IgGs from Human Milk Hydrolyze microRNAs
Abstract
1. Introduction
2. Results
2.1. Purification and Characterizing of IgGs
2.2. Application of the Strict Criteria
2.3. Hydrolysis of Homo-Oligonucleotides
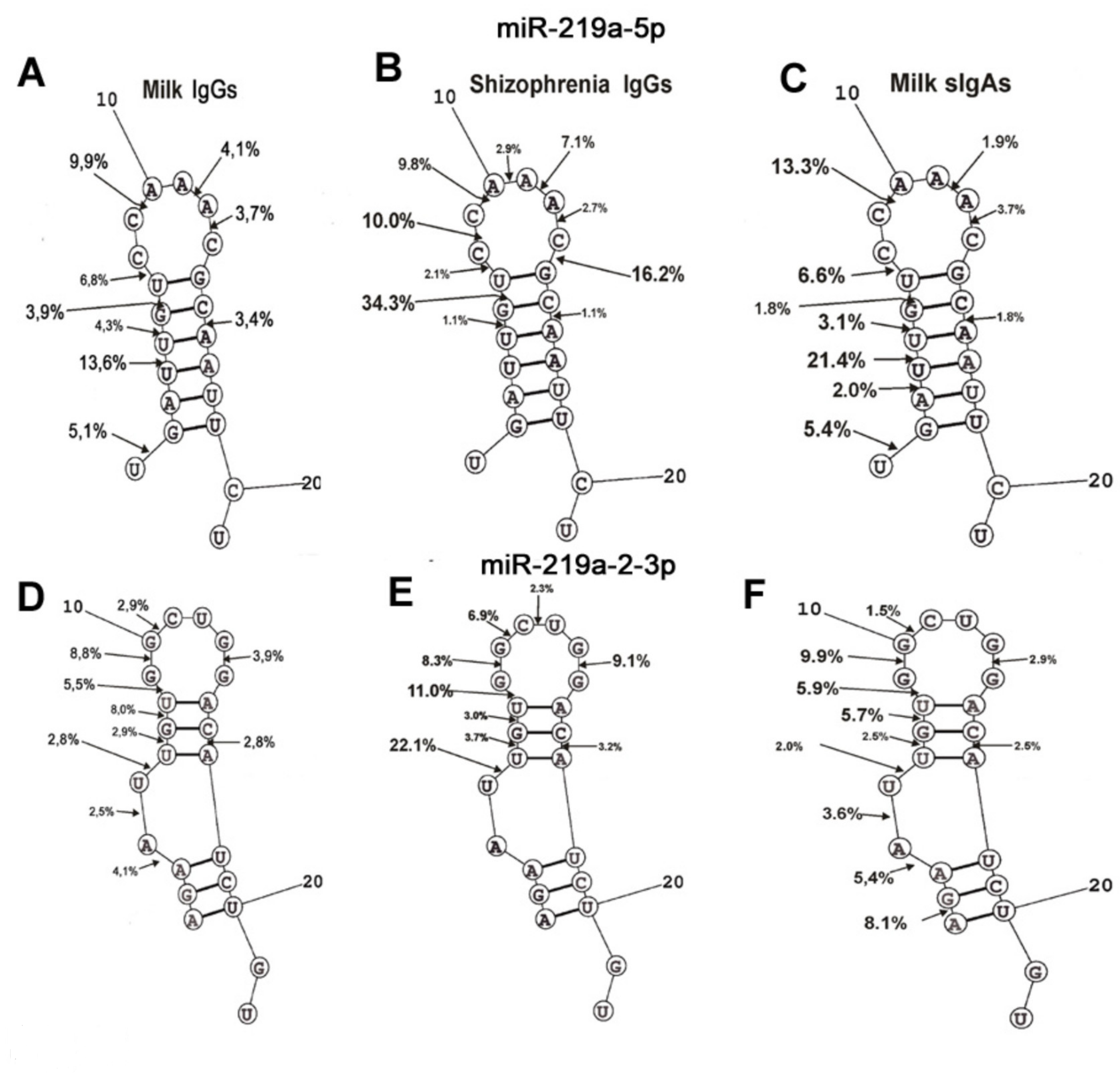
2.4. Hydrolysis of miRNAs
3. Discussion
4. Materials and Methods
4.1. Chemicals and Donors
4.2. Purification and Analysis of Antibodies
4.3. Analysis of microRNA Hydrolysis by IgGs
4.4. In Situ RNase Activity Assay
4.4.1. Spatial Model of microRNAs
4.4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bodo, C.; Melnic, B.C.; John, S.M.; Schmitz, G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr. J. 2013, 12, 103. [Google Scholar]
- Reinhardt, T.A.; Lippolis, J.D.; Nonnecke, B.J.; Sacco, R.E. Bovine milk exosome proteome. J. Proteom. 2012, 75, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Praticò, G.; Capuani, G.; Tomassini, A.; Baldassarre, M.E.; Delfini, M.; Miccheli, A. Exploring human breast milk composition by NMR-based metabolomics. Nat. Prod. Res. 2014, 28, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Catalytic Antibodies. Keinan, E. (Ed.) Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2005; pp. 1–586. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies–abzymes. In Catalytic Antibodies; Keinan, E., Ed.; VCH-Wiley press: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers, Inc.: New York, USA, 2010; pp. 1–107. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century; Kasenga, F.H., Ed.; InTech.: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar]
- Nevinsky, G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In Multiple Sclerosis; Conzalez-Quevedo, A., Ed.; InTech.: Rijeka, Croatia, 2016; pp. 100–147. [Google Scholar]
- Nevinsky, G.A. Catalytic antibodies in norm and systemic lupus erythematosus. In Lupus; Khan, W.A., Ed.; InTech.: Rijeka, Croatia, 2017; pp. 41–101. [Google Scholar]
- Shuster, A.M.; Gololobov, G.V.; Kvashuk, O.A.; Bogomolova, A.E.; Smirnov, I.V.; Gabibov, A.G. DNA hydrolyzing autoantibodies. Science 1992, 256, 665–667. [Google Scholar] [CrossRef]
