Abstract
The present study demonstrated a sustainable and cost-effective approach to depolymerize/oxidize softwood (SW) and hardwood (HW) kraft lignins using concentrated hydrogen peroxide at temperatures ranging from 25 to 35 °C, in the absence of catalysts or organic solvents. The degree of lignin depolymerization could be simply controlled by reaction time, and no further separation process was needed at the completion of the treatment. The obtained depolymerized lignin products were comprehensively characterized by GPC–UV, FTIR, 31P-NMR, TGA, Py-GC/MS and elemental analysis. The weight-average molecular weights (Mw) of the depolymerized lignins obtained from SW or HW lignin at a lignin/H2O2 mass ratio of 1:1 after treatment for 120 h at room temperature (≈25 °C) were approximately 1420 Da. The contents of carboxylic acid groups in the obtained depolymerized lignins were found to significantly increase compared with those of the untreated raw lignins. Moreover, the depolymerized lignin products had lower thermal decomposition temperatures than those of the raw lignins, as expected, owing to the greatly reduced Mw. These findings represent a novel solution to lignin depolymerization for the production of chemicals that can be utilized as a bio-substitute for petroleum-based polyols in polyurethane production.
1. Introduction
The forestry and agricultural sectors worldwide are generating considerable amounts of lignocellulosic materials in the form of residues, an attractive carbon-neutral source for fuels and chemicals, which can be an economically important alternative to fossil fuels. Recently, significant advances have been made in the development of economically viable biorefineries involving the fractionation of lignocellulose into its three major constituents (i.e., cellulose, hemicellulose and lignin), and full valorization of these constituents [1]. It should be noted here that biomass is the only renewable source of carbon for chemicals and materials [2]. Kraft pulping is the dominant chemical pulping process in the world. In this process, lignin and hemicellulose in wood chips are dissolved by sodium hydroxide (NaOH) and sodium sulfide (Na2S) to form black liquor, which is subsequently concentrated and burned in the recovery boiler to recover chemicals and heat, both of which are crucial to the functioning of a kraft mill. In North America, however, there are many mills in which the recovery boiler is the production bottleneck with respect to pulp production. Hence, removing a portion of the lignin from the black liquor can be an effective way to offload the recovery boiler with respect to calorific load, thereby allowing for an increase in pulp production without enormous capital spending. Moreover, the isolated portion of lignin can provide an additional value-added revenue stream for the mill by valorizing lignin for bio-products.
Lignin is an aromatic heterogeneous biopolymer, with a three-dimensional, crossed-linked network structure [3]. The structural diversity of lignin arises mainly from the combination of three linked phenylpropane derivatives that are the main building blocks of lignin’s complex architecture. The three building blocks are the lignols p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, all of which are phenylpropane (C9) units differing from each other in the substitutions at the 3- and 5-positions. These lignols are linked into a lignin aromatic network in the form of phenyl propanoids, namely p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units, respectively. Different sources of lignin (e.g., softwood, hardwood, grasses, etc.) contain different amounts of methoxyl groups depending on how much of each of the three lignols is incorporated into the lignin macromolecules [4,5]. Technical lignins, e.g., kraft lignin extