Exploiting the Indole Scaffold to Design Compounds Binding to Different Pharmacological Targets †
Abstract
1. Introduction
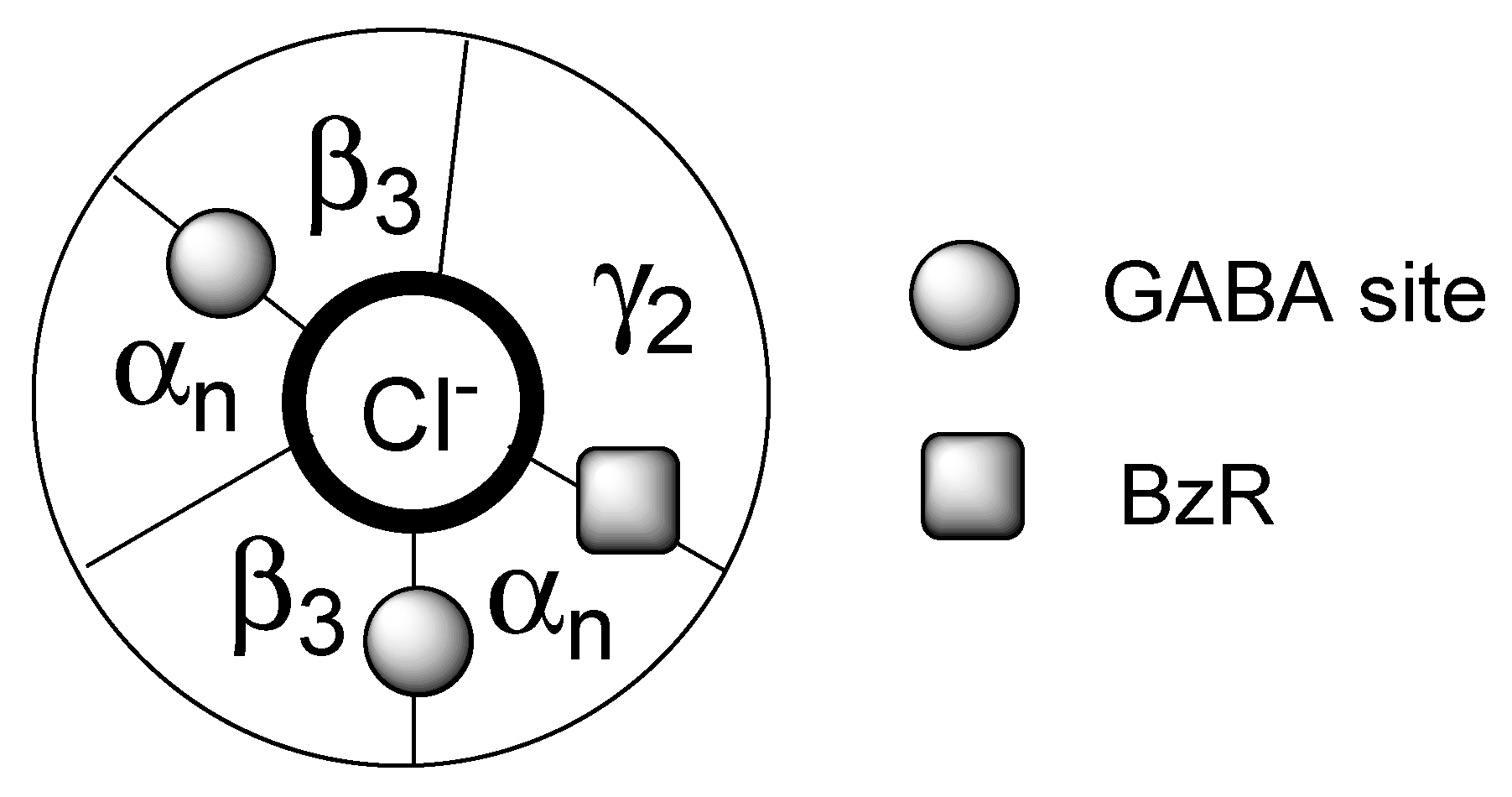
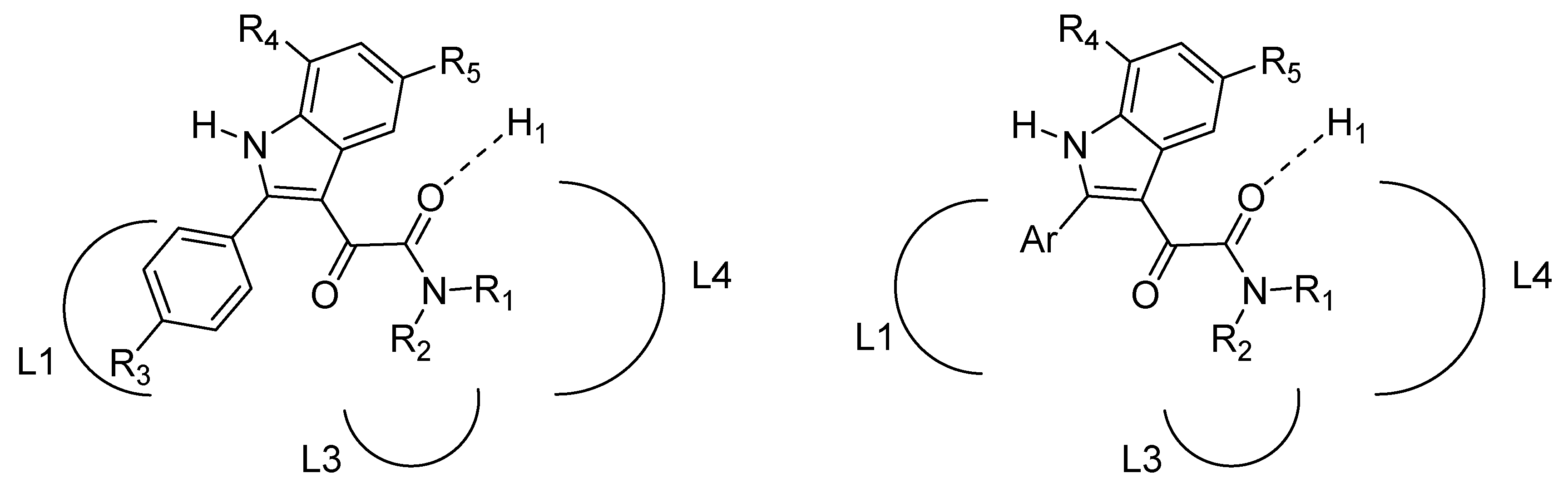
2. Indole Derivatives as Ligands of the Benzodiazepine Receptor
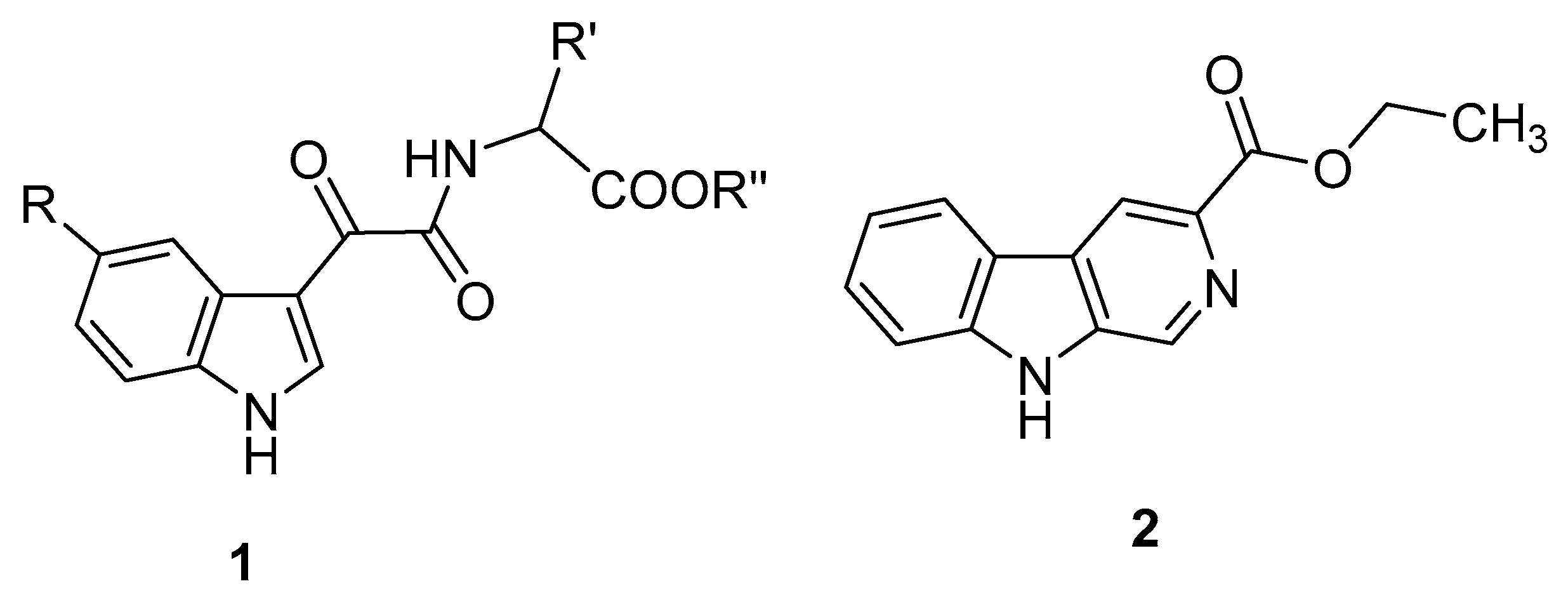
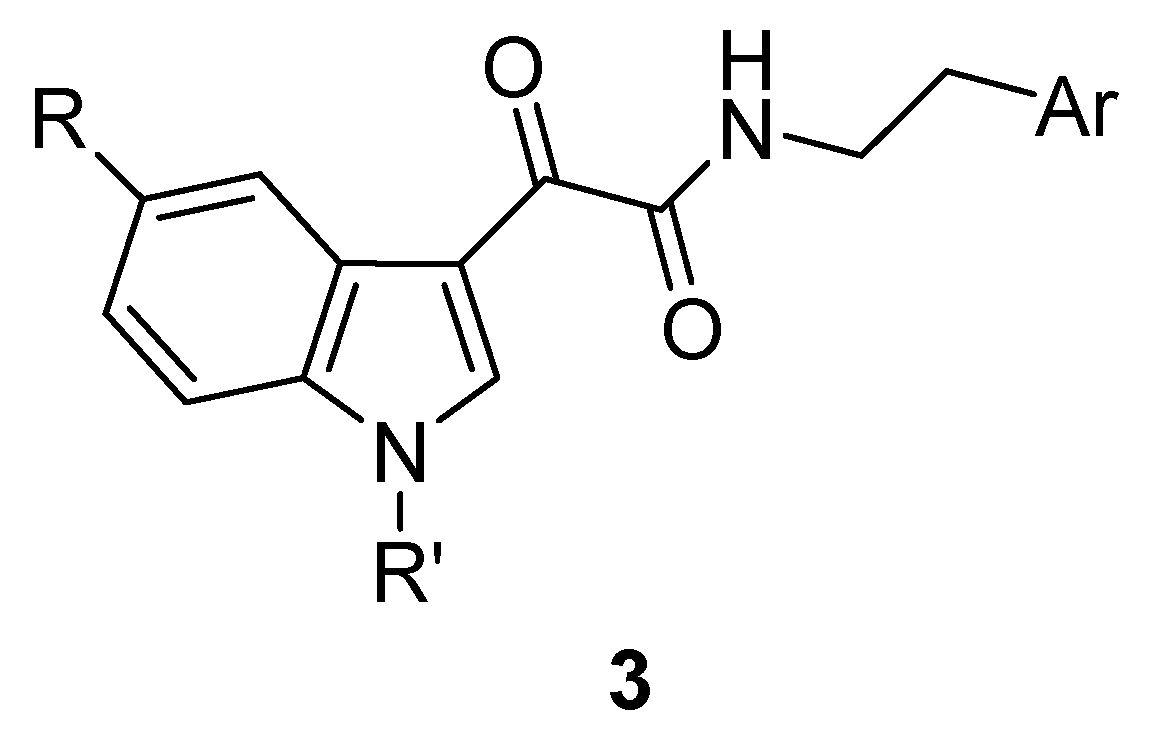
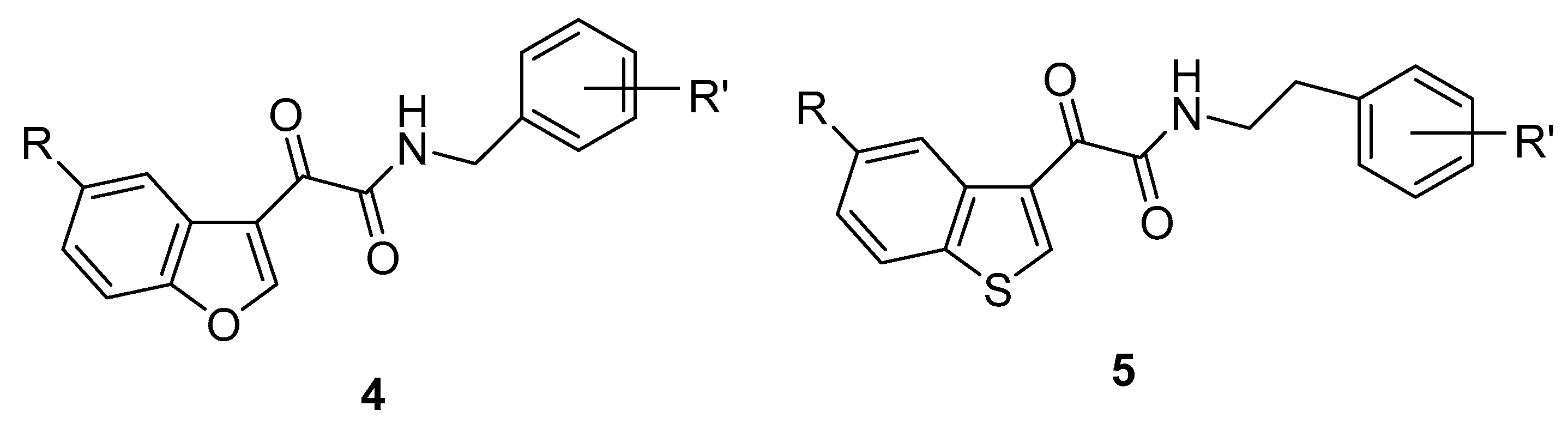
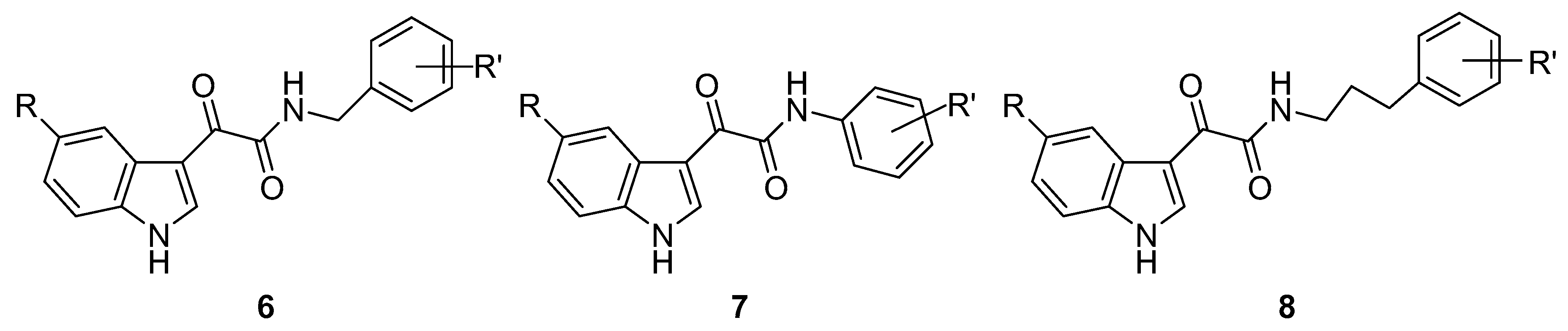
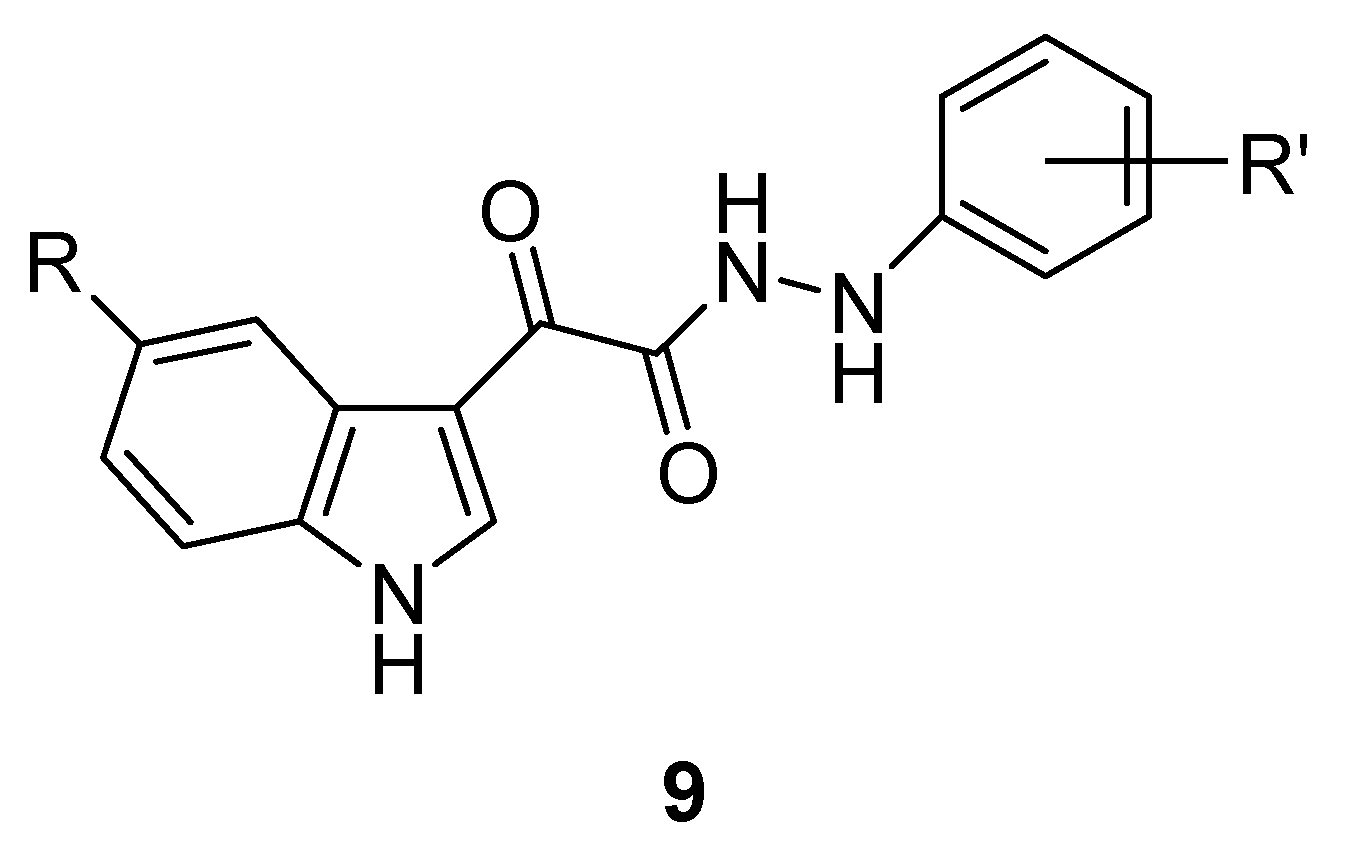

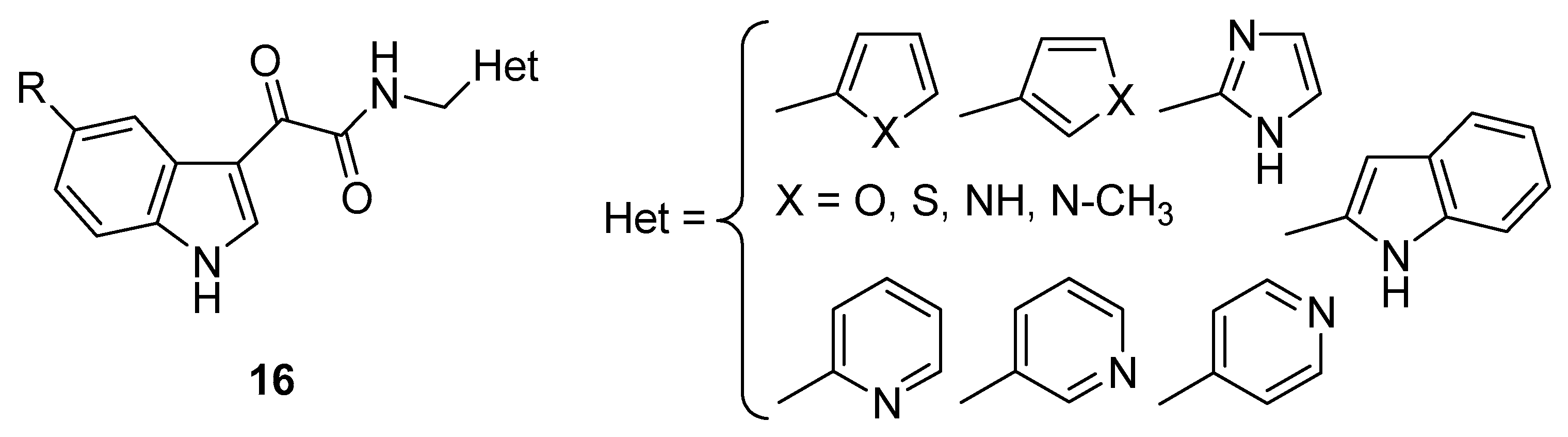
3. Indole Derivatives as Ligands of the Translocator Protein
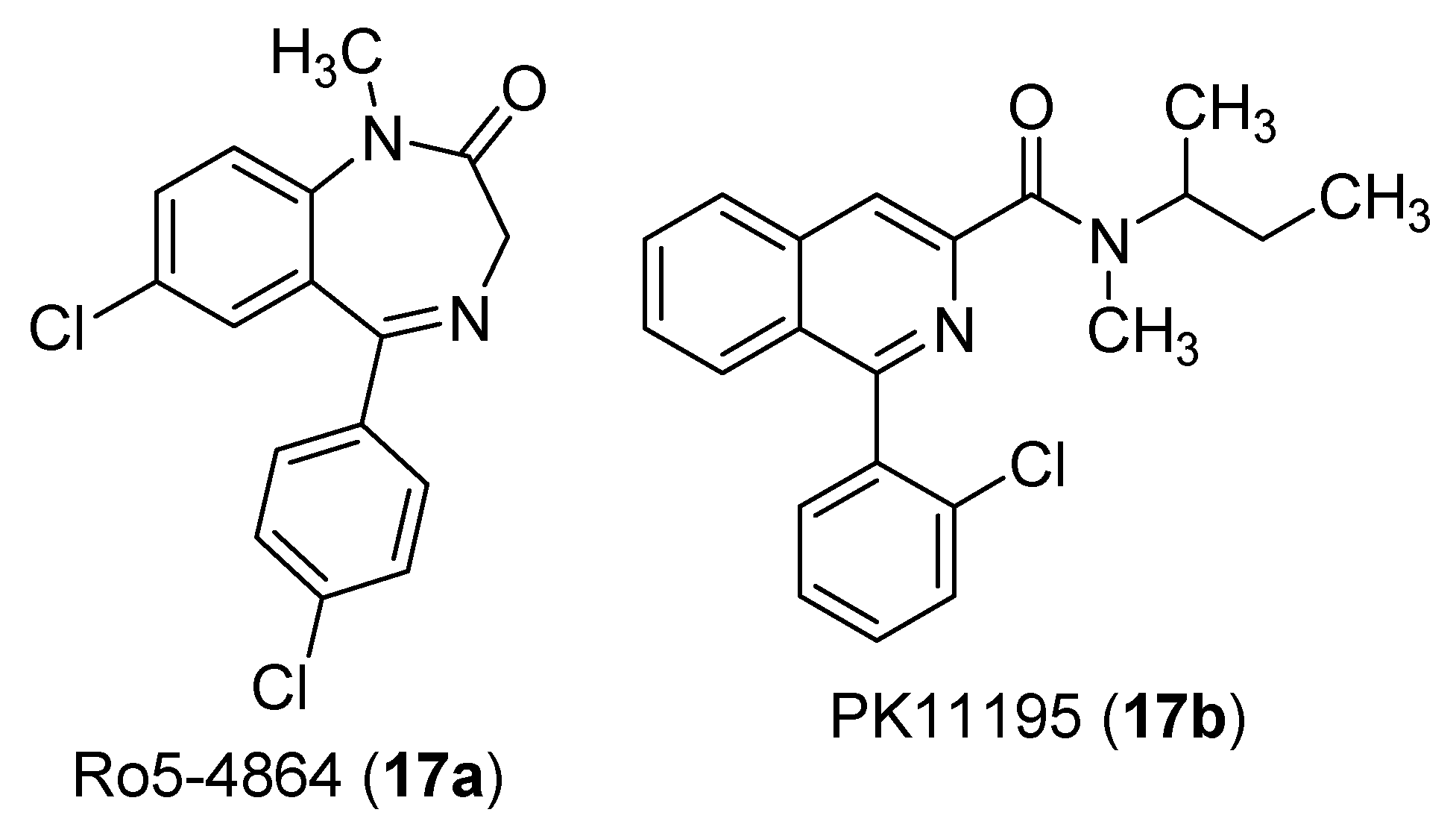


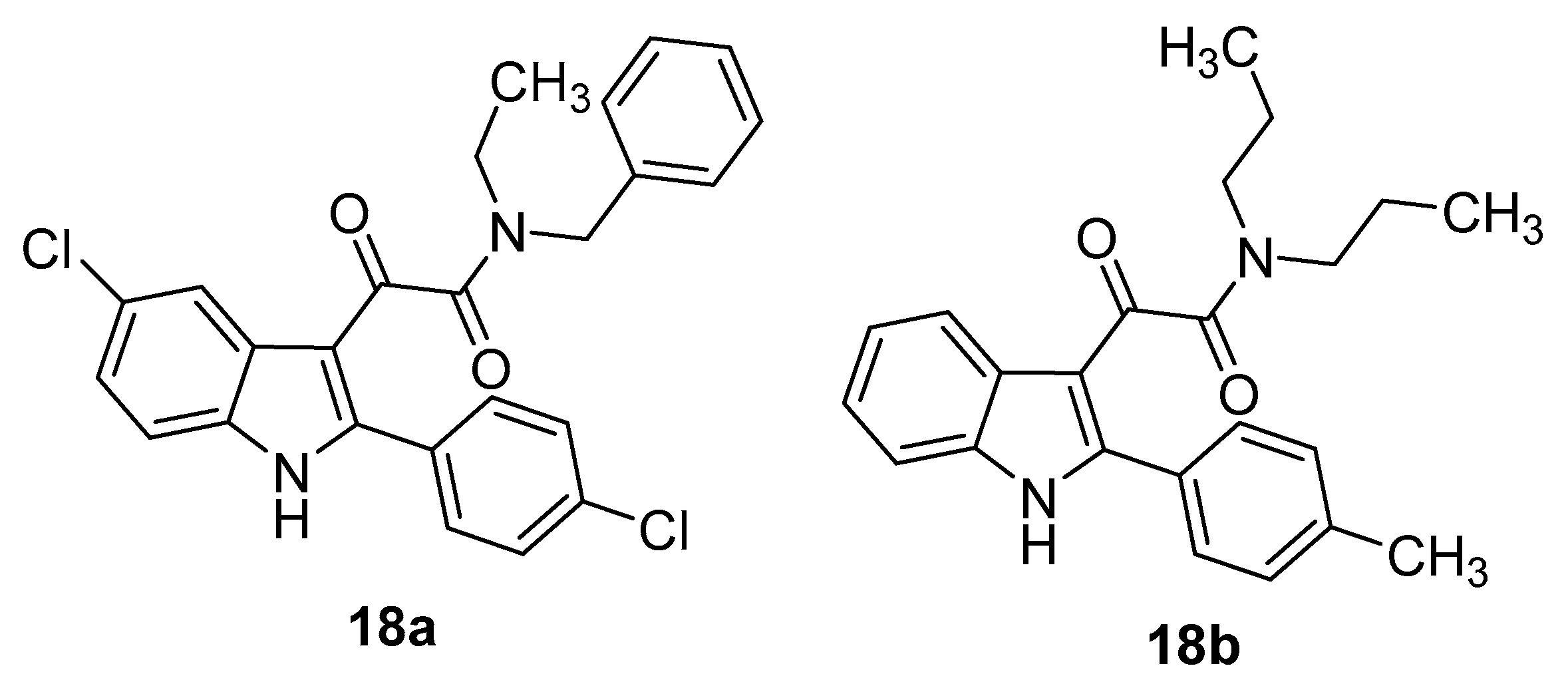
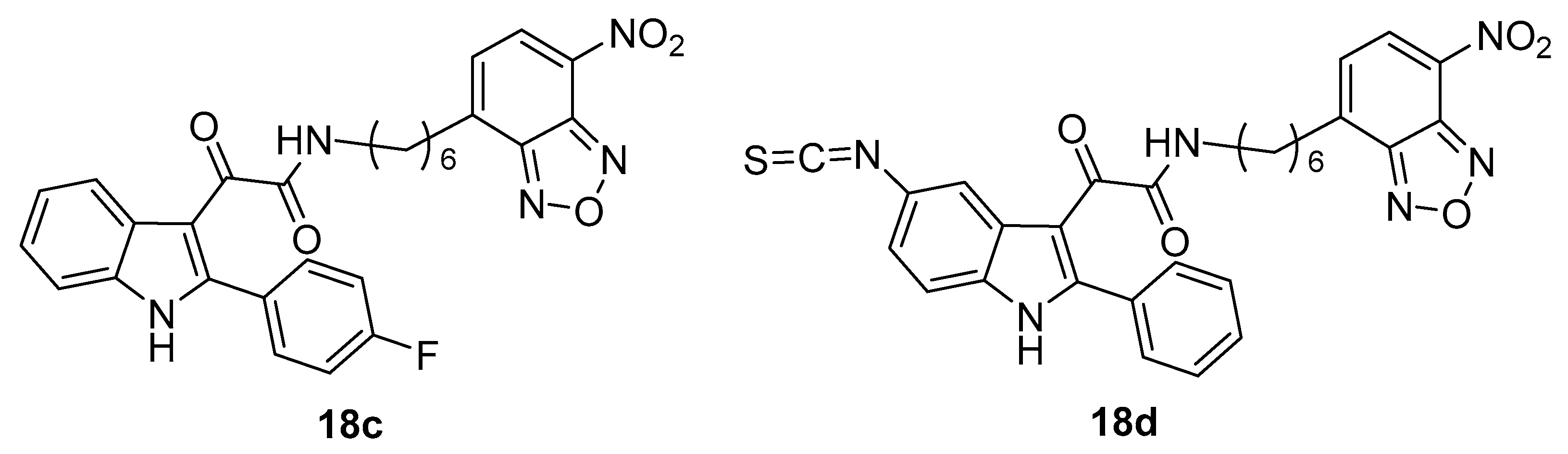

4. Indole Derivatives as Dual Ligands of the Translocator Protein and the Murine Double Minute 2 Protein
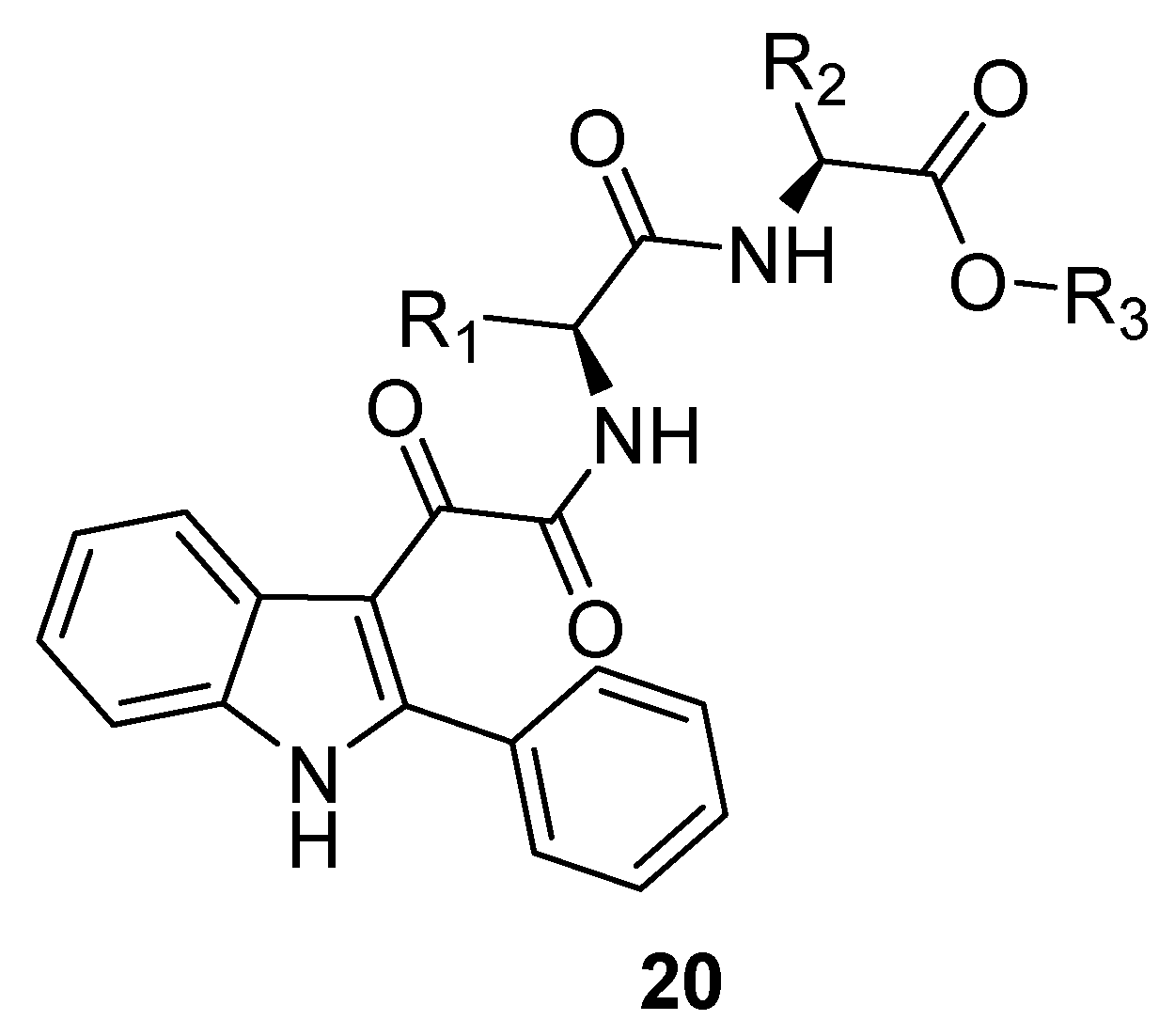

5. Indole Derivatives as Allosteric Modulators of the Human Adenosine A2B Receptor

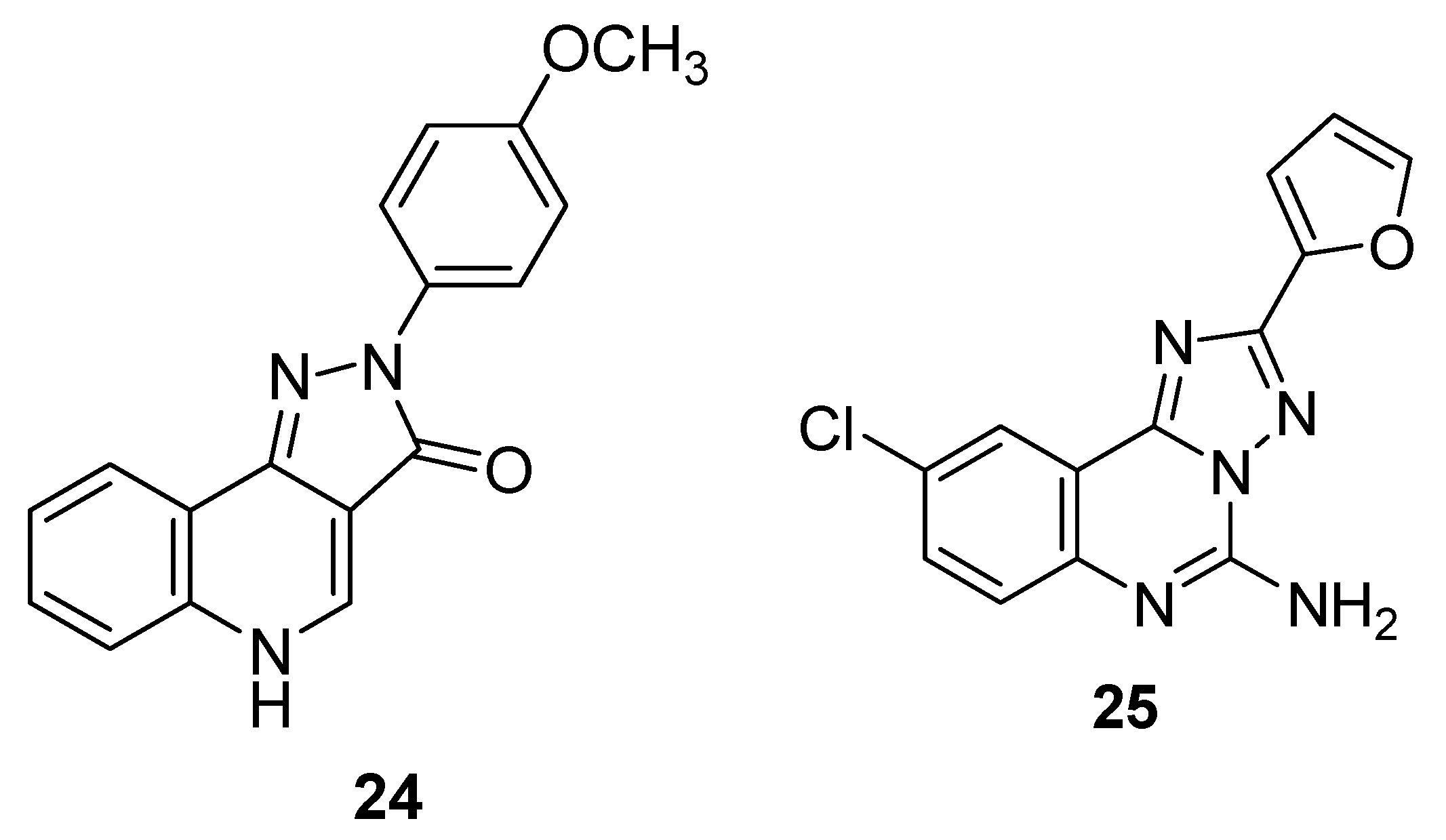
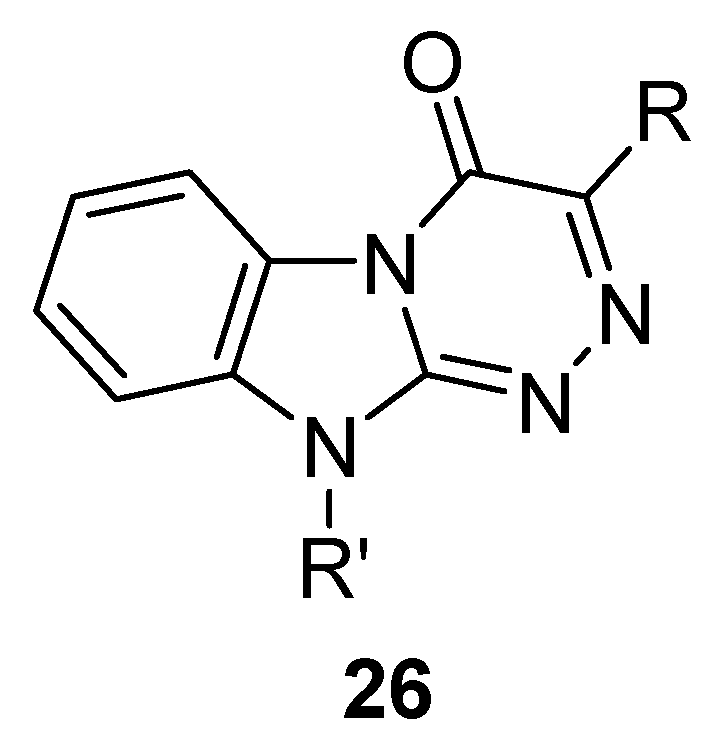
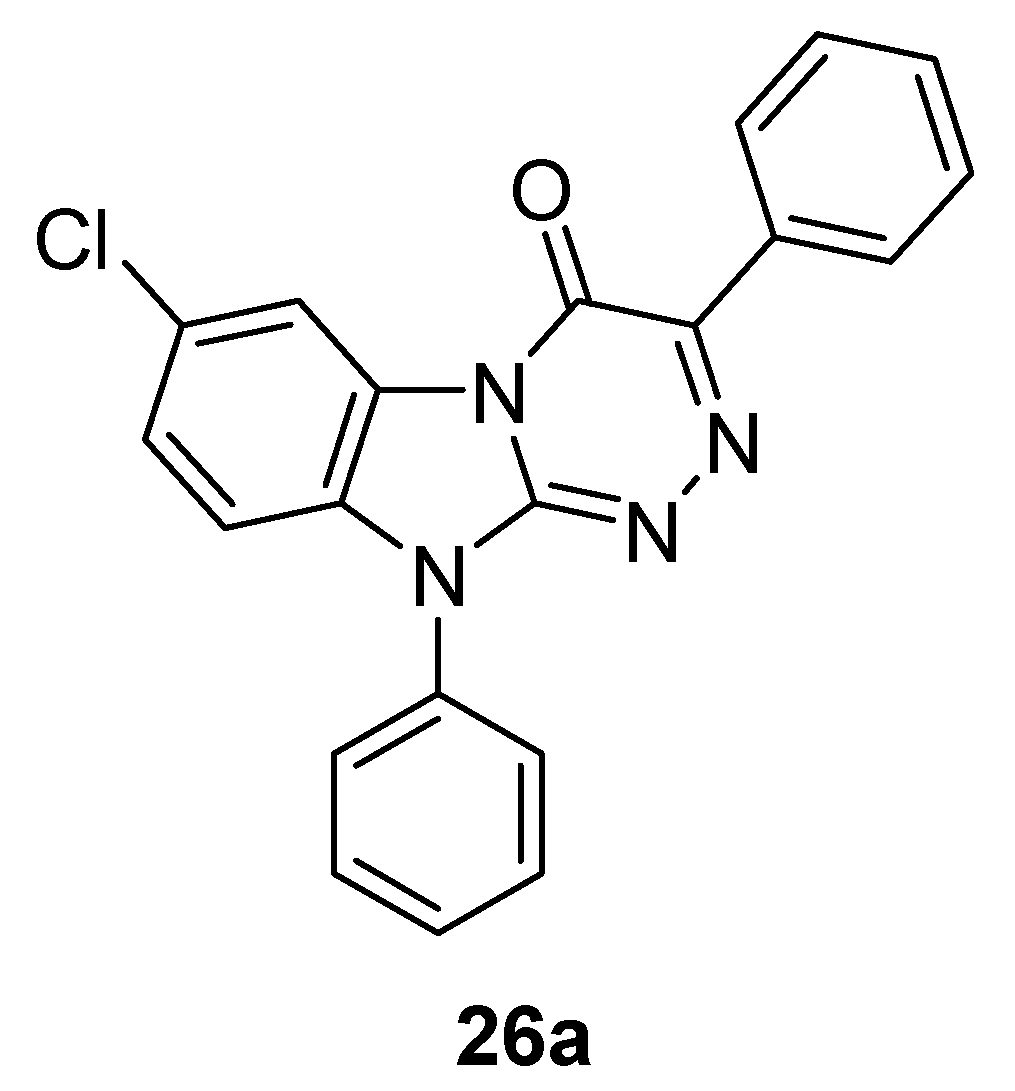
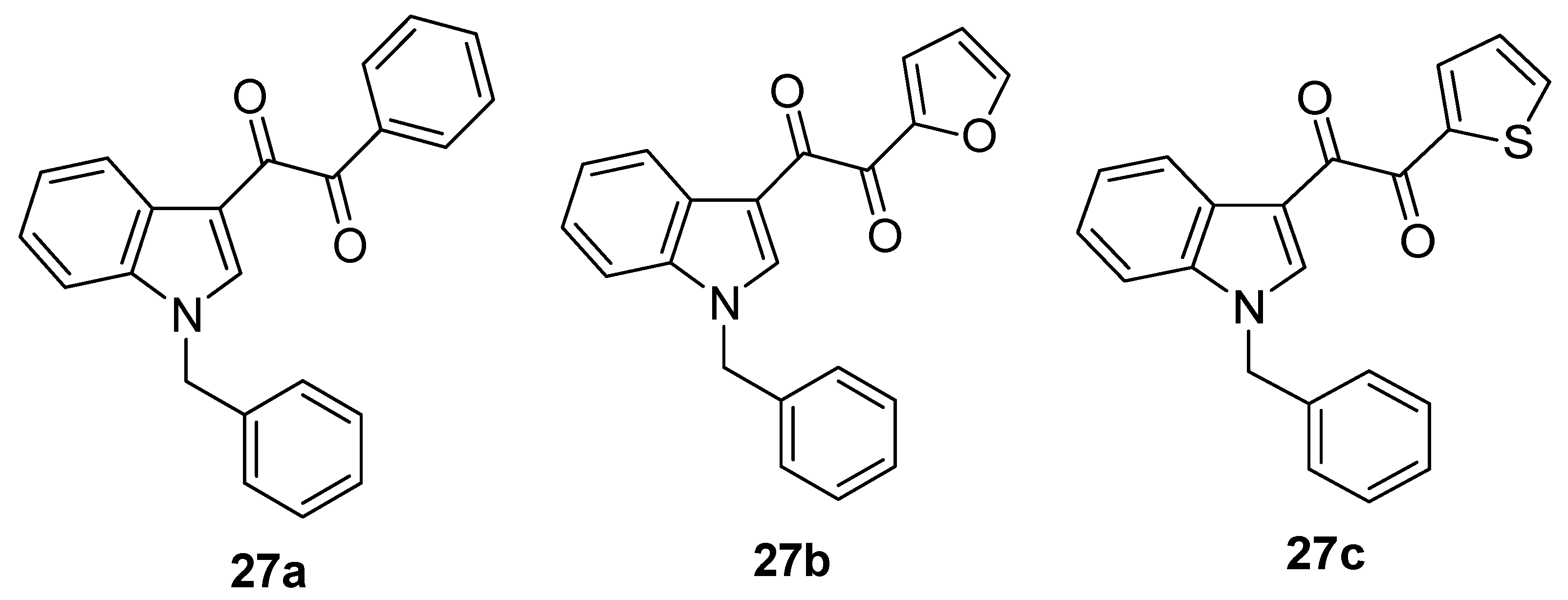
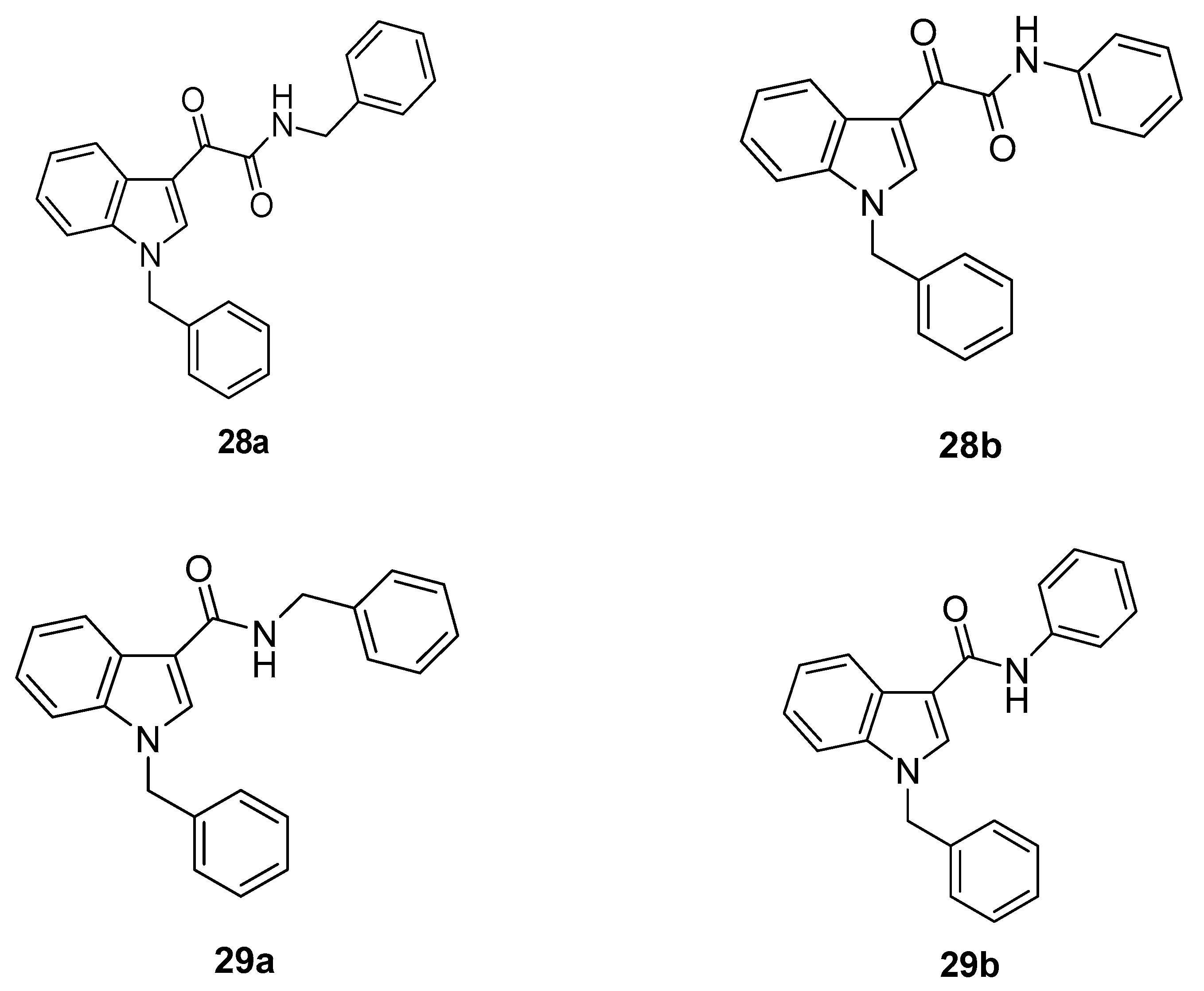
6. Indole Derivatives as Ligands of the Kelch-like ECH-Associated Protein 1
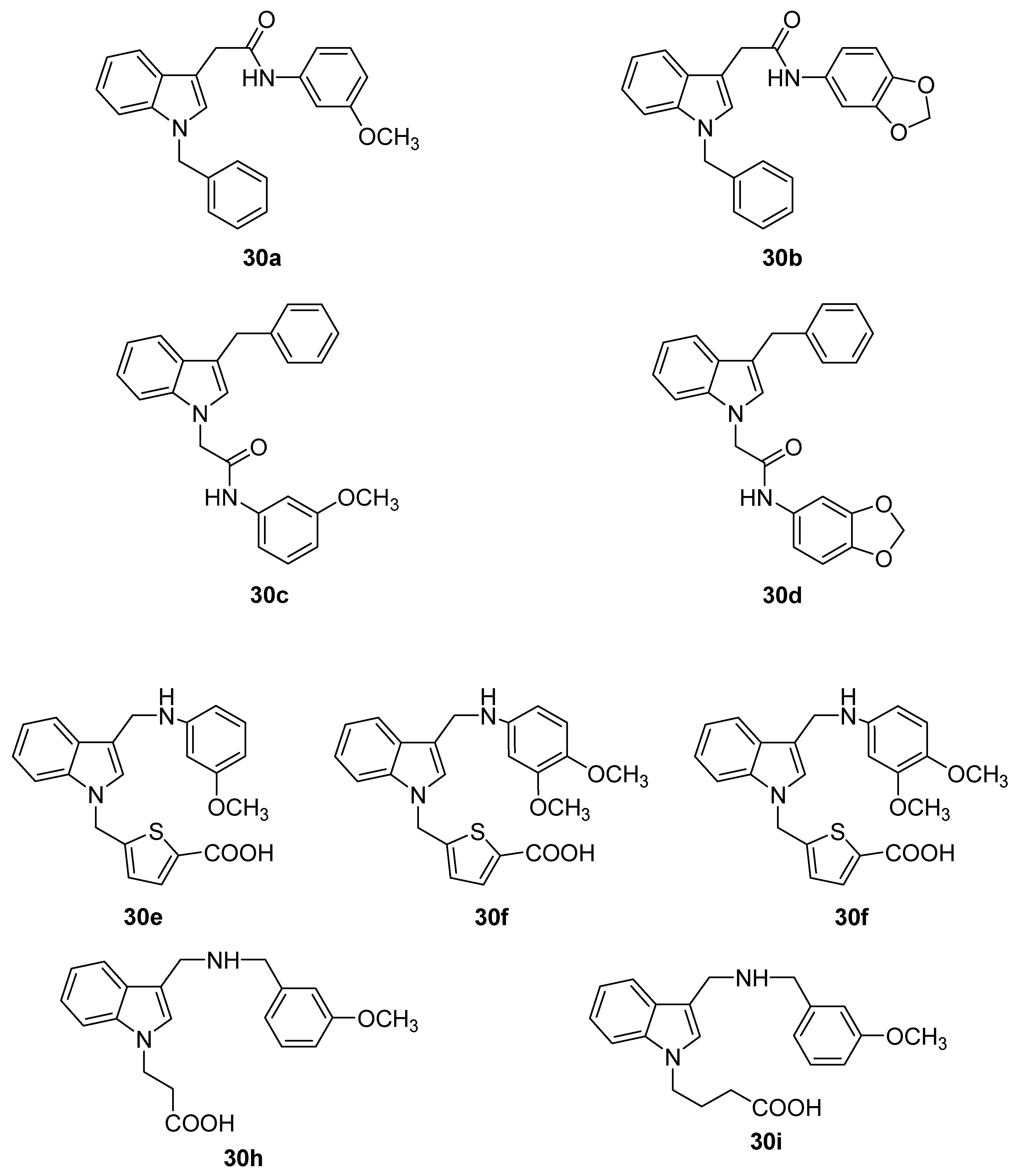
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Fraga, C.A.M.; Barreiro, E.J.; de Sa Alves, F.R. From nature to drug discovery: The indole scaffold as a ‘privileged structure’. Mini Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar]
- Barnard, E.A.; Skolnick, P.; Olsen, R.W.; Mohler, H.; Sieghart, W.; Biggio, G.; Braestrup, C.; Bateson, A.N.; Langer, S.Z. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: Classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998, 50, 291–313. [Google Scholar] [PubMed]
- Mohler, H. GABA(A) Receptor diversity and pharmacology. Cell Tissue Res. 2006, 326, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Cell Tissue Res. 2006, 326, 795–816. [Google Scholar] [CrossRef]
- Sieghart, W.; Savic, M.M. International Union of Basic and Clinical Pharmacology. CVI: GABAA receptor subtype- and function-selective ligands: Key issues in translation to humans. Pharmacol. Rev. 2018, 70, 836–878. [Google Scholar] [CrossRef]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef]
- Mohler, H.; Fritschy, J.M.; Rudolph, U. A New benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002, 300, 2–8. [Google Scholar] [CrossRef]
- Whiting, P.J. GABA-A receptor subtypes in the brain: A paradigm for CNS drug discovery? Drug Discovery Today 2003, 8, 445–450. [Google Scholar] [CrossRef]
- Wafford, K.A.; Thompson, S.A.; Thomas, D.; Sikela, J.; Wilcox, A.S.; Whiting, P.J. Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol. Pharmacol. 1996, 50, 670–678. [Google Scholar]
- Accardi, M.V.; Brown, P.M.; Miraucourt, L.S.; Orser, B.A.; Bowie, D. α6-Containing GABAA receptors are the principal mediators of inhibitory synapse strengthening by insulin in cerebellar granular cells. J. Neurosci. 2015, 35, 9676–9688. [Google Scholar] [CrossRef]
- Sigel, E. Mapping of the benzodiazepine recognition site on GABAA receptors. Curr. Top. Med. Chem. 2002, 2, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Teuber, L.; Watjens, F.; Jensen, L.H. Ligands for the benzodiazepine binding site-a survey. Curr. Pharm. Des. 1999, 5, 317–343. [Google Scholar] [PubMed]
- Gardner, C.R. Interpretation of behavioral effects of benzodiazepine receptor ligands. Drugs Future 1989, 14, 51–67. [Google Scholar]
- Schofield, P.R.; Darlison, M.G.; Fijita, N.; Burt, D.R.; Sthepenson, F.A.; Rodriguez, H.; Rhee, L.M.; Ramachandran, J.; Reale, V.; Glencorse, T.A.; et al. Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature 1987, 328, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.S.; Schofield, P.R.; Burt, D.R.; Rhee, L.M.; Wisden, W.; Kohler, M.; Fujita, N.; Rodriguez, H.; Stephenson, A.; Darlison, M.G.; et al. Structural and functional basis for GABAA receptor heterogeneity. Nature 1988, 335, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Tobin, A.J. Molecular biology of GABAA receptor. FASEB J. 1990, 4, 1469–1480. [Google Scholar] [CrossRef]
- Burt, D.; Kamatachi, G. GABAA receptor subtypes: From pharmacology to molecular biology. FASEB J. 1991, 5, 2916–2923. [Google Scholar] [CrossRef]
- McKernan, R.M.; Rosahl, T.W.; Reynolds, D.S.; Sur, C.; Wafford, K.A.; Atack, J.R.; Farrar, S.; Myers, J.; Cook, G.; Ferris, P.; et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat. Neurosci. 2000, 3, 587–592. [Google Scholar] [CrossRef]
- Rudolph, U.; Möhler, H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 475–498. [Google Scholar] [CrossRef]
- Lüscher, B.; Keller, C.A. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 2004, 102, 195–221. [Google Scholar] [CrossRef]
- Rudolph, U.; Möhler, H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006, 6, 18–23. [Google Scholar] [CrossRef]
- Da Settimo, F.; Taliani, S.; Trincavelli, M.L.; Montali, M.; Martini, C. GABAA/Bz receptor subtypes as targets for selective drugs. Curr. Med. Chem. 2007, 14, 2680–2701. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Gervasio, T.; Lucacchini, A.; Da Settimo, A.; Primofiore, G.; Marini, A.M. Specific inhibition of benzodiazepine receptor binding by some N-(indol-3-ylglyoxylyl)amino acid derivatives. J. Med. Chem. 1985, 28, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Braestrup, C.; Nielsen, M.C.; Olsen, C.E. Urinary and brain β-carboline carboxylates as potent inhibitors of brain benzodiazepine receptors. Proc. Natl. Acad. Sci. USA 1980, 77, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Primofiore, G.; Marini, A.M.; Da Settimo, F.; Martini, C.; Bardellini, A.; Giannaccini, G.; Lucacchini, A. Specific inhibition of benzodiazepine receptor binding by some N-(indol-3-ylglyoxylyl)amino acid derivatives: Stereoselective interactions. J. Med. Chem. 1989, 32, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, A.M.; Da Settimo, A.; Da Settimo, F.; Primofiore, G.; Martini, C.; Giannaccini, G.; Lucacchini, A. Benzodiazepine receptor affinity and interaction of some N-(indol-3-ylglyoxylyl)amine derivatives. J. Med. Chem. 1992, 35, 2214–2220. [Google Scholar] [CrossRef]
- Da Settimo, A.; Lucacchini, A.; Marini, A.M.; Martini, C.; Primofiore, G.; Senatore, G.; Taliani, S. Isosteric replacement of the indole nucleus by benzothiophene and benzofuran in a series of indolylglyoxylylamine derivatives with partial agonist activity at the benzodiazepine receptor. Eur. J. Med. Chem. 1996, 31, 951–956. [Google Scholar] [CrossRef]
- Da Settimo, A.; Primofiore, G.; Da Settimo, F.; Marini, A.M.; Novellino, E.; Greco, G.; Martini, C.; Giannaccini, G.; Lucacchini, A. Synthesis, structure-activity relationships, and molecular modeling studies of N-(indol-3-ylglyoxylyl)benzylamine derivatives acting at the benzodiazepine receptor. J. Med. Chem. 1996, 39, 5083–5091. [Google Scholar] [CrossRef]
- Da Settimo, A.; Primofiore, G.; Da Settimo, F.; Marini, A.M.; Novellino, E.; Greco, G.; Gesi, M.; Martini, C.; Giannaccini, G.; Lucacchini, A. N’-Phenylindol-3-ylglyoxylohydrazide derivatives: Synthesis, structure-activity relationships, molecular modeling studies and pharmacological action on brain benzodiazepine receptors. J. Med. Chem. 1998, 41, 3821–3830. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoo, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Zhang, W.; Koehler, K.F.; Zhang, P.; Cook, J.M. Development of a comprehensive pharmacophore model for the benzodiazepine receptor. Drug. Des. Discov. 1995, 12, 193–248. [Google Scholar] [PubMed]
- Primofiore, G.; Da Settimo, F.; Taliani, S.; Marini, A.M.; Novellino, E.; Greco, G.; Lavecchia, A.; Besnard, F.; Trincavelli, L.; Costa, B.; et al. Novel N-(arylalkyl)indol-3-ylglyoxylamides targeted as ligands of the benzodiazepine receptor: Synthesis, biological evaluation, and molecular modeling analysis of the structure-activity relationships. J. Med. Chem. 2001, 44, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Primofiore, G.; Da Settimo, F.; Marini, A.M.; Taliani, S.; La Motta, C.; Simorini, F.; Novellino, E.; Greco, G.; Cosimelli, B.; Ehlardo, M.; et al. Refinement of the benzodiazepine receptor site topology by structure-activity relationships of new N-(heteroarylmethyl)indol-3-ylglyoxylamides. J. Med. Chem. 2006, 49, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Primofiore, G.; Taliani, S.; Da Settimo, F.; Marini, A.M.; La Motta, C.; Simorini, F.; Patrizi, M.P.; Sergianni, V.; Novellino, E.; Greco, G.; et al. Novel N-substituted indol-3-ylglyoxylamides probing the LDi and L1/L2 lipophilic regions of the benzodiazepine receptor site in search for subtype-selective ligands. J. Med. Chem. 2007, 50, 1627–1634. [Google Scholar] [CrossRef]
- Huang, Q.; He, X.; Ma, C.; Liu, R.; Yu, S.; Dayer, C.A.; Wenger, G.R.; McKernan, R.; Cook, J.M. Pharmacophore/receptor models for GABAA/BzR subtypes (α1β3γ2, α5β3γ2, and α6β3γ2) via a comprehensive ligand-mapping approach. J. Med. Chem. 2000, 43, 71–95. [Google Scholar] [CrossRef]
- He, X.; Huang, Q.; Ma, C.; Yu, S.; McKernan, R.; Cook, J.M. Pharmacophore/receptor models for GABAA/BzR α2β3γ2, α3β3γ2, and α4β3γ2 recombinant subtypes. Included volume analysis and comparison to α1β3γ2, α5β3γ2, and α6β3γ2. Drug Des. Discov. 2000, 17, 131–171. [Google Scholar]
- Yu, S.; He, X.; Ma, C.; McKernan, R.; Cook, J.M. Studies in search of α2 Selective ligands for GABAA/BzR receptor subtypes. Part I. Evidence for the conservation of pharmacophoric descriptors for DS subtypes. Med. Chem. Res. 1999, 9, 186–202. [Google Scholar]
- Atack, J.R. GABAA receptor subtype-selective modulators. I. α2/α3-Selective agonists as non-sedating anxiolytics. Curr. Top. Med. Chem. 2011, 11, 1176–1202. [Google Scholar] [CrossRef]
- Taliani, S.; Cosimelli, B.; Da Settimo, F.; Marini, A.M.; La Motta, C.; Simorini, F.; Salerno, S.; Novellino, E.; Greco, G.; Cosconati, S.; et al. Identification of anxiolytic/nonsedative agents among indol-3-ylglyoxylamides acting as functionally selective agonists at the γ-aminobutyric acid-A (GABAA) α2 benzodiazepine receptor. J. Med. Chem. 2009, 52, 5798–5806. [Google Scholar] [CrossRef]
- Costall, B.; Jones, B.J.; Kelly, M.E.; Naylor, R.J.; Tomlins, D.M. Exploration of mice in a black and white test box: Validation as a model of anxiety. Pharmacol. Biochem. Behav. 1989, 32, 777–785. [Google Scholar] [CrossRef]
- Cromer, B.A.; Morton, C.J.; Parker, M.W. Anxiety over GABA(A) receptor structure relieved by AChBP. Trends Biochem. Sci. 2002, 27, 280–287. [Google Scholar] [CrossRef]
- Squires, R.F.; Braestrup, C. Benzodiazepine receptors in rat brain. Nature 1977, 266, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Aghazadeh, Y.; Fan, J.; Campioli, E.; Zirkin, B.; Midzak, A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Papadopoulos, V. Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS ONE 2013, 8, e76701. [Google Scholar] [CrossRef]
- Costa, B.; Da Pozzo, E.; Martini, C. Translocator protein and steroidogenesis. Biochem. J. 2018, 475, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Middleton, R.J.; Hatty, C.R.; Kam, W.W.; Chan, R.; Pham, T.; Harrison-Brown, M.; Dodson, E.; Veale, K.; Banati, R.B. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014, 24, 631–653. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Tremolanti, C.; Costa, B.; Giacomelli, C.; Milenkovic, V.M.; Bader, S.; Wetzel, C.H.; Rupprecht, R.; Taliani, S.; Da Settimo, F.; et al. Microglial pro-inflammatory and anti-inflammatory phenotypes are modulated by translocator protein activation. Int. J. Mol. Sci. 2019, 20, 4467. [Google Scholar] [CrossRef]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef]
- Taliani, S.; Pugliesi, I.; Da Settimo, F. Structural requirements to obtain highly potent and selective 18 kDa translocator protein (TSPO) ligands. Curr. Top. Med. Chem. 2011, 11, 860–886. [Google Scholar] [CrossRef] [PubMed]
- Farges, R.; Joseph-Liauzun, E.; Shire, D.; Caput, D.; Le Fur, G.; Ferrara, P. Site-directed mutagenesis of the peripheral benzodiazepine receptor: Identification of amino acids implicated in the binding site of Ro5-4864. Mol. Pharmacol. 1994, 46, 1160–1167. [Google Scholar] [PubMed]
- Le Fur, G.; Guilloux, F.; Rufat, P.; Benavides, J.; Uzan, A.; Renault, C.; Dubroeucq, M.C.; Gueremy, C. Peripheral benzodiazepine binding sites: Effect of PK11195, 1-(2-chlorophenyl)-N-methyl-(1-methylpropyl)-3-isoquinolinecarboxamide. II. In vivo studies. Life Sci. 1983, 32, 1849–1856. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Giacomelli, C.; Barresi, E.; Costa, B.; Taliani, S.; Da Settimo, F.; Martini, C. Targeting the 18-kDa translocator protein: Recent perspectives for neuroprotection. Biochem. Soc. Trans. 2015, 43, 559–565. [Google Scholar] [CrossRef]
- Taliani, S.; Da Settimo, F.; Da Pozzo, E.; Chelli, B.; Martini, C. Translocator protein ligands as promising therapeutic tools for anxiety disorders. Curr. Med. Chem. 2009, 16, 3359–3380. [Google Scholar] [CrossRef]
- Rupprecht, R.; Rammes, G.; Eser, D.; Baghai, T.C.; Schüle, C.; Nothdurfter, C.; Troxler, T.; Gentsch, C.; Kalkman, H.O.; Chaperon, F.; et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 2009, 325, 490–493. [Google Scholar] [CrossRef]
- Costa, B.; Da Pozzo, E.; Martini, C. Translocator protein as a promising target for novel anxiolytics. Curr. Top. Med. Chem. 2012, 12, 270–285. [Google Scholar] [CrossRef]
- Kita, A.; Kinoshita, T.; Kohayakawa, H.; Furukawa, K.; Akaike, A. Lack of tolerance to anxiolysis and withdrawal symptoms in mice repeatedly treated with AC-5216, a selective TSPO ligand. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1040–1045. [Google Scholar] [CrossRef]
- Nguyen, N.; Fakra, E.; Pradel, V.; Jouve, E.; Alquier, C.; Le Guern, M.E.; Micallef, J.; Blin, O. Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: A doubleblind controlled study in general practice. Hum. Psychopharmacol. 2006, 21, 139–149. [Google Scholar] [CrossRef]
- Primofiore, G.; Da Settimo, F.; Taliani, S.; Simorini, F.; Patrizi, M.P.; Novellino, E.; Greco, G.; Abignente, E.; Costa, B.; Chelli, B.; et al. N,N-Dialkyl-2-phenylindol-3-ylglyoxylamides. a new class of potent and selective ligands at the peripheral benzodiazepine receptor. J. Med. Chem. 2004, 47, 1852–1855. [Google Scholar] [CrossRef]
- Da Settimo, F.; Simorini, F.; Taliani, S.; La Motta, C.; Marini, A.M.; Salerno, S.; Bellandi, M.; Novellino, E.; Greco, G.; Cosimelli, B.; et al. Anxiolytic-like effects of N,N-dialkyl-2-phenylindol-3-ylglyoxylamides by modulation of translocator protein promoting neurosteroid biosynthesis. J. Med. Chem. 2008, 51, 5798–5806. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Ma, D.; Brewer, J.; Sun, S.; Costa, E.; Romeo, E.; Guidotti, A. Chemistry, binding affinity, and behavioral properties of a new class of “antineophobic” mitochondrial DBI receptor complex (mDRC) ligands. J. Med. Chem. 1993, 36, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Da Pozzo, E.; Chelli, B.; Simola, N.; Morelli, M.; Luisi, M.; Maccheroni, M.; Taliani, S.; Simorini, F.; Da Settimo, F.; et al. Anxiolytic properties of a 2-phenylindolglyoxylamide TSPO ligand: Stimulation of in vitro neurosteroid production affecting GABAA receptor activity. Psychoneuroendocrinology 2011, 36, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Simorini, F.; Marini, A.M.; Taliani, S.; La Motta, C.; Salerno, S.; Pugliesi, I.; Da Settimo, F. Medicinal chemistry of indolylglyoxylamide TSPO high affinity ligands with anxiolytic-like effects. Curr. Top. Med. Chem. 2012, 12, 333–351. [Google Scholar] [CrossRef]
- Barresi, E.; Bruno, A.; Taliani, S.; Cosconati, S.; Da Pozzo, E.; Salerno, S.; Simorini, F.; Daniele, S.; Giacomelli, C.; Marini, A.M.; et al. Deepening the topology of the translocator protein binding site by novel N,N-dialkyl-2-arylindol-3-ylglyoxylamides. J. Med. Chem. 2015, 58, 6081–6092. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef]
- This code univocally identifies the quoted protein. PDB code: 2MGY.
- Da Pozzo, E.; Giacomelli, C.; Costa, B.; Cavallini, C.; Taliani, S.; Barresi, E.; Da Settimo, F.; Martini, C. TSPO PIGA ligands promote neurosteroidogenesis and human astrocyte well-being. Int. J. Mol. Sci. 2016, 17, 1028. [Google Scholar] [CrossRef]
- Santoro, A.; Mattace Raso, G.; Taliani, S.; Da Pozzo, E.; Simorini, F.; Costa, B.; Martini, C.; Laneri, S.; Sacchi, A.; Cosimelli, B.; et al. TSPO-ligands prevent oxidative damage and inflammatory response in C6 glioma cells by neurosteroid synthesis. Eur. J. Pharm. Sci. 2016, 88, 124–131. [Google Scholar] [CrossRef]
- Taliani, S.; Simorini, F.; Sergianni, V.; La Motta, C.; Da Settimo, F.; Cosimelli, B.; Abignente, E.; Greco, G.; Novellino, E.; Rossi, L.; et al. New fluorescent 2-phenylindolglyoxylamide derivatives as probes targeting the peripheral type benzodiazepine receptor: Design, synthesis, and biological evaluation. J. Med. Chem. 2007, 50, 404–407. [Google Scholar] [CrossRef]
- Taliani, S.; Da Pozzo, E.; Bellandi, M.; Bendinelli, S.; Pugliesi, I.; Simorini, F.; La Motta, C.; Salerno, S.; Marini, A.M.; Da Settimo, F.; et al. Novel irreversible fluorescent probes targeting the 18 kDa translocator protein: Synthesis and biological characterization. J. Med. Chem. 2010, 53, 4085–4093. [Google Scholar] [CrossRef]
- Lin, R.; Angelin, A.; Da Settimo, F.; Martini, C.; Taliani, S.; Zhu, S.; Wallace, D.C. Genetic analysis of dTSPO, an outer mitochondrial membrane protein, reveals its functions in apoptosis, longevity, and Ab42-induced neurodegeneration. Aging Cell 2014, 13, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Pike, V.W.; Taliani, S.; Lohith, T.G.; Owen, D.R.; Pugliesi, I.; Da Pozzo, E.; Hong, J.; Zoghbi, S.S.; Gunn, R.N.; Parker, C.A.; et al. Evaluation of novel N1-methyl-2-phenylindol-3-ylglyoxylamides as a new chemotype of 18 kDa translocator protein-selective ligand suitable for the development of positron emission tomography radioligands. J. Med. Chem. 2011, 54, 366–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scarf, A.M.; Auman, K.M.; Kassiou, M. Is there any correlation between binding and functional effects at the translocator protein (TSPO) (18 kDa)? Curr. Mol. Med. 2012, 12, 387–397. [Google Scholar] [PubMed]
- Copeland, R.A.; Pompliano, D.L.; Meek, T.D. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov. 2006, 5, 730–739. [Google Scholar] [CrossRef]
- Costa, B.; Da Pozzo, E.; Giacomelli, C.; Barresi, E.; Taliani, S.; Da Settimo, F.; Martini, C. TSPO ligand residence time: A new parameter to predict compound neurosteroidogenic efficacy. Sci. Rep. 2016, 6, 18164. [Google Scholar] [CrossRef]
- Costa, B.; Taliani, S.; Da Pozzo, E.; Barresi, E.; Robello, M.; Cavallini, C.; Cosconati, S.; Da Settimo, F.; Novellino, E.; Martini, C. Residence time, a new parameter to predict neurosteroidogenic efficacy of translocator protein (TSPO) ligands: The case study of N,N-dialkyl-2-arylindol-3-ylglyoxylamides. ChemMedChem 2017, 12, 1275–1278. [Google Scholar] [CrossRef]
- Costa, B.; Da Pozzo, E.; Cavallini, C.; Taliani, S.; Da Settimo, F.; Martini, C. Long residence time at the neurosteroidogenic 18 kDa translocator protein characterizes the anxiolytic ligand XBD173. ACS Chem. Neurosci. 2016, 7, 1041–1046. [Google Scholar] [CrossRef]
- Costa, B.; Cavallini, C.; Da Pozzo, E.; Taliani, S.; Da Settimo, F.; Martini, C. The anxiolytic etifoxine binds to TSPO Ro5−4864 binding site with long residence time showing a high neurosteroidogenic activity. ACS Chem. Neurosci. 2017, 8, 1448–1454. [Google Scholar] [CrossRef]
- Bruno, A.; Barresi, E.; Simola, N.; Da Pozzo, E.; Costa, B.; Novellino, E.; Da Settimo, F.; Martini, C.; Taliani, S.; Cosconati, S. Unbinding of Translocator Protein 18 kDa (TSPO) Ligands: From in Vitro Residence Time to in Vivo Efficacy via in Silico Simulations. ACS Chem. Neurosci. 2019, 10, 3805–3814. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Meegan, M.J. Designed multiple ligands for cancer therapy. Curr. Med. Chem. 2001, 18, 4722–4737. [Google Scholar]
- Petrelli, A.; Giordano, S. From single- to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr. Med. Chem. 2008, 15, 422–432. [Google Scholar] [PubMed]
- Petrelli, A.; Valabrega, G. Multitarget drugs: The present and the future of cancer therapy. Expert Opin. Pharmacother. 2009, 10, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Lisitsa, A.; Knight, R.A.; Melino, G.; Antonov, A.V. Polypharmacology of Approved Anticancer Drugs. Curr. Drug Targets 2017, 18, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Griguer, C.E.; Oliva, C.R. Bioenergetics pathways and therapeutic resistance in gliomas: Emerging role of mitochondria. Curr. Pharm. Des. 2011, 17, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Walczak, H. Apoptosis therapy: Driving cancers down the road to ruin. Nat. Med. 2013, 19, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef]
- Austin, C.J.; Kahlert, J.; Kassiou, M.; Rendina, L.M. The translocator protein (TSPO): A novel target for cancer chemotherapy. Int. J. Biochem. Cell Biol. 2013, 45, 1212–1216. [Google Scholar] [CrossRef]
- Chelli, B.; Rossi, L.; Da Pozzo, E.; Costa, B.; Spinetti, F.; Rechichi, M.; Salvetti, A.; Lena, A.; Simorini, F.; Vanacore, R.; et al. PIGA (N,N-di-n-butyl-5-chloro-2-(4-chlorophenyl)indol-3-ylglyoxylamide), a new mitochondrial benzodiazepine-receptor ligand, induces apoptosis in C6 glioma cells. ChemBioChem 2005, 6, 1082–1088. [Google Scholar] [CrossRef]
- England, B.; Huang, T.; Karsy, M. Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumor Biol. 2013, 34, 2063–2074. [Google Scholar] [CrossRef]
- Villalonga-Planells, R.; Coll-Mullet, L.; Martinez-Soler, F.; Castano, E.; Acebes, J.J.; Gimenéz-Bonafé, P.; Gil, J.; Tortosa, A. Activation of p53 by nutlin-3a induces apoptosis and cellular senescence in human glioblastoma multiforme. PLoS ONE 2011, 6, e18588. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-moleculea ntagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Taliani, S.; Da Pozzo, E.; Giacomelli, C.; Costa, B.; Trincavelli, M.L.; Rossi, L.; La Pietra, V.; Barresi, E.; Carotenuto, A.; et al. Apoptosis therapy in cancer: The first single-molecule coactivating p53 and the translocator protein in glioblastoma. Sci. Rep. 2014, 4, 4749. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; La Pietra, V.; Barresi, E.; Di Maro, S.; Da Pozzo, E.; Robello, M.; La Motta, C.; Cosconati, S.; Taliani, S.; Marinelli, L.; et al. Lead optimization of 2-phenylindolylglyoxylyldipeptide murine double minute (MDM)2/translocator protein (TSPO) dual inhibitors for the treatment of gliomas. J. Med. Chem. 2016, 59, 4526–4538. [Google Scholar] [CrossRef] [PubMed]
- Smalley, M.; Piggott, L.; Clarkson, R. Breast cancer stem cells: Obstacles to therapy. Cancer Lett. 2013, 338, 57–62. [Google Scholar] [CrossRef]
- Tjin Tham Sjin, R.; Lee, K.; Walter, A.O.; Dubrovskiy, A.; Sheets, M.; Martin, T.S.; Labenski, M.T.; Zhu, Z.; Tester, R.; Karp, R.; et al. In vitro and in vivo characterization of irreversible mutant-selective EGFR inhibitors that are wild-type sparing. Mol. Cancer Ther. 2014, 13, 1468–1479. [Google Scholar] [CrossRef]
- Daniele, S.; Barresi, E.; Zappelli, E.; Marinelli, L.; Novellino, E.; Da Settimo, F.; Taliani, S.; Trincavelli, M.L.; Martini, C. Long lasting MDM2/translocator protein modulator: A new strategy for irreversible apoptosis of human glioblastoma cells. Oncotarget 2016, 7, 7866–7884. [Google Scholar] [CrossRef]
- Daniele, S.; Giacomelli, C.; Pietrobono, D.; Barresi, E.; Piccarducci, R.; La Pietra, V.; Taliani, S.; Da Settimo, F.; Marinelli, L.; Novellino, E.; et al. Long lasting inhibition of Mdm2-p53 interaction potentiates mesenchymal stem cell differentiation into osteoblasts. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 737–749. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, J.K.A.; Linden, K.N.; Muller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors-an update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Muller, C.E.; Jacobson, K.A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta 2011, 1808, 1290–1308. [Google Scholar] [CrossRef]
- Schulte, G.; Fredholm, B.B. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 2003, 15, 813–827. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drugs Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Primofiore, G.; Da Settimo, F.; Taliani, S.; Marini, A.M.; La Motta, C.; Novellino, E.; Greco, G.; Gesi, M.; Trincavelli, L.; Martini, C. 3-Aryl-[1,2,4]triazino [4,3-a]benzimidazol-4(10H)-ones: Tricyclic heteroaromatic derivatives as a new class of benzodiazepine receptor ligands. J. Med. Chem. 2000, 43, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Primofiore, G.; Da Settimo, F.; Taliani, S.; Marini, A.M.; Simorini, F.; Novellino, E.; Greco, G.; Trincavelli, L.; Martini, C. Geometrically constrained analogues of N-benzylindolylglyoxylylamides: [1,2,4]triazino[4,3-a]benzimidazol-4(10H)-one derivatives as potential new ligands at the benzodiazepine receptor. Arch. Pharm. 2003, 336, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Ritter, B.; Neubert, A.D. 2-Arylpyrazolo[4,3-c]quinolin-3-ones: A novel agonist, a partial agonist and an antagonist of benzodiazepines. J. Med. Chem. 1982, 25, 337–339. [Google Scholar] [CrossRef]
- Francis, J.E.; Cash, W.D.; Psychoyos, S.; Ghai, G.; Friedmann, R.C.; Atkins, C.; Warren, V.; Furness, P.; Hyun, J.L.; Stone, G.A.; et al. Structure-activity profile of novel triazoloquinazoline adenosine antagonists. J. Med. Chem. 1988, 31, 1014–1020. [Google Scholar] [CrossRef]
- Da Settimo, F.; Primofiore, G.; Taliani, S.; Marini, A.M.; La Motta, C.; Novellino, E.; Greco, G.; Lavecchia, A.; Trincavelli, L.; Claudia, M. 3-Aryl[1,2,4]triazino[4,3-a]benzimidazol-4(10H)-ones: A new class of selective A1 adenosine receptor antagonists. J. Med. Chem. 2001, 44, 316–327. [Google Scholar] [CrossRef]
- Da Settimo, F.; Primofiore, G.; Taliani, S.; La Motta, C.; Novellino, E.; Greco, G.; Lavecchia, A.; Cosimelli, B.; Iadanza, M.; Klotz, K.N.; et al. A1 adenosine receptor antagonists, 3-aryl[1,2,4]triazino[4,3-a]benzimidazol-4-(10H)-ones (ATBIs) and N-alkyl and N-acyl-(7-substituted-2-phenylimidazo[1,2-a][1,3,5]triazin-4-yl)amines (ITAs): Different recognition of bovine and human binding sites. Drug Dev. Res. 2004, 63, 1–7. [Google Scholar] [CrossRef]
- Taliani, S.; Pugliesi, I.; Barresi, E.; Simorini, F.; Salerno, S.; La Motta, C.; Marini, A.M.; Cosimelli, B.; Cosconati, S.; Di Maro, S.; et al. Aryl-[1,2,4]triazino[4,3-a]benzimidazol-4(10H)-one: A novel template for the design of highly selective A2B adenosine receptor antagonists. J. Med. Chem. 2012, 55, 1490–1499. [Google Scholar] [CrossRef]
- Kalla, R.V.; Zablocki, J.; Tabrizi, M.A.; Baraldi, P.G. Recent developments in A2B adenosine receptor ligands. Handb. Exp. Pharmacol. 2009, 193, 99–122. [Google Scholar]
- Eckle, T.; Grenz, A.; Laucher, S.; Eltzschig, H.K. A2B adenosine receptor signalling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Investig. 2008, 118, 3301–3315. [Google Scholar]
- Eckle, T.; Hartmann, K.; Bonney, S.; Reithel, S.; Mittelbronn, M.; Walker, L.A.; Lowes, B.D.; Han, J.; Borchers, C.H.; Buttrick, P.M.; et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med. 2012, 18, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, S.; Novitskiy, S.V.; Zaynagetdinov, R.; Goldstein, A.E.; Carbone, D.P.; Biaggioni, I.; Dikov, M.M.; Feoktistov, I. Host A2B adenosine receptors promote carcinoma growth. Neoplasia 2008, 10, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-G.; Jacobson, K.A. A2B Adenosine Receptor and Cancer. Int. J. Mol. Sci. 2019, 20, 5139. [Google Scholar] [CrossRef] [PubMed]
- Kolachala, V.; Asamoah, V.; Wang, L.; Obertone, T.S.; Ziegler, T.R.; Merlin, D.; Sitaraman, S.V. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: A basis for A2bR overexpression in colitis. Cell. Mol. Life Sci. 2005, 62, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Kolachala, V.; Ruble, B.; Vijay-Kumar, M.; Wang, L.; Mwangi, S.; Figler, H.; Figler, R.; Srinivasan, S.; Gewirtz, A.; Linden, J.; et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br. J. Pharmacol. 2008, 155, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Zablocki, J.; Elzein, E.; Kalla, R.V. A2B adenosine receptor antagonists and their potential indications. Expert Opin. Ther. Pat. 2006, 16, 1347–1357. [Google Scholar] [CrossRef]
- Kalla, R.V.; Zablocki, J. Progress in the discovery of selective, high affinity A2B adenosine receptor antagonists as clinical candidates. Purinergic Signal. 2009, 5, 21–29. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Samarneh, S.; Jaber, A.M.Y.; Kassab, G.; Agrawal, N. Therapeutic Potentials of A2B Adenosine Receptor Ligands: Current Status and Perspectives. Curr. Pharm. Des. 2019, 25, 2741–2771. [Google Scholar] [CrossRef]
- Taliani, S.; Trincavelli, M.L.; Cosimelli, B.; Laneri, S.; Severi, E.; Barresi, E.; Pugliesi, I.; Daniele, S.; Giacomelli, C.; Greco, G.; et al. Modulation of A2B adenosine receptor by 1-benzyl-3-ketoindole derivatives. Eur. J. Med. Chem. 2013, 69, 331–337. [Google Scholar] [CrossRef]
- Trincavelli, M.L.; Giacomelli, C.; Daniele, S.; Taliani, S.; Cosimelli, B.; Laneri, S.; Severi, E.; Barresi, E.; Pugliesi, I.; Greco, G.; et al. Allosteric modulators of human A2B adenosine receptor. Biochim. Biophys. Acta 2014, 1840, 1194–1203. [Google Scholar] [CrossRef]
- Keov, P.; Sexton, P.M.; Christopoulos, A. Allosteric modulation of G protein-coupled receptors: A pharmacological perspective. Neuropharmacology 2011, 60, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.G.; Jacobson, K.A. Allosteric modulation and functional selectivity of G protein coupled receptors. Drug Discov. Today 2013, 10, 237–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trincavelli, M.L.; Daniele, S.; Giacomelli, C.; Taliani, S.; Da Settimo, F.; Cosimelli, B.; Greco, G.; Novellino, E.; Martini, C. Osteoblast differentiation and survival: A role for A2B adenosine receptor. Biochim. Biophys. Acta 2014, 1843, 2957–2966. [Google Scholar] [CrossRef]
- Cosimelli, B.; Greco, G.; Laneri, S.; Novellino, E.; Sacchi, A.; Amendola, G.; Cosconati, S.; Bortolozzi, R.; Viola, G. Identification of novel indole derivatives acting as inhibitors of the Keap1-Nrf2 interaction. J. Enzym. Inhib. Med. Chem. 2019, 34, 1152–1157. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweir, J.L.; Yunbo, L. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef]
- Xu, I.M.; Lai, R.K.; Lin, S.H.; Tse, A.P.; Chiu, D.K.; Koh, H.Y.; Law, C.T.; Wong, C.M.; Cai, Z.; Wong, C.C.; et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl. Acad. Sci. USA 2016, 113, E725–E734. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2 related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1−Nrf2−ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Lu, M.C.; You, Q.D. Discovery and development of Kelch-like ECH-associated protein 1. Nuclear factor erythroid 2-related factor 2 (Keap1:Nrf2) protein-protein interaction inhibitors: Achievements, challenges, and future directions. J. Med. Chem. 2016, 59, 10837–10858. [Google Scholar] [CrossRef]
- Marcotte, D.; Zeng, W.; Hus, J.C.; McKenzie, A.; Hession, C.; Jin, P.; Bergeron, C.; Lugovskoy, A.; Enyedy, I.; Hernan, C.; et al. Small molecules inhibit the interaction of Nrf2 and the Keap1 Kelch domain through a non-covalent mechanism. Bioorg. Med. Chem. 2013, 21, 4011–4019. [Google Scholar] [CrossRef]
- Zhuang, C.; Narayanapillai, S.; Zhang, W.; Sham, Y.Y.; Xing, C. Rapid identification of Keap1-Nrf2 small-molecule inhibitors through structure-based virtual screening and hit-based substructure search. J. Med. Chem. 2014, 57, 1121–1126. [Google Scholar] [CrossRef]
- Wang, X.J.; Hayes, J.D.; Wolf, C.R. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of Nrf2 by cancer chemotherapeutic agents. Cancer Res. 2006, 66, 10983–10994. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Haskew-Layton, R.E.; Basso, M.; Hushpulian, D.M.; Payappilly, J.B.; Speer, R.E.; Ahn, Y.H.; Rakhman, I.; Cole, P.A.; Pinto, J.T.; et al. Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem. Biol. 2011, 18, 752–765. [Google Scholar] [CrossRef]
- This code univocally identifies the quoted protein. PDB codes: 3VNG, 3WNK, 4IFN, 4IQK, 4L7B, AL7D, 4N1B, 4L7D and 4XMB.


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taliani, S.; Da Settimo, F.; Martini, C.; Laneri, S.; Novellino, E.; Greco, G. Exploiting the Indole Scaffold to Design Compounds Binding to Different Pharmacological Targets. Molecules 2020, 25, 2331. https://doi.org/10.3390/molecules25102331
Taliani S, Da Settimo F, Martini C, Laneri S, Novellino E, Greco G. Exploiting the Indole Scaffold to Design Compounds Binding to Different Pharmacological Targets. Molecules. 2020; 25(10):2331. https://doi.org/10.3390/molecules25102331
Chicago/Turabian StyleTaliani, Sabrina, Federico Da Settimo, Claudia Martini, Sonia Laneri, Ettore Novellino, and Giovanni Greco. 2020. "Exploiting the Indole Scaffold to Design Compounds Binding to Different Pharmacological Targets" Molecules 25, no. 10: 2331. https://doi.org/10.3390/molecules25102331
APA StyleTaliani, S., Da Settimo, F., Martini, C., Laneri, S., Novellino, E., & Greco, G. (2020). Exploiting the Indole Scaffold to Design Compounds Binding to Different Pharmacological Targets. Molecules, 25(10), 2331. https://doi.org/10.3390/molecules25102331







