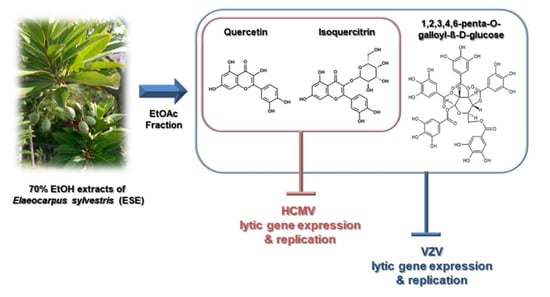
Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses
Abstract
1. Introduction
2. Results
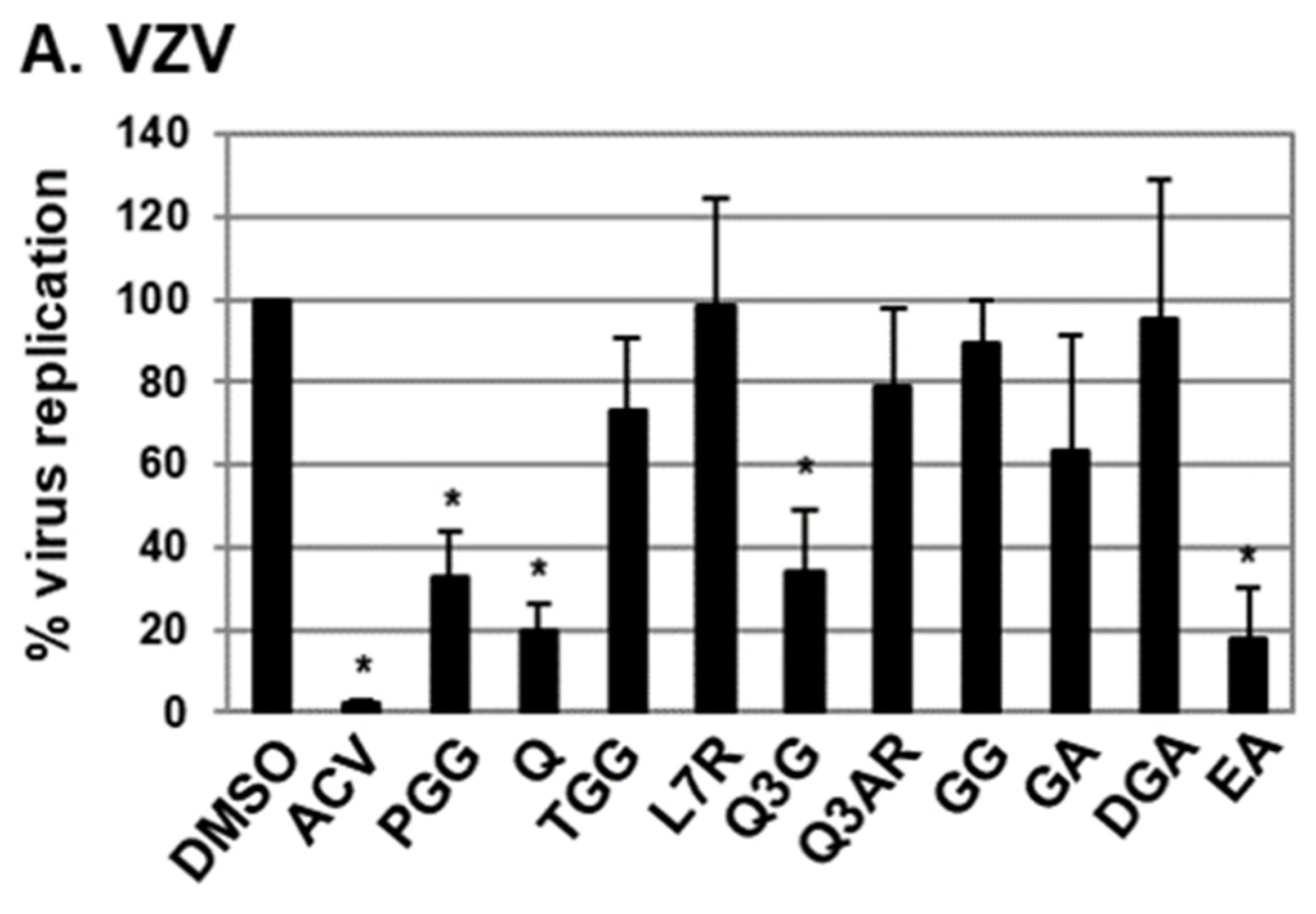
2.1. Antiviral Activities of Chemical Compounds Identified in EtOAc Fraction of ESE against VZV and HCMV
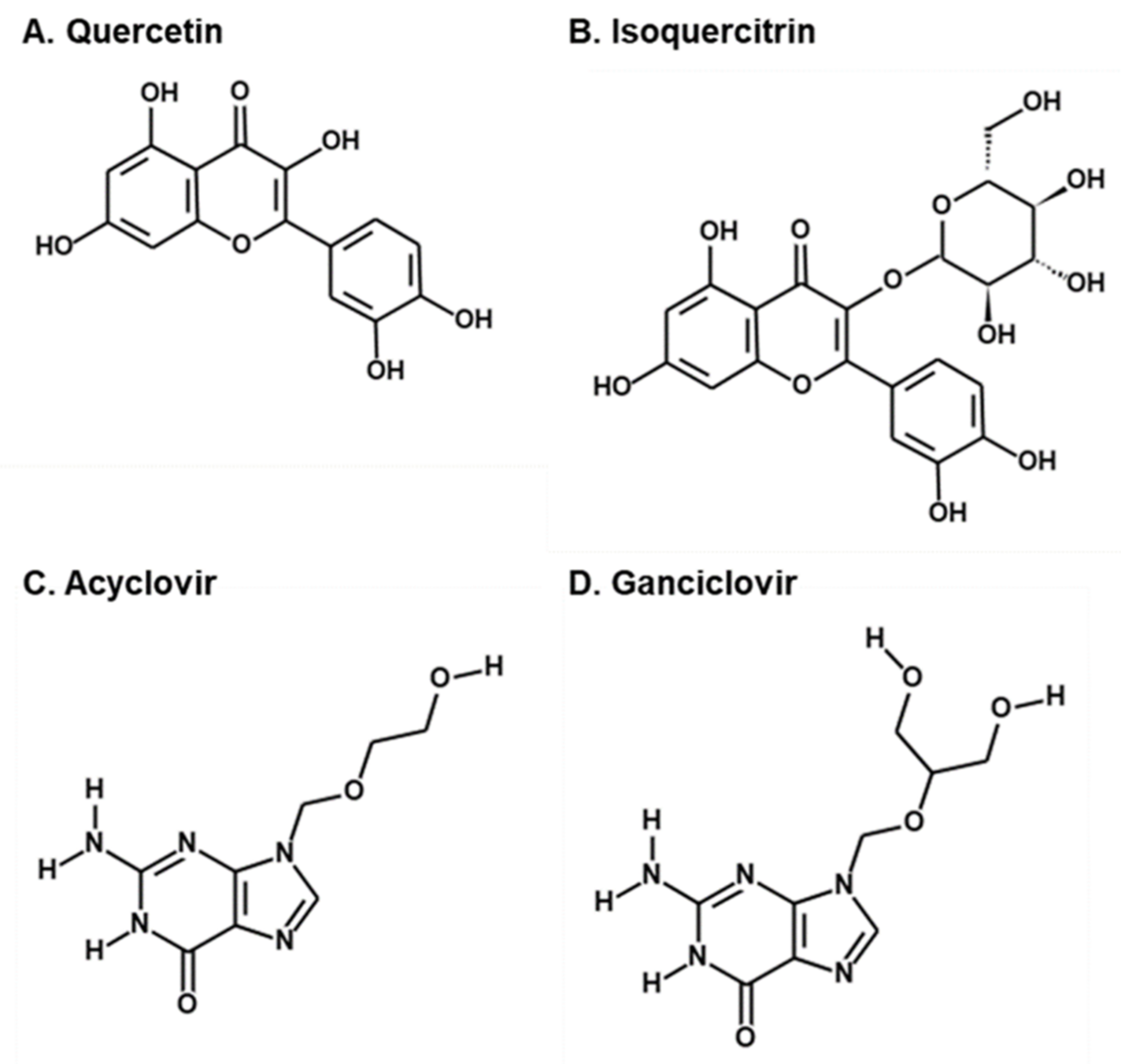
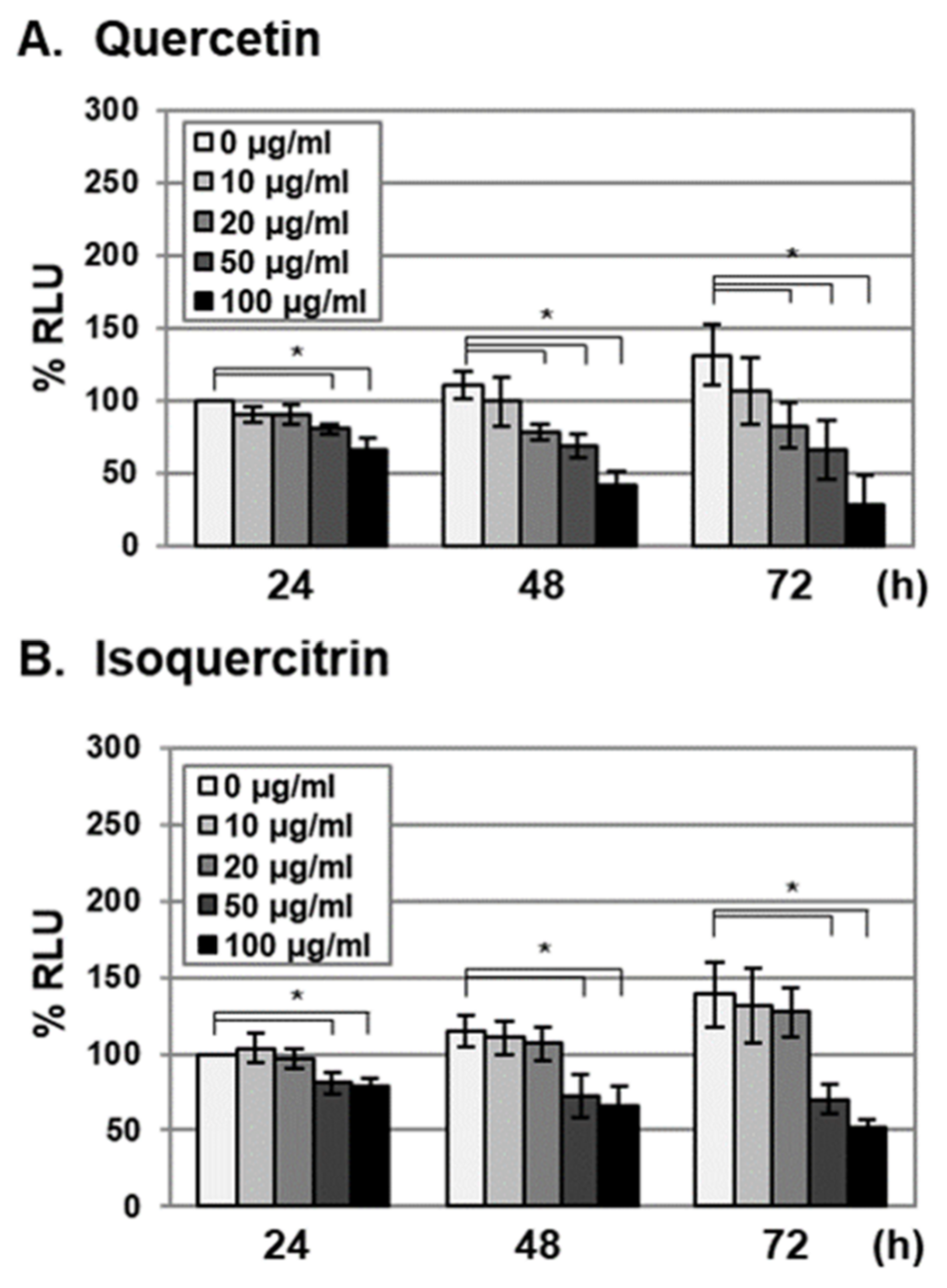
2.2. Antiviral Activities of Quercetin and Isoquercitrin Against VZV and HCMV
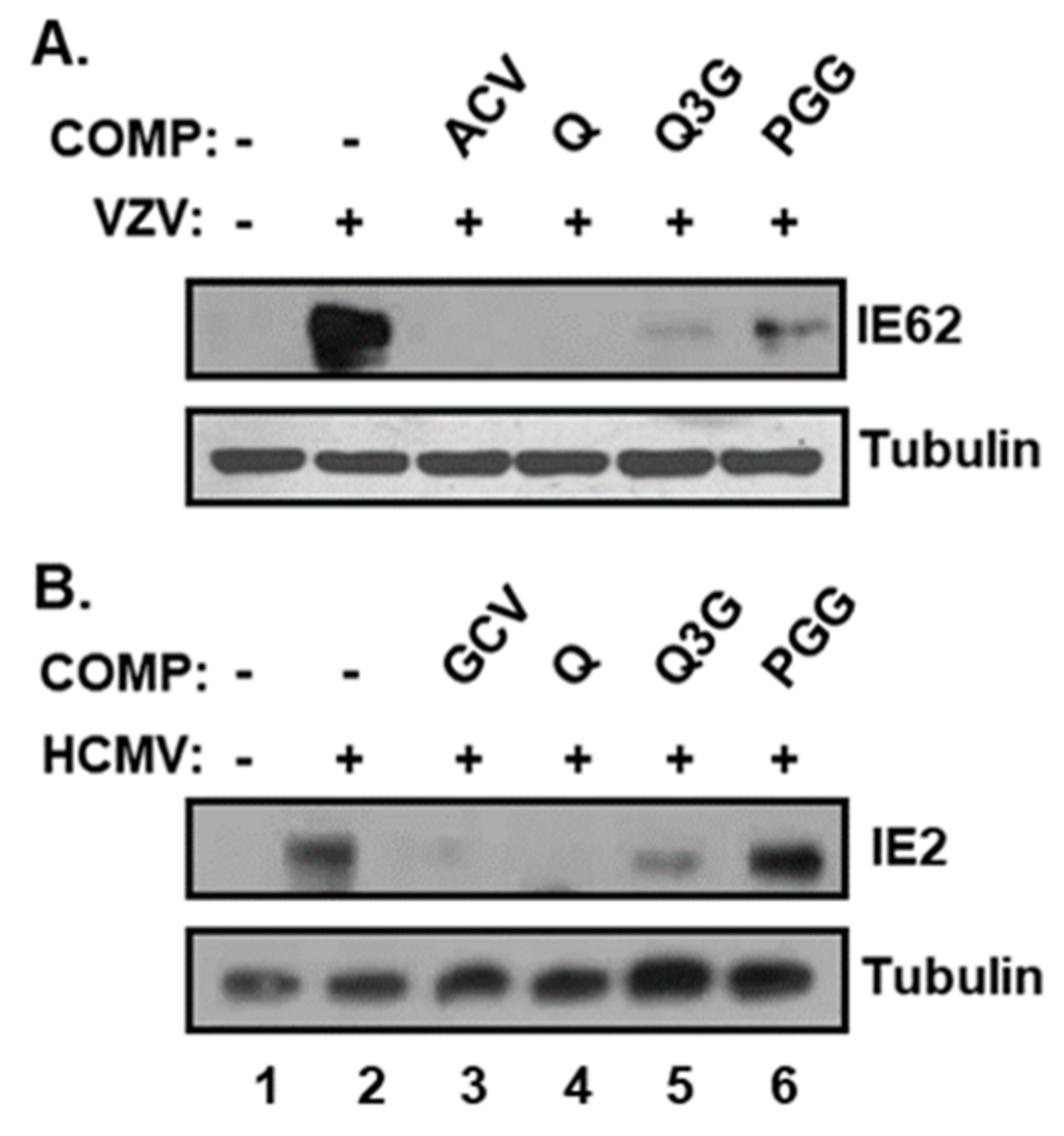
2.3. Quercetin and Isoquercitrin Inhibits VZV and HCMV Lytic-Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cells, Viruses, and Chemicals
4.2. Plaque-Reduction Assay
4.3. Cell-Viability Assay
4.4. Quantification of Viral DNA and RNA
4.5. Western Blot Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Roizman, B.; Baines, J. The diversity and unity of herpesviridae. Comp. Immunol. Microbiol. Infect. Dis. 1991, 14, 63–79. [Google Scholar] [CrossRef]
- Bowman, B.R.; Baker, M.L.; Rixon, F.J.; Chiu, W.; Quiocho, F.A. Structure of the herpesvirus major capsid protein. EMBO J. 2003, 22, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J. Herpesvirus systematics. Veter- Microbiol. 2010, 143, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kang, S.C.; Song, Y.-J. Inhibition of human cytomegalovirus immediate-early gene expression and replication by the ethyl acetate (EtOAc) fraction of Elaeocarpus sylvestris in vitro. BMC Complement. Altern. Med. 2017, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.-J. 1,2,3,4,6-Penta-O-galloyl-ß-D-glucose, a bioactive compound in Elaeocarpus sylvestris extract, inhibits varicella-zoster virus replication. Antivir. Res. 2017, 144, 266–272. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Zhang, R.; Ko, D.O.; Wang, Z.H.; Lee, K.H.; Chang, W.Y.; Chae, S.; Jee, Y.; Shin, T.; et al. Antioxidant properties of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose from Elaeocarpus sylvestris var. ellipticus. Food Chem. 2009, 115, 412–418. [Google Scholar] [CrossRef]
- Moffat, J.; Ku, C.-C.; Zerboni, L.; Sommer, M. VZV: Pathogenesis and the disease consequences of primary infection. In Human Herpesviruses; Cambridge University Press: Cambridge, UK, 2010; pp. 675–688. [Google Scholar]
- Reichelt, M.; Brady, J.; Arvin, A.M. The Replication Cycle of Varicella-Zoster Virus: Analysis of the Kinetics of Viral Protein Expression, Genome Synthesis, and Virion Assembly at the Single-Cell Level. J. Virol. 2009, 83, 3904–3918. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Baraniak, I.; Reeves, M.B. The pathogenesis of human cytomegalovirus. J. Pathol. 2014, 235, 288–297. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of Human Cytomegalovirus: From Bench to Bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Cobbs, C.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar]
- Halwachs-Baumann, G.; Genser, B.; Pailer, S.; Engele, H.; Rosegger, H.; Schalk, A.; Kessler, H.H.; Truschnig-Wilders, M. Human cytomegalovirus load in various body fluids of congenitally infected newborns. J. Clin. Virol. 2002, 25, 81–87. [Google Scholar] [CrossRef]
