.; Ross, M.M.; Waite, J.H. Novel 3,4-Di- and 3,4,5-Trihydroxyphenylalanine-Containing Polypeptides from the Blood Cells of the Ascidians Ascidia ceratodes and Molgula manhattensis. Arch. Biochem. Biophy. 1995, 324, 228–240. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Quinn, R.J. Longithorones J and K, Two New Cyclofarnesylated Quinone Derived Metabolites from the Australian Ascidian Aplidium longithorax. J. Nat. Prod. 1999, 62, 158–160. [Google Scholar] [CrossRef]
- Mauro, M.; Lazzara, V.; Punginelli, D.; Arizza, V.; Vazzana, M. Antitumoral compounds from vertebrate sister group: A review of Mediterranean ascidians. Dev. Comp. Immunol. 2020, 108, 103669. [Google Scholar] [CrossRef] [PubMed]
- Christy, H.K.S.; Margret, R.J.; Meenakshi, K. Evaluation of wound healing activity of Phallusia Arabica. World J. Pharm. Res. 2015, 4, 13. [Google Scholar]
- Christy, H.K.S.; Margret, R.J.; Meenakshi, V.K. Infrared and gas chromatogram-mass spectral studies of the ethanolic extract of Phallusia arabica Savigny, 1816. Arch. Appl. Sci. Res. 2013, 5, 17–23. [Google Scholar]
- Gopalakrishnan, S.; Meenakshi, V.K.; Shanmuga Priya, D. Chemical Investigation Of The Simple Ascidian Phallusia Nigra Savigny, 1816 Of Tuticorin Coast By Gc-Ms. Int. J. Pharm. Bio. Sci. 2011, 2, 382–387. [Google Scholar]
- Gopalakrishnan, S.B.; Priya, D.S.; Meenakshi, V. Wound healing activity of the methanolic extract of Phallusia nigra Sav. Int. J. Chem. Pharm. Sci. 2012, 3, 8. [Google Scholar]
- Jung, Y.J. Properties of Regenerated Cellulose Films Prepared from the Tunicate Styela clava. Korean J. Fish. Aquat. Sci. 2008, 41, 237–242. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Sung, G.Y.; Kwon, S.H.; Son, H.J.; Lee, H.S.; Jung, Y.J.; Hwang, D.Y. Cellulose film regenerated from Styela clava tunics have biodegradability, toxicity and biocompatibility in the skin of SD rats. J. Mater. Sci. Mater. Med. 2014, 25, 1519–1530. [Google Scholar] [CrossRef]
- Song, S.H.; Seong, K.Y.; Kim, J.E.; Go, J.; Koh, E.K.; Sung, J.E.; Son, H.J.; Jung, Y.J.; Kim, H.S.; Hong, J.T.; et al. Effects of different cellulose membranes regenerated from Styela clava tunics on wound healing. Int. J. Mol. Med. 2017, 39, 1173–1187. [Google Scholar] [CrossRef] [Green Version]
- Song, S.H.; Kim, J.E.; Koh, E.K.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Hong, J.T.; Hwang, D.Y. Selenium-loaded cellulose film derived from Styela clava tunic accelerates the healing process of cutaneous wounds in streptozotocin-induced diabetic Sprague–Dawley rats. J. Dermatol. Treat. 2018, 29, 606–616. [Google Scholar] [CrossRef]
- Sundaram, G.M.; Quah, S.; Sampath, P. Cancer: The dark side of wound healing. FEBS J. 2018, 285, 4516–4534. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications: Antarctic krill (Euphausia superba) oil. Comprehen. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [Green Version]
- Wahyuningtyas, E.; Hsu, L.-C.; Lan, W.-C.; Wen, S.-C.; Ou, K.-L.; Chou, H.-H.; Huang, M.-S.; Sugiatno, E. Application of a Promising Bone Graft Substitute in Bone Tissue Regeneration: Characterization, Biocompatibility, and In vivo Animal Study. BioMed. Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
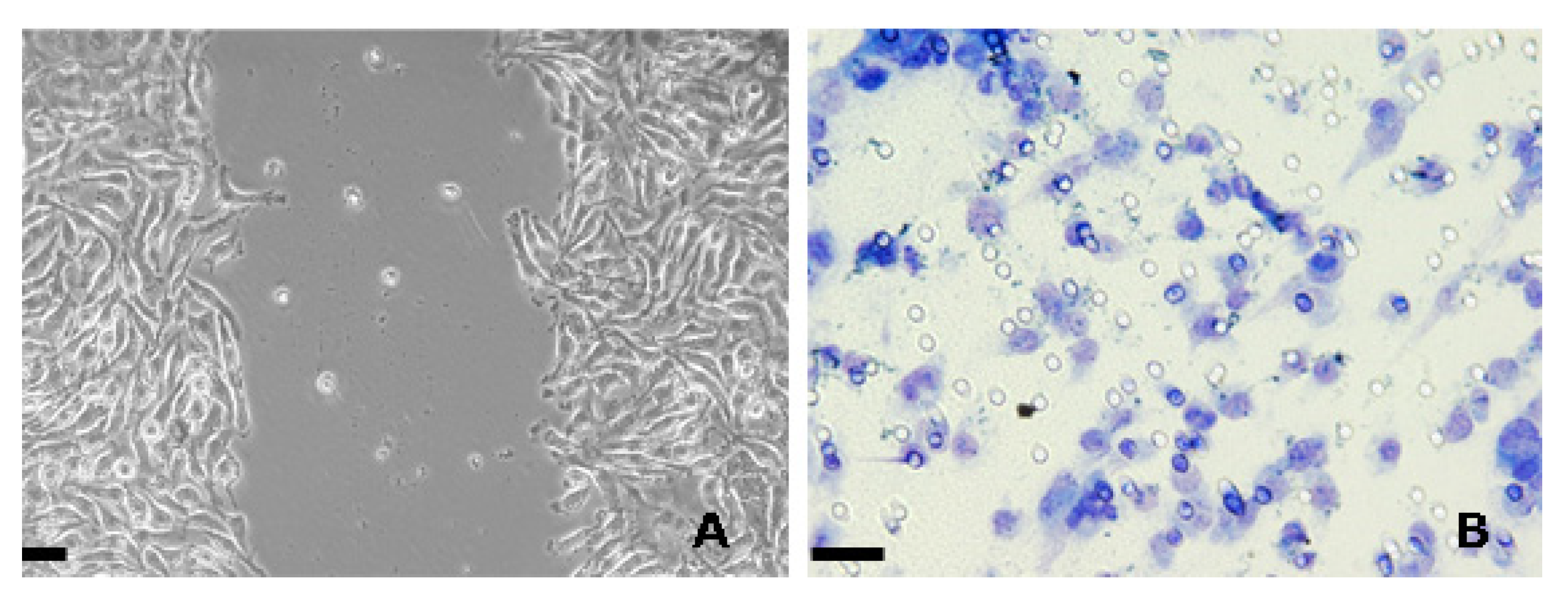

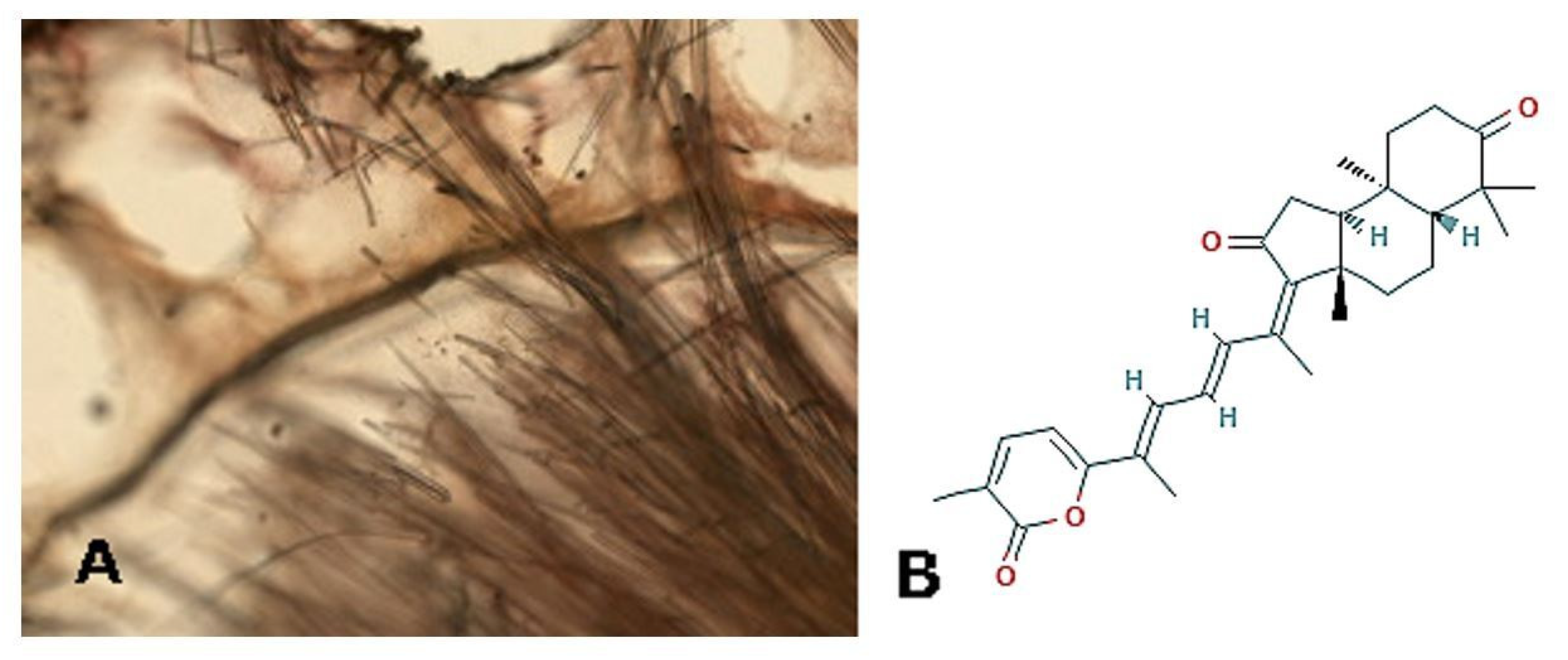
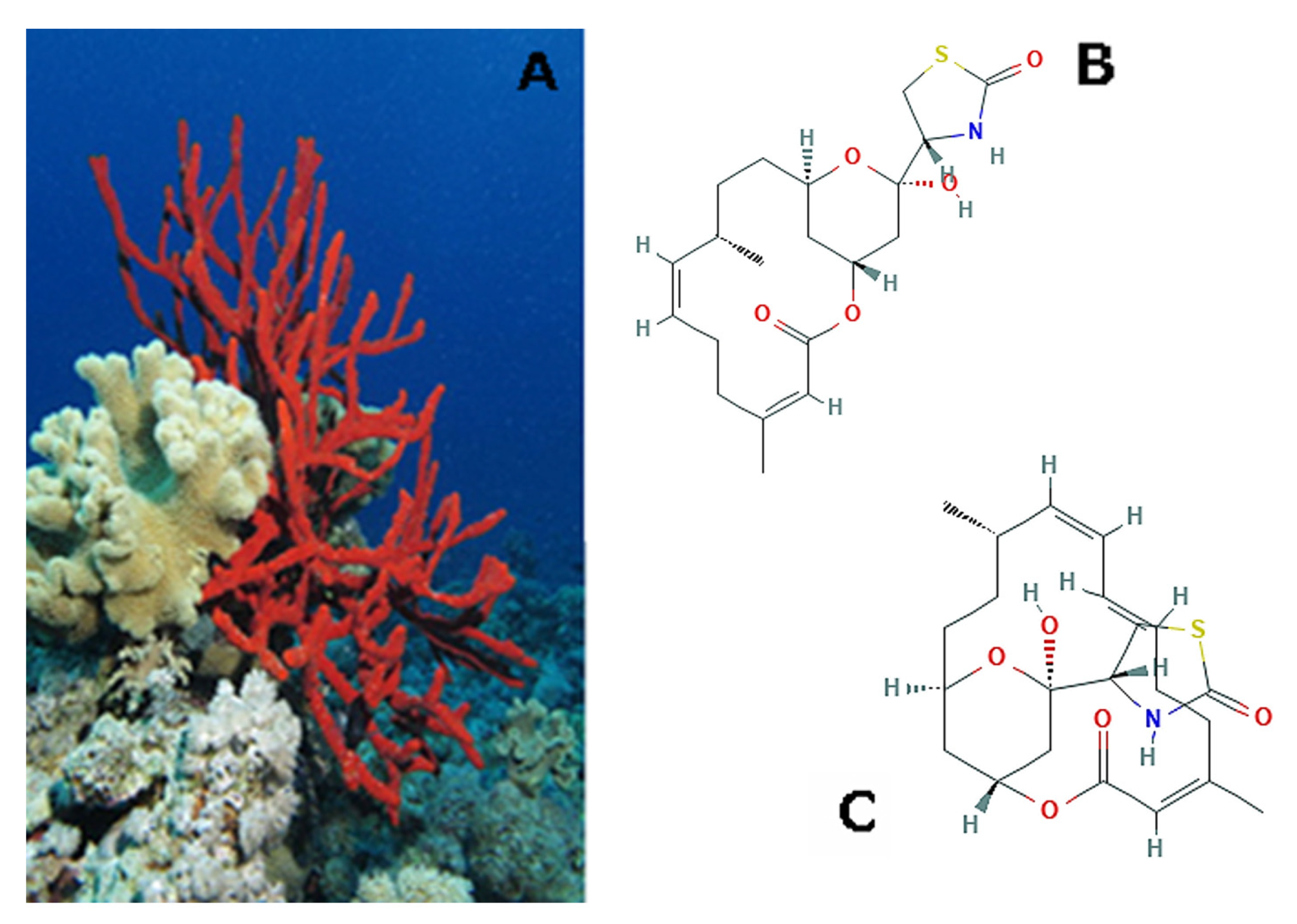
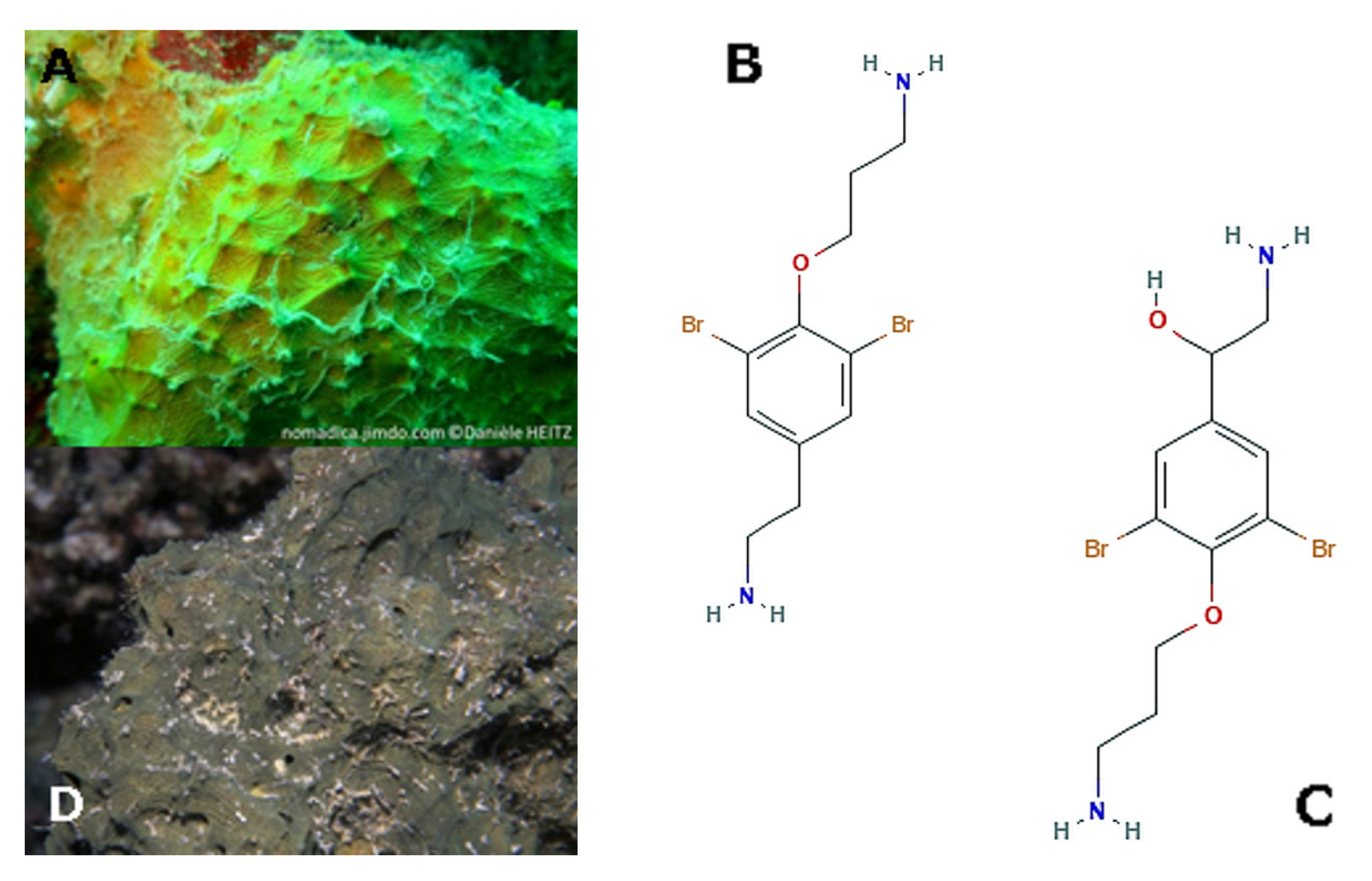




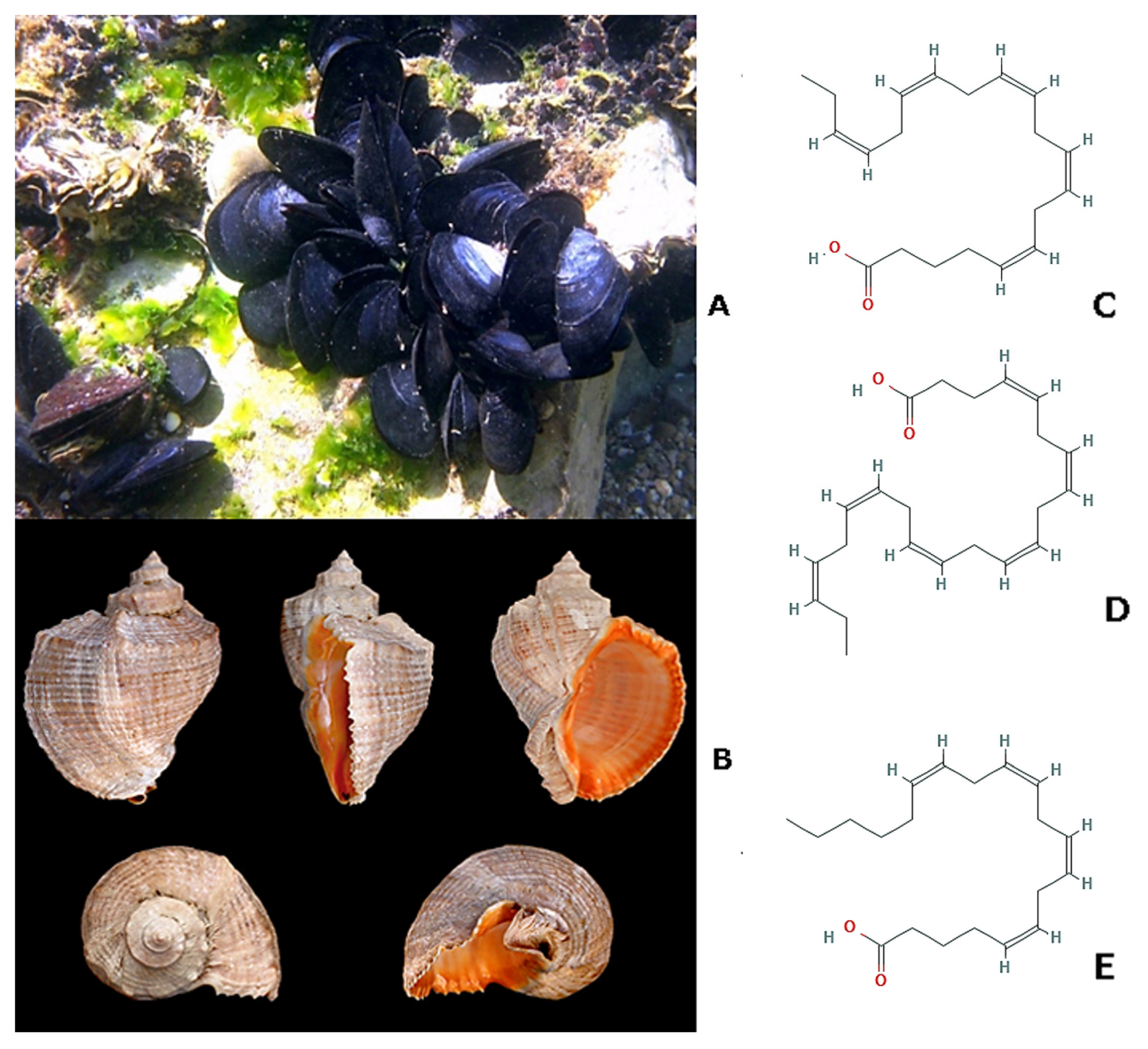


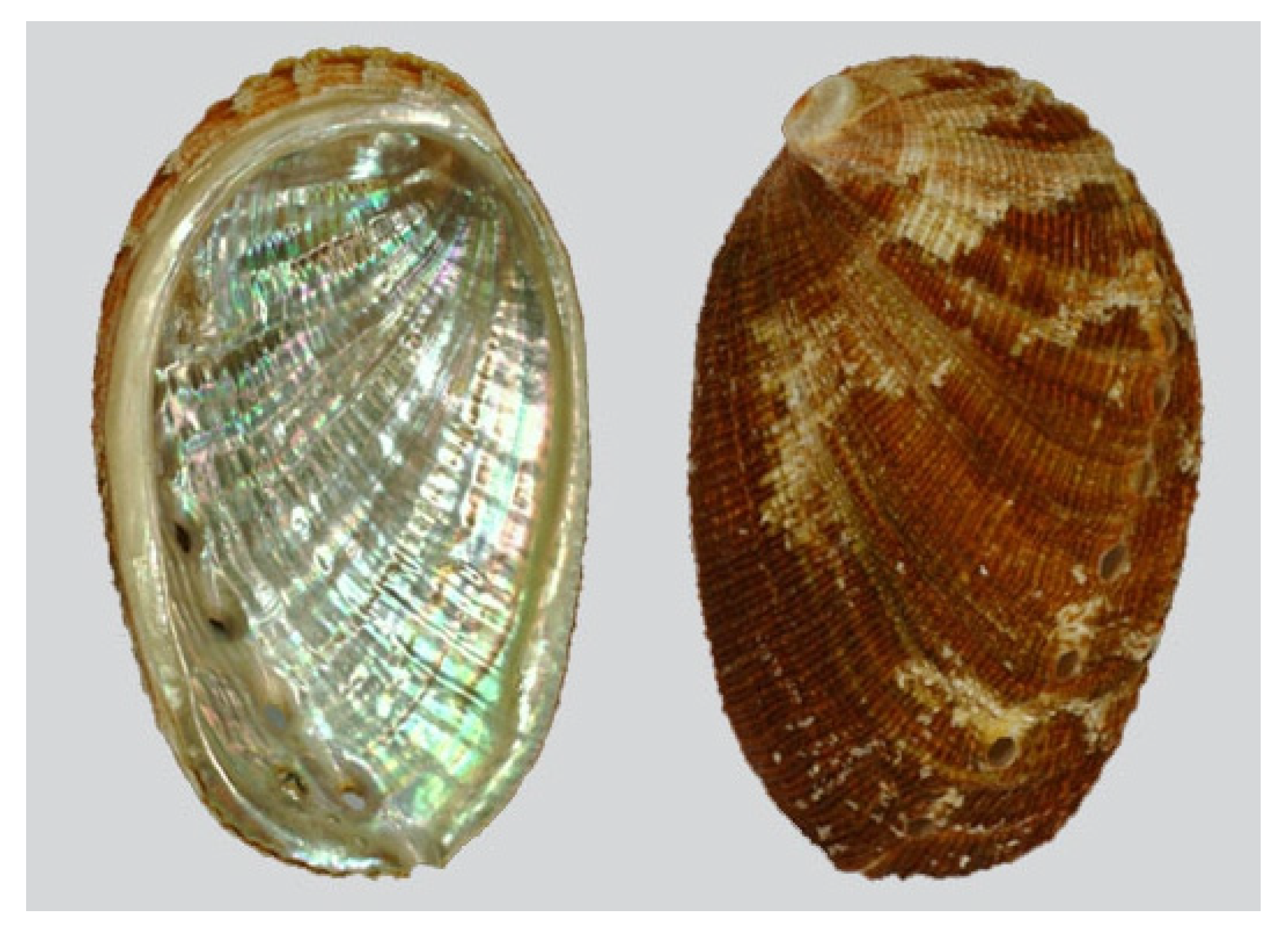


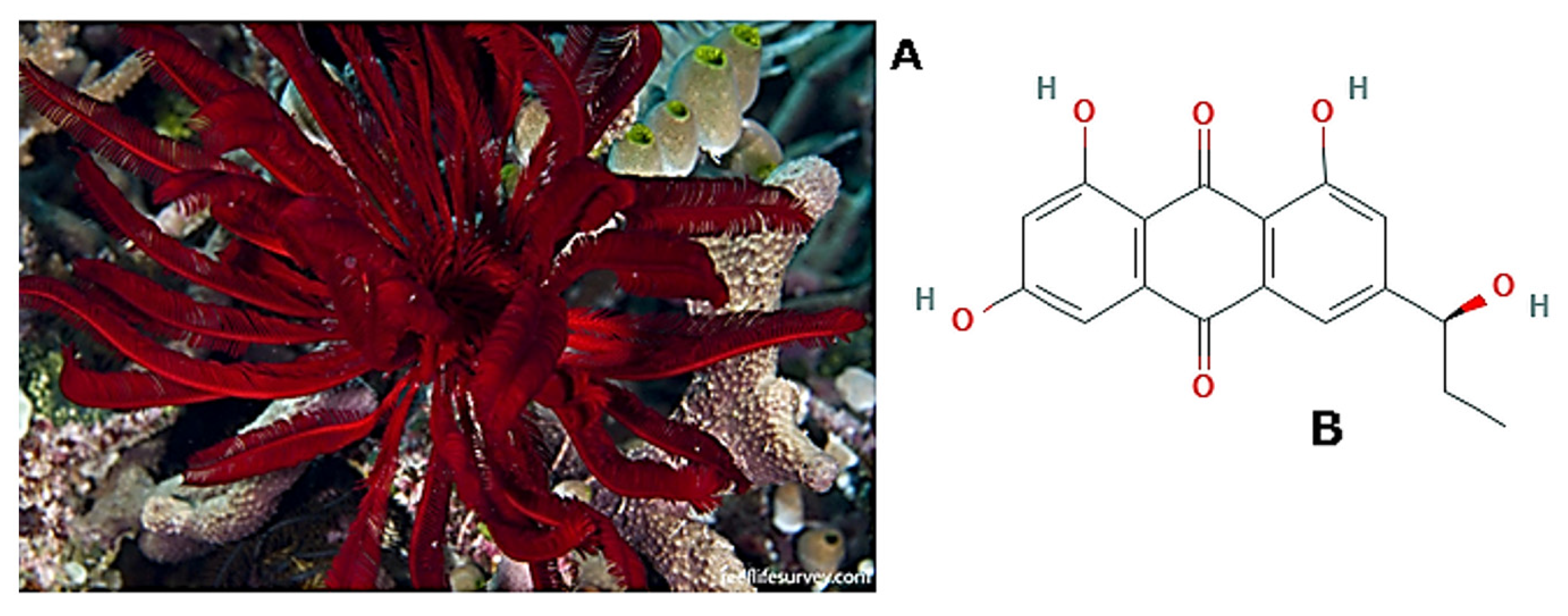







| Effects | Phylum | Species | Compounds | References |
|---|---|---|---|---|
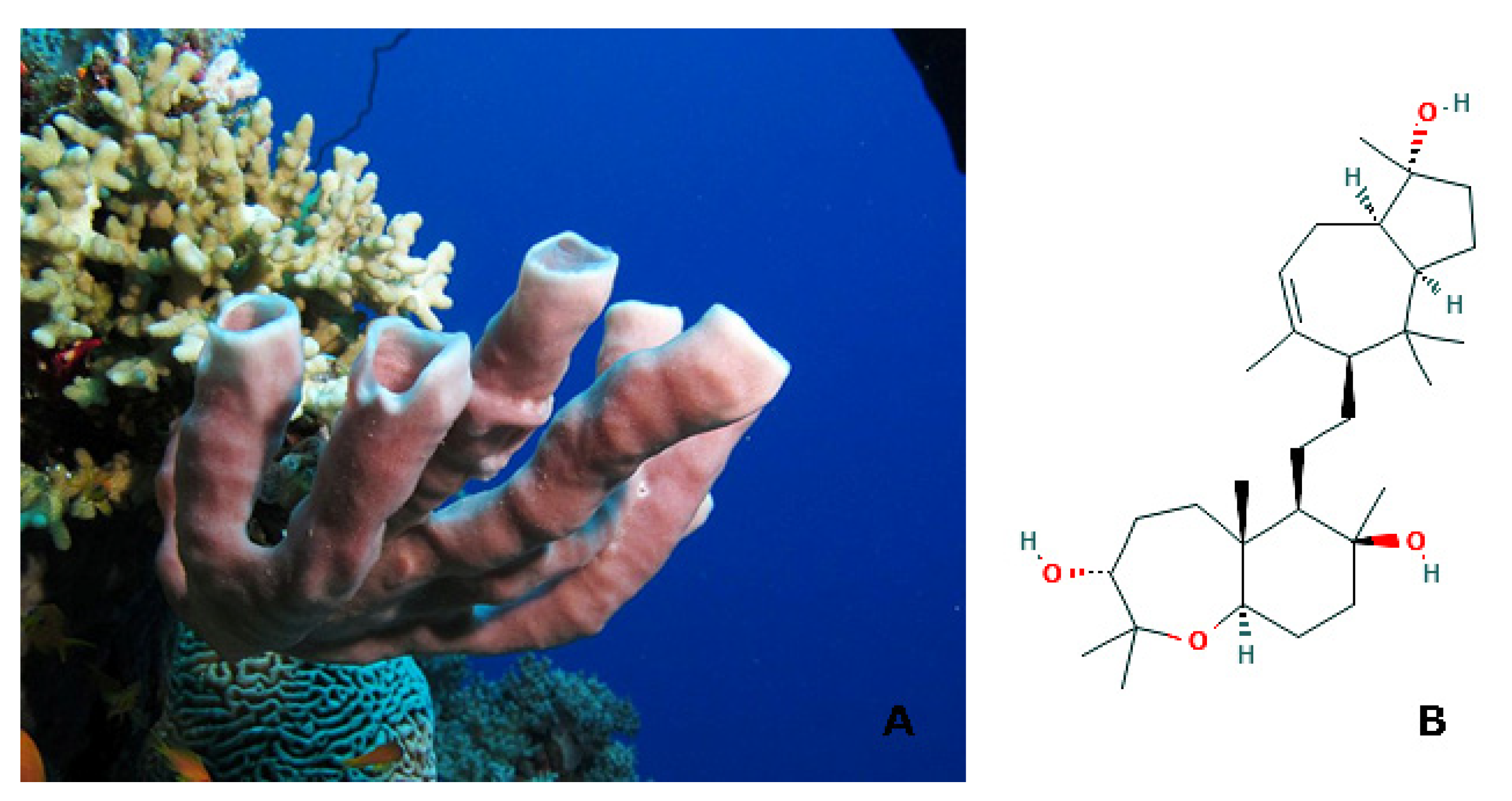
| Anti-cell migration/invasion properties | Porifera | Jaspis stellifera Negombata magnifica Pseudoceratina arabica Suberea mollis Siphonocalina (Callyspongia) siphonella Xestospongia sp. | Stellettin B Latrunculin B and 15-O-methyl-latrunculin B Latrunculin A Bromotyrosine derivative (1’R,5’S,6’S)-2-(3’,5’-dibromo-1’,6’ -dihydroxy-4’-oxocyclohex-2’-enyl) acetonitrile Moloka’iamine Ceratinin A Ceratinin D hydroxymoloka’iamine subereamolline A Sipholenol A and its aliphatic polar and aromatic ester derivatives Sipholenol A-4-O-3′,4′-dichlorobenzoate derivative Ranieramycins (i.e ranieramycin M) | [42] [47] [48] [53] [54] [62,64] [71] |
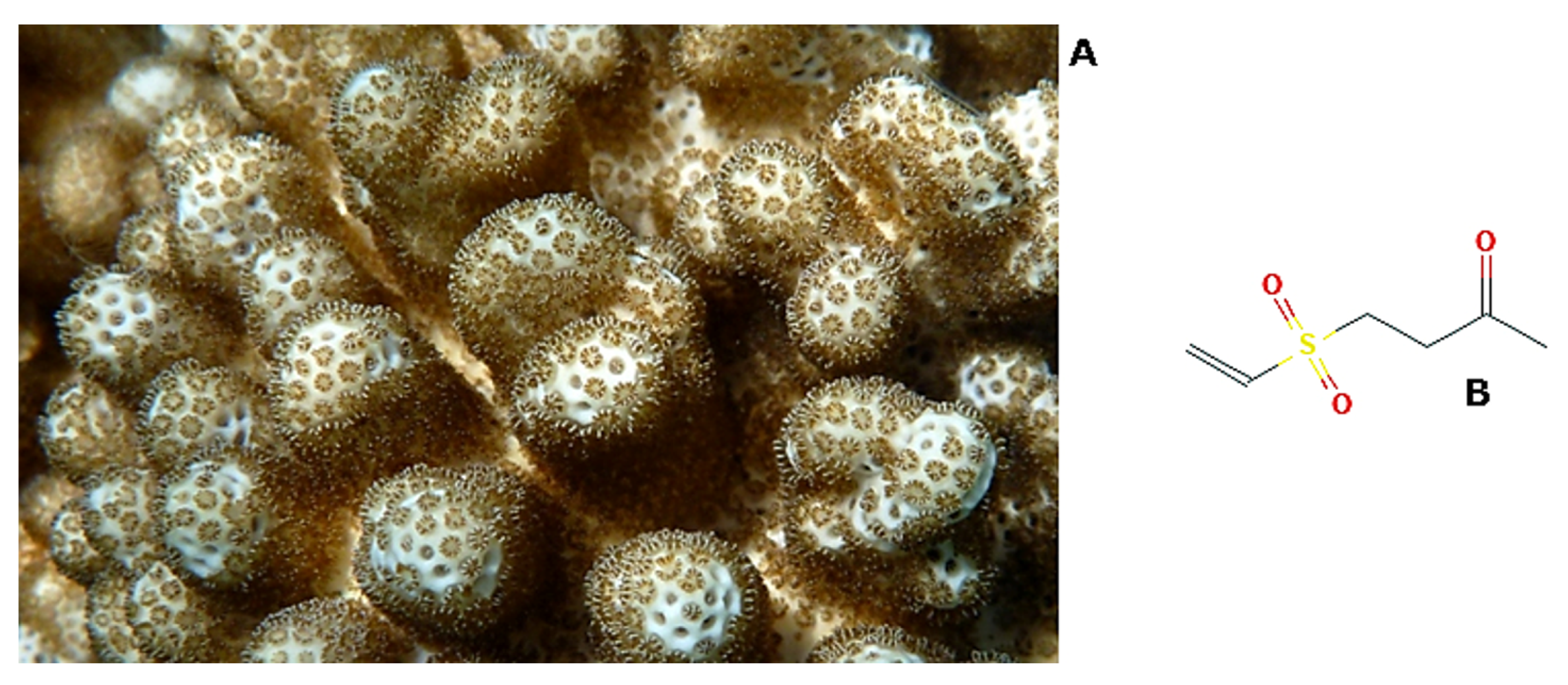
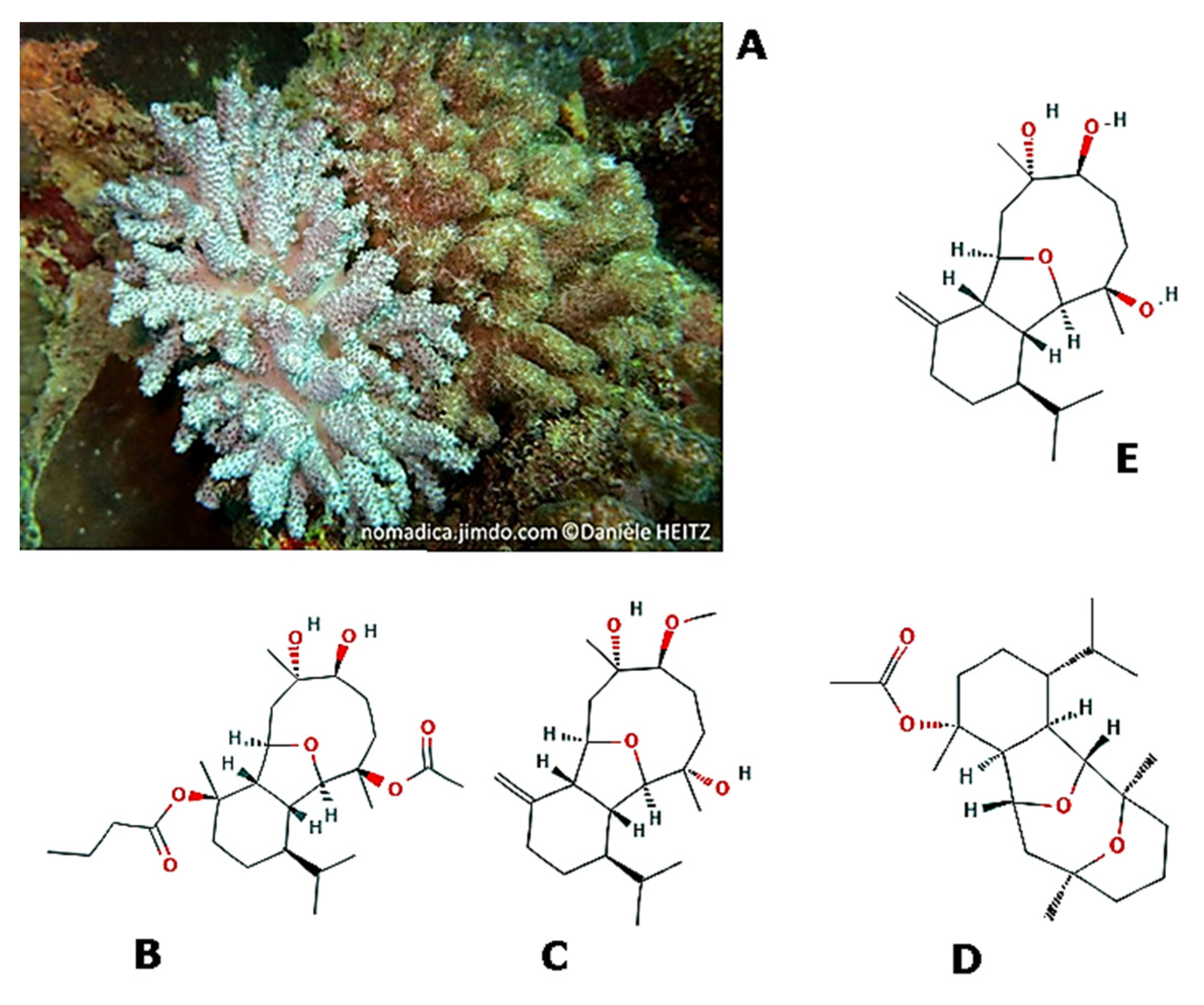