- Andrievskaya, O.A.; Buneva, V.N.; Naumov, V.A.; Nevinsky, G.A. Catalytic heterogeneity of polyclonal RNA-hydrolyzing IgM from sera of patients with lupus erythematosus. Med. Sci. Monit. 2000, 6, 460–470. [Google Scholar]
- Andrievskaya, O.A.; Buneva, V.N.; Baranovskii, A.G.; Gal’vita, A.V.; Benzo, E.S.; Naumov, V.A.; Nevinsky, G.A. Catalytic diversity of polyclonal RNA-hydrolyzing IgG antibodies from the sera of patients with systemic lupus erythematosus. Immunol. Lett. 2002, 81, 191–198. [Google Scholar] [CrossRef]
- Baranovskii, A.G.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Naumov, V.A.; Buneva, V.N.; Gusev, E.I.; Boiko, A.N.; Zargarova, T.A.; Favorova, O.O.; Nevinsky, G.A. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry (Moscow) 1998, 63, 1239–1248. [Google Scholar]
- Baranovskii, A.G.; Ershova, N.A.; Buneva, V.N.; Kanyshkova, T.G.; Mogelnitskii, A.S.; Doronin, B.M.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunol. Lett. 2001, 76, 163–167. [Google Scholar] [CrossRef]
- Savel’ev, A.N.; Eneyskaya, E.V.; Shabalin, K.A.; Filatov, M.V.; Neustroev, K. Autoantibodies with amylolytic activity. Prot. Pept. Lett. 1999, 6, 179–184. [Google Scholar]
- Savel’ev, A.N.; Kanyshkova, T.G.; Kulminskaya, A.A.; Buneva, V.N.; Eneyskaya, E.V.; Filatov, M.V.; Nevinsky, G.A.; Neustroev, K.N. Amylolytic activity of IgG and sIgA immunoglobulins from human milk. Clin. Chim. Acta 2001, 314, 141–152. [Google Scholar] [CrossRef]
- Savel’iev, A.N.; Ivanen, D.R.; Kulminskaya, A.A.; Ershova, N.A.; Kanyshkova, T.G.; Buneva, V.N.; Mogelnitskii, A.S.; Doronin, B.M.; Favorova, O.O.; Nevinsky, G.A.; et al. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol. Lett. 2003, 86, 291–297. [Google Scholar] [CrossRef]
- Neustoev, K.N.; Ivanen, D.R.; Kulminskaya, A.A.; Brumer, I.H.; Saveliev, A.N.; Nevinsky, G.A. Amylolytic activity and catalytic properties of IgM and IgG antibodies from patients with systemic lupus erythematosus. Hum. Antibodies 2003, 12, 31–34. [Google Scholar]
- Paul, S.; Volle, D.J.; Beach, C.M.; Johnson, D.R.; Powell, M.J.; Massey, R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. [Google Scholar] [CrossRef]
- Li, L.; Paul, S.; Tyutyulkova, S.; Kazatchkine, M.D.; Kaveri, S. Catalytic activity of anti-thyroglobulin antibodies. J. Immunol. 1995, 154, 3328–3332. [Google Scholar] [PubMed]
- Kalaga, R.; Li, L.; O’Dell, J.R.; Paul, S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. J. Immunol. 1995, 155, 2695–2702. [Google Scholar]
- Thiagarajan, P.; Dannenbring, R.; Matsuura, K.; Tramontane, A.; Gololobov, G.; Paul, S. Monoclonal antibody light chain with prothrombinase activity. Biochemistry 2000, 39, 6459–6465. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, S.; Moreau, A.; Sooryanarayana, B.C.; Stieltjes, N.; Pashov, A.; Sultan, Y.; Hoebeke, J.; Kazatchkine, M.D.; Kaveri, S.V.; Sooryanarayana, B.C. Catalytic activity of antibodies against factor VIII in patients with hemophilia A. Nat. Med. 1999, 5, 1044–1047. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Favorova, O.O.; Nevinsky, G.A. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J. Cell Mol. Med. 2004, 8, 359–368. [Google Scholar] [CrossRef]
- Polosukhina, D.I.; Kanyshkova, T.G.; Doronin, B.M.; Tyshkevich, O.B.; Buneva, V.N.; Boiko, A.N.; Gusev, E.I.; Nevinsky, G.A.; Favorova, O.O. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol. Lett. 2006, 103, 75–81. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.L.; Parissi, V.; Nevinsky, G.A. HIV-1 integrase-hydrolyzing antibodies from sera of HIV-infected patients. Biochimie 2009, 91, 1081–1086. [Google Scholar] [CrossRef]
- Mestecky, J.M.; Russell, W.; Jackson, S.; Brown, T.A. The human IgA system: A reassessment. Clin. Immunol. Immunopathol. 1986, 40, 105–114. [Google Scholar] [CrossRef]
- Hanson, L.A.; Hahn-Zoric, M.; Berndes, M.; Ashraf, R.; Herias, V.; Jalil, F.; Bhutta, T.I.; Laeeq, A.; Mattsby-Baltzer, I. Breast feeding: Overview and breast milk immunology. Acta Paediatr. Jpn. 1994, 36, 557–561. [Google Scholar] [CrossRef]
- Buneva, V.N.; Kanyshkova, T.G.; Vlassov, A.V.; Semenov, D.V.; Khlimankov, D.; Breusova, L.R.; Nevinsky, G.A. Catalytic DNA- and RNA-hydrolyzing antibodies from milk of healthy human mothers. Appl. Biochem. Biotechnol. 1998, 75, 63–76. [Google Scholar] [CrossRef]
- Buneva, V.N.; Kudryavtseva, A.N.; Gal’vita, A.V.; Dubrovskaya, V.V.; Khokhlova, O.V.I.; Kalinina, A.; Galenok, V.A.; Nevinsky, G.A. Dynamics of antibody nuclease activity in blood of women during pregnancy and lactation. Biochemistry 2003, 68, 890–900. [Google Scholar]
- Semenov, D.V.; Kanyshkova, T.G.; Karotaeva, N.A.; Krasnorutskii, M.A.; Kuznetsova, I.A.; Buneva, V.N.; Nevinsky, G.A. Catalytic nucleotide-hydrolyzing antibodies in milk and serum of clinically healthy human mothers. Med. Sci. Monit. 2004, 10, BR23–BR33. [Google Scholar]
- Ivanen, D.R.; Kulminskaya, A.A.; Shabalin, K.A.; Isaeva-Ivanova, L.V.; Saveliev, A.N.; Nevinsky, G.A.; Shabalin, K.A. Catalytic properties of IgMs with amylolytic activity isolated from patients with multiple sclerosis. Med. Sci. Monit. 2004, 10, BR273–BR280. [Google Scholar]
- Odintsova, E.S.; Buneva, V.N.; Nevinsky, G.A. Casein-hydrolyzing activity of sIgA antibodies from human milk. J. Mol. Recognit. 2005, 18, 413–421. [Google Scholar] [CrossRef]
- Amino, N.; Tada, H.; Hidaka, Y. Postpartum autoimmune thyroid syndrome: A model of aggravation of autoimmune disease. Thyroid 1999, 9, 705–713. [Google Scholar] [CrossRef]
- Hazes, J.M. Pregnancy and its effect on the risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 1991, 50, 71–72. [Google Scholar] [CrossRef]
- Dayan, C.M.; Daniels, G.H. Chronic autoimmune thyroiditis. N. Engl. J. Med. 1996, 335, 99–107. [Google Scholar] [CrossRef]
- Tanaka, A.; Lindor, K.; Ansari, A.; Gershwin, M.E. Fetal microchimerisms in the mother: Immunologic implications. Liver Transpl. 2000, 6, 138–143. [Google Scholar] [CrossRef]
- Freeman, R.; Rosen, H.; Thysen, B. Incidence of thyroid dysfunction in an unselected postpartum population. Arch. Intern. Med. 1986, 146, 1361–1364. [Google Scholar] [CrossRef]
- Kazakov, V.I.; Bozhkov, V.M.; Linde, V.A.; Repina, M.A.; Mikhaĭlov, V.M. Extracellular DNA in the blood of pregnant women. Tsitologiia 1995, 37, 232–236. [Google Scholar]
- Nevinsky, G.A.; Kanyshkova, T.G.; Semenov, D.V.; Vlassov, A.V.; Gal’vita, A.V.; Buneva, V.N. Secretory immunoglobulin A from healthy human mothers’ milk catalyzes nucleic acid hydrolysis. Appl. Biochem. Biotechnol. 2000, 83, 115–129. [Google Scholar] [CrossRef]
- Watanabe-Fukunada, R.; Brannan, C.I.; Copeland, N.G. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992, 356, 314–317. [Google Scholar] [CrossRef]
- Andryushkova, A.A.; Kuznetsova, I.A.; Orlovskaya, I.A.; Buneva, V.N.; Nevinsky, G.A. Antibodies with amylase activity from the sera of autoimmune-prone MRL/MpJ-lpr mice. FEBS Lett. 2006, 580, 5089–5095. [Google Scholar] [CrossRef]
- Andryushkova, A.S.; Kuznetsova, I.A.; Buneva, V.N.; Toporkova, L.B.; Sakhno, L.V.; Tikhonova, M.A.; Chernykh, E.R.; Orlovskaya, I.A.; Nevinsky, G.A. Formation of different abzymes in autoimmune-prone MRL-lpr/lpr mice is associated with changes in colony formation of haematopoetic progenitors. J. Cell Mol. Med. 2007, 11, 531–551. [Google Scholar] [CrossRef]
- Andryushkova, A.A.; Kuznetsova, I.A.; Orlovskaya, I.A.; Buneva, V.N.; Nevinsky, G.A. Nucleotide-hydrolyzing antibodies from the sera of autoimmune-prone MRL-lpr/lpr mice. Int. Immunol. 2009, 21, 935–945. [Google Scholar] [CrossRef][Green Version]
- Doronin, V.B.; Parkhomenko, T.A.; Korablev, A.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaja, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; et al. Changes in different parameters, lymphocyte proliferation and hematopoietic progenitor colony formation in EAE mice treated with myelin oligodendrocyte glycoprotein. J. Cell Mol. Med. 2016, 20, 81–94. [Google Scholar] [CrossRef]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in haematopoietic progenitor colony differentiation and proliferation and the production of different abzymes in EAE mice treated with DNA. J. Cell Mol. Med. 2017, 21, 3795–3809. [Google Scholar] [CrossRef]