- Sinclair, J. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 2006, 87, 1763–1779. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004, 30, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Littler, E.; Stuart, A.D.; Chee, M.S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 1992, 358, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, R.; Johnson, M. Acyclovir Nephrotoxicity: A Case Report Highlighting the Importance of Prevention, Detection, and Treatment of Acyclovir-Induced Nephropathy. Case Rep. Med. 2010, 2010, 1–3. [Google Scholar] [CrossRef]
- Gunness, P.; Aleksa, K.; Bend, J.; Koren, G. Acyclovir-induced nephrotoxicity: The role of the acyclovir aldehyde metabolite. Transl. Res. 2011, 158, 290–301. [Google Scholar] [CrossRef]
- Lyall, E.G.; Ogilvie, M.M.; Smith, N.M.; Burns, S. Acyclovir resistant varicella zoster and HIV infection. Arch. Dis. Child. 1994, 70, 133–135. [Google Scholar] [CrossRef]
- Balzarini, J.; Ostrowski, T.; Goslinski, T.; De Clercq, E.; Golankiewicz, B. Pronounced cytostatic activity and bystander effect of a novel series of fluorescent tricyclic acyclovir and ganciclovir derivatives in herpes simplex virus thymidine kinase gene-transduced tumor cell lines. Gene Ther. 2002, 9, 1173–1182. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Gnann Jr, J.W. Antiviral therapy of varicella-zoster virus infections. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- McSharry, J.M.; Lurain, N.S.; Drusano, G.L.; Landay, A.; Manischewitz, J.; Nokta, M.; O’Gorman, M.; Shapiro, H.M.; Weinberg, A.; Reichelderfer, P.; et al. Flow Cytometric Determination of Ganciclovir Susceptibilities of Human Cytomegalovirus Clinical Isolates. J. Clin. Microbiol. 1998, 36, 958–964. [Google Scholar] [CrossRef]