| Cnidaria | Cladiella australis Cladiella pachyclados Sarcophyton crassocaule Sinularia nanolobata Clavularia koellikeri | Dihydroaustrasulfone alcohol (synthetic precursor of Austrasulfone) Pachycladin A Sclerophytin F methyl ether Polyanthelin A Sclerophytin A Cembrenolide diterpene 13-acetoxysarcocrassolide 11-dehydrosinulariolide Sinularin Clavukoellian A and clavukoellian D-related 4-O-Deacetylparalemnolin D | [76,77,78] [79] [81,82] [83,84] [87] | |
| Mollusca | Euchelus asper | Methanol extracts | [145] | |
| Echinodermata | Ophiocoma erinaceus | Saponins | [204,205] | |
| Pro wound-healing properties | Porifera | Chondrosia reniformis | Fraction enriched in hydroxyproline- containing collagenous peptides | [33] |
| Cnidaria | Cyanea capillata Rhopilema esculentum | Tentacle extracts, Granulin Collagen peptides | [96,97] [103] | |
| Mollusca | Uroteuthis (Doryteuthis) singhalensis Sepia kobiensis Sepia officinalis Mytilus galloprovincialis and Rapana venosa Pinctada martensii Haliotis diversicolor Euphausia superba | Radical scavenging-carotenoid astaxhantin Chitosan and collagen Collagen Lipid extracts; Aminoacid mixture from protein extract Peptides extracted from P. martensii’s mantle H. diversicolor’s shell powder Krill oil | [109] [115] [116] [132,133] [140] [151] [164] | |
| Echinodermata | Astropecten indicus Himerometra magnipinna Stichopus japonicus Isostichopus badionotus Ophiocoma erinaceus Stichopus herrmanni | AiP1 metalloproteinase Anthraquinone (+)-rhodoptilometrin Collagen Oligopeptides Polysaccharides “Gamat Hydrogel” (S. hermannii’s extract-containing hydrogel dressing) | [171] [178] [188] [198] [203] [210] | |
| Tunicata | Phallusia arabica Phallusia nigra Styela clava | Ethanolic extracts Methanolic extract Cellulose films manufactured from S. clava’s tunic (SCT) selenium-loaded STC-cellulose films | [219] [221,222] [225,226] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luparello, C.; Mauro, M.; Lazzara, V.; Vazzana, M. Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates. Molecules 2020, 25, 2471. https://doi.org/10.3390/molecules25112471
Luparello C, Mauro M, Lazzara V, Vazzana M. Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates. Molecules. 2020; 25(11):2471. https://doi.org/10.3390/molecules25112471
Chicago/Turabian StyleLuparello, Claudio, Manuela Mauro, Valentina Lazzara, and Mirella Vazzana. 2020. "Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates" Molecules 25, no. 11: 2471. https://doi.org/10.3390/molecules25112471
APA StyleLuparello, C., Mauro, M., Lazzara, V., & Vazzana, M. (2020). Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates. Molecules, 25(11), 2471. https://doi.org/10.3390/molecules25112471