- Correia, I.R. Stability of IgG isotypes in serum. MAbs 2010, 2, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nevinsky, G.A.; Kit, Y.; Semenov, D.V.; Khlimankov, D.; Buneva, V.N. Secretory immunoglobulin A from human milk catalyses milk protein phosphorylation. Appl. Biochem. Biotechnol. 1998, 75, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Kit, Y.Y.; Semenov, D.V.; Nevinsky, G.A. Phosphorylation of different human milk proteins by human catalytic secretory immunoglobulin A. Biochem. Mol. Biol. Int. 1996, 39, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, D.V.; Karataeva, N.A.; Buneva, V.N.; Nevinsky, G.A. Lipid kinase activity of antibodies from milk of clinically healthy human mothers. Biochim. Biophys. Acta 2005, 1735, 153–166. [Google Scholar] [CrossRef]
- Karataeva, N.A.; Gorbunov, D.; Prokudin, I.V.; Buneva, V.N.; Kulminskaya, A.A.; Neustroev, K.N.; Nevinsky, G.A. Human milk antibodies with polysaccharide kinase activity. Immunol. Lett. 2006, 103, 58–67. [Google Scholar] [CrossRef]
- Karataeva, N.A.; Buneva, V.N.; Nevinsky, G.A. Polysaccharide kinase activity of human milk IgG antibodies. Biochemistry (Mosc.) 2006, 71, 1207–1221. [Google Scholar] [CrossRef]
- Matsuyama, H.; Suzuki, H.I. Systems and synthetic microRNA biology: From biogenesis to disease pathogenesis. Int. J. Mol. Sci. 2019, 2, 132. [Google Scholar] [CrossRef]
- Ferro, E.C.; Enrico Bena, S.; Grigolon, C. From endogenous to synthetic microRNA-mediated regulatory circuits: An overview. Cells 2019, 8, 1540. [Google Scholar] [CrossRef]
- Miller, B.H.; Wahlestedt, C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010, 1338, 89–99. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best. Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Alsaweed, M.A.; Hepworth, R.; Lefèvre, C.; Hartmann, P.E.; Geddes, D.T.; Hassiotou, F. Human milk microRNA and total RNA differ depending on milk fractionation. J. Cell Biochem. 2015, 116, 2397–2407. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in breast milk and the lactating breast: Potential immunoprotectors and developmental regulators for the Infant and the mother. Int. J. Environ. Res. Public Health. 2015, 12, 13981–14020. [Google Scholar] [CrossRef]