- Birlea, M.; Nagel, M.A.; Khmeleva, N.; Choe, A.; Kleinschmidt-DeMasters, B.; Hevner, R.; Boyer, P.; Lear-Kaul, K.C.; Bos, N.; Wellish, M.; et al. Varicella-zoster virus trigeminal ganglioneuritis without rash. Neurol. 2013, 82, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Mocarski, E.S.; Shenk, T.; Griffiths, P.D.; Pass, R.F. Cytomegaloviruses, 6th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2013; Volume 2. [Google Scholar]
- Parasuraman, S.; David, A.V.A.; Arulmoli, R. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancirova, M.; Ulrichová, J.; Kren, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Cotin, S.; Calliste, C.-A.; Mazeron, M.-C.; Hantz, S.; Duroux, J.-L.; Rawlinson, W.D.; Ploy, M.-C.; Alain, S. Eight flavonoids and their potential as inhibitors of human cytomegalovirus replication. Antivir. Res. 2012, 96, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.X.; Li, E. Houttuynia cordata blocks HSV infection through inhibition of NF-kappaB activation. Antiviral Res. 2011, 92, 341–345. [Google Scholar] [CrossRef]
- Chiang, L.C.; Liu, M.C.; Lin, C.C. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J. Antimicrob. Chemother. 2003, 52, 194–198. [Google Scholar] [CrossRef]
- Hung, P.-Y.; Ho, B.-C.; Lee, S.-Y.; Chang, S.-Y.; Kao, C.-L.; Lee, S.-S.; Lee, C.-N. Houttuynia cordata Targets the Beginning Stage of Herpes Simplex Virus Infection. PLoS ONE 2015, 10, e0115475. [Google Scholar] [CrossRef]
- Kaul, T.N.; Middleton, E.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.H.; Shin, Y.S.; Kang, H.; Cho, H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharmacal Res. 2017, 40, 623–630. [Google Scholar] [CrossRef]
- Lyu, S.-Y.; Rhim, J.-Y.; Park, W.-B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2)in vitro. Arch. Pharmacal Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Chaumorcel, M.; Lussignol, M.; Mouna, L.; Cavignac, Y.; Fahie, K.; Cotte-Laffitte, J.; Geballe, A.; Brune, W.; Beau, I.; Codogno, P.; et al. The Human Cytomegalovirus Protein TRS1 Inhibits Autophagy via Its Interaction with Beclin 1. J. Virol. 2011, 86, 2571–2584. [Google Scholar] [CrossRef] [PubMed]
- Zapata, H.J.; Nakatsugawa, M.; Moffat, J.F. Varicella-Zoster Virus Infection of Human Fibroblast Cells Activates the c-Jun N-Terminal Kinase Pathway. J. Virol. 2006, 81, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, P.; Xu, Y.; Li, Y.; Tang, B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol. Rep. 2014, 33, 840–848. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Bishop-Bailley, D.; Lodi, F.; Duarte, J.; Cogolludo, A.; Moreno, L.; Boscá, L.; Mitchell, J.A.; Warner, T.D. The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 346, 919–925. [Google Scholar] [CrossRef]
- Jeon, J.S.; Won, Y.H.; Kim, I.K.; Ahn, J.-H.; Shin, O.S.; Kim, J.-H.; Lee, C.H. Analysis of single nucleotide polymorphism among Varicella-Zoster Virus and identification of vaccine-specific sites. Virology 2016, 496, 277–286. [Google Scholar] [CrossRef]