- Alsaweed, M.C.; Lai, T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells contain numerous mirnas that may change with milk removal and regulate multiple physiological processes. Int. J. Mol. Sci. 2016, 17, 956. [Google Scholar] [CrossRef]
- Alsaweed, M.C.; Lai, T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells and lipids conserve numerous known and novel mirnas, some of which are differentially expressed during lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef]
- Alsaweed, M.C.; Lai, T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Hydrolysis by catalytic IgGs of microRNA specific for patients with schizophrenia. IUBMB Life 2018, 70, 153–164. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Blood-derived RNA- and microRNA-hydrolyzing IgG antibodies in schizophrenia patients. Biochemistry 2018, 83, 507–526. [Google Scholar]
- Kompaneets, I.Y.; Ermakov, E.A.; Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. IgGs from human milk hydrolyze microRNAs. Molecules 2020, 25, 2366. [Google Scholar] [CrossRef]
- Murai, K.G.; Ye, P.; Sun, E.; Tian, S.; Yang, Q.; Cui, G.; Sun, G.; Trinh, D.; Sun, O.; Hong, T.; et al. The TLX-miR-219 cascade regulates neural stem cell proliferation in neurodevelopment and schizophrenia iPSC model. Nat. Commun. 2016, 7, 10965. [Google Scholar] [CrossRef]
- Vlassov, A.; Florentz, V.; Helm, M.; Naumov, V.; Buneva, V.; Nevinsky, G.; Giegé, R. Characterization and selectivity of catalytic antibodies from human serum with RNase activity. Nucl. Acid Res. 1998, 26, 5243–5250. [Google Scholar] [CrossRef]
- Vlasov, A.V.; Baranovskii, A.G.; Kanyshkova, T.G.; Prints, A.V.; Zabara, V.G.; Naumov, V.A.; Breusov, A.A.; Giege, R.; Buneva, V.; Nevinskii, G.A. Substrate specificity of serum DNA- and RNA-hydrolyzing antibodies of patients with polyarthritis and autoimmune thyroiditis. Mol. Biol. 1998, 32, 559–569. [Google Scholar]
- Vlasov, A.V.; Helm, M.; Naumov, V.A.; Breusov, A.A.; Buneva, V.N.; Florentz, C.; Giege, R.; Nevinskii, G.A. Features of tRNA hydrolysis by autoantibodies from blood serum of patients with certain autoimmune and virus diseases. Mol. Biol. 1999, 33, 866–872. [Google Scholar]
- Chernikov, I.V.; Gladkikh, D.V.; Meschaninova, M.I.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V.V.; Chernolovskaya, E.L. Cholesterol-containing nuclease-resistant siRNA. Accumulates in tumors in a carrier-free mode and silences MDR1 gene. Mol. Ther. Nucleic Acids 2017, 6, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. All data are given in the article. |
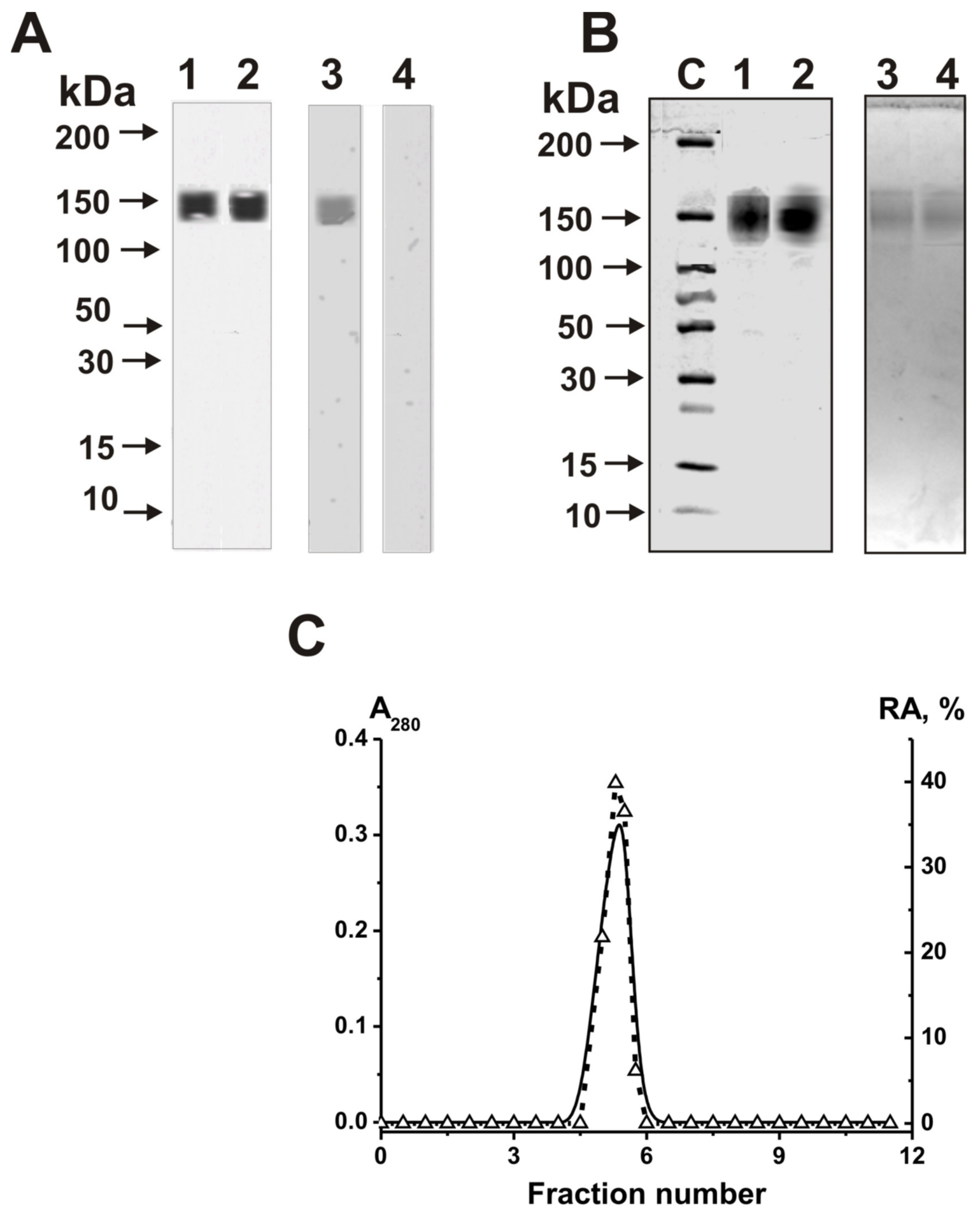
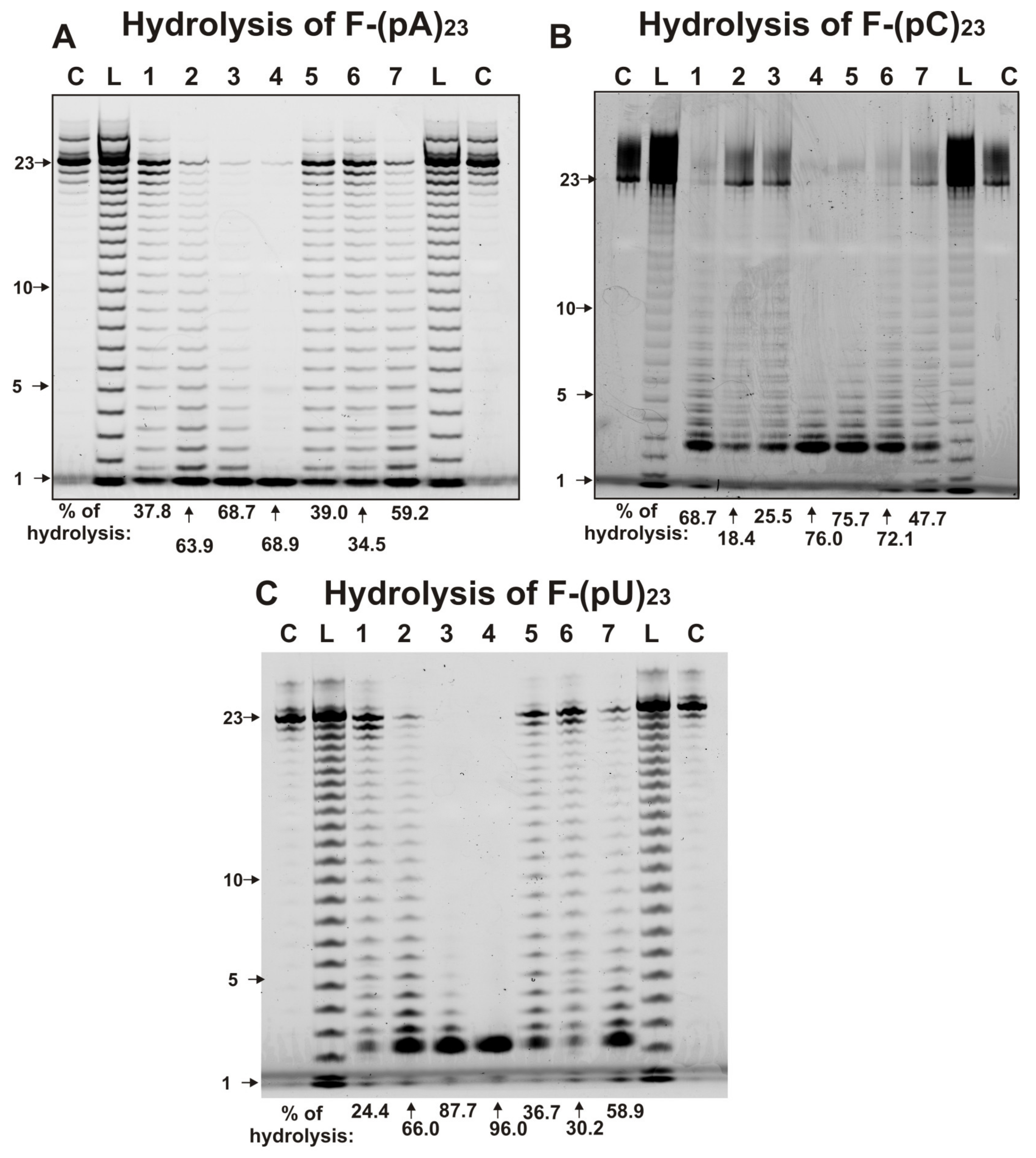

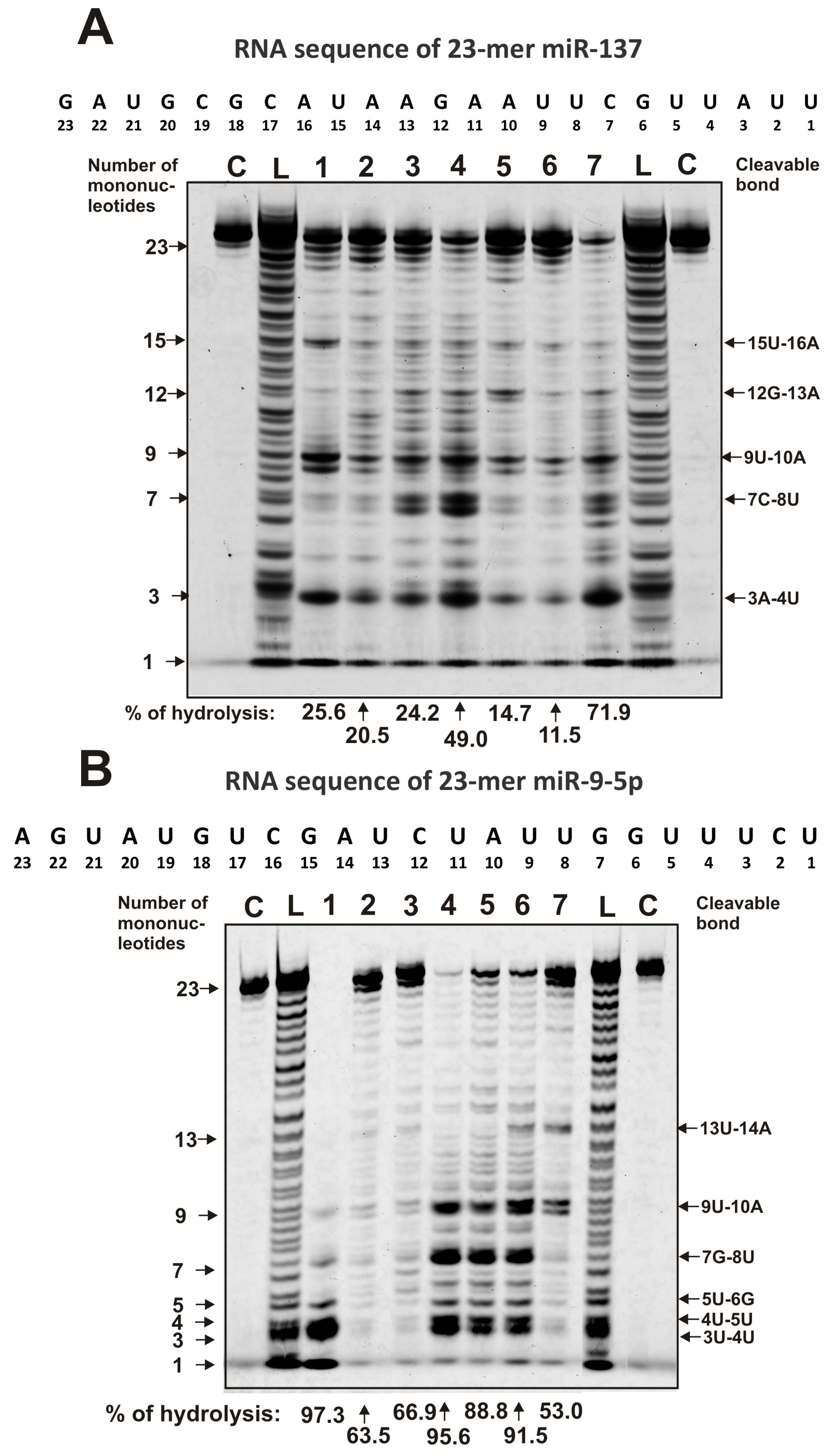
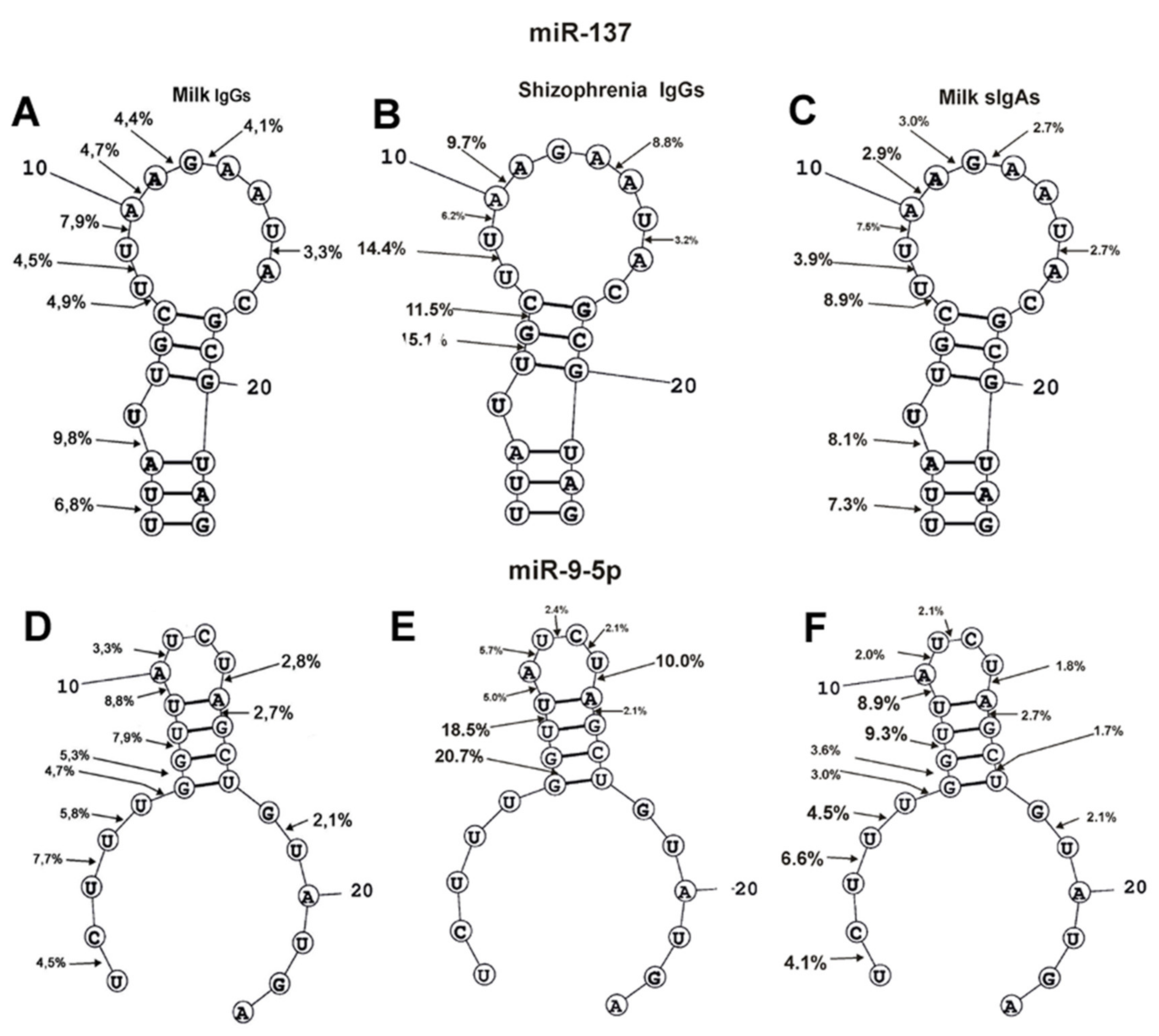
| Substrate | (pU)23 | (pC)23 | miR-219a-5p | miR-219-2-3p | miR-137 | miR-9-5p |
|---|---|---|---|---|---|---|
| (pA)23 | +0.96 | −0.42 | +0.75 | −0.27 | +0.49 | −0.55 |
| (pU)23 | −0.42 | +0.80 | −0.03 | +0.40 | −0.35 | |
| (pC)23 | −0.22 | +0.88 | −0.02 | +0.81 | ||
| Substrate | miR-219-2-3p | miR-137 | miR-9-5p | |||
| miR-219a-5p | +0.2 | +0.78 | −0.17 | |||
| miR-219-2-3p | −0.07 | +0.86 | ||||
| miR-137 | −0.43 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompaneets, I.Y.; Ermakov, E.A.; Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. IgGs from Human Milk Hydrolyze microRNAs. Molecules 2020, 25, 2366. https://doi.org/10.3390/molecules25102366
Kompaneets IY, Ermakov EA, Sedykh SE, Buneva VN, Nevinsky GA. IgGs from Human Milk Hydrolyze microRNAs. Molecules. 2020; 25(10):2366. https://doi.org/10.3390/molecules25102366
Chicago/Turabian StyleKompaneets, Ivan Yu., Evgeny A. Ermakov, Sergey E. Sedykh, Valentina N. Buneva, and Georgy A. Nevinsky. 2020. "IgGs from Human Milk Hydrolyze microRNAs" Molecules 25, no. 10: 2366. https://doi.org/10.3390/molecules25102366
APA StyleKompaneets, I. Y., Ermakov, E. A., Sedykh, S. E., Buneva, V. N., & Nevinsky, G. A. (2020). IgGs from Human Milk Hydrolyze microRNAs. Molecules, 25(10), 2366. https://doi.org/10.3390/molecules25102366