- Stinski, M.F. Synthesis of Proteins and Glycoproteins in Cells Infected with Human Cytomegalovirus. J. Virol. 1977, 23, 751–767. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.Y.; Lim, S.Y.; Kieff, E.; Song, Y.J. Role of Ca2+/calmodulin-dependent kinase II-IRAK1 interaction in LMP1-induced NF-kappaB activation. Mol. Cell Biol. 2014, 34, 325–334. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.-E.; Won, J.; Song, Y.-J. Characterization of the rapamycin-inducible EBV LMP1 activation system. J. Microbiol. 2015, 53, 732–738. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Virus | IC50(μg/mL) | |
|---|---|---|
| Quercetin | Isoquercitrin | |
| VZV | 3.835 ± 0.56 | 14.4 ± 2.77 |
| HCMV | 5.931 ± 1.195 | 1.852 ± 1.115 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.H.; Kim, J.-E.; Song, Y.-J. Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses. Molecules 2020, 25, 2379. https://doi.org/10.3390/molecules25102379
Kim CH, Kim J-E, Song Y-J. Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses. Molecules. 2020; 25(10):2379. https://doi.org/10.3390/molecules25102379
Chicago/Turabian StyleKim, Chae Hyun, Jung-Eun Kim, and Yoon-Jae Song. 2020. "Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses" Molecules 25, no. 10: 2379. https://doi.org/10.3390/molecules25102379
APA StyleKim, C. H., Kim, J.-E., & Song, Y.-J. (2020). Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses. Molecules, 25(10), 2379. https://doi.org/10.3390/molecules25102379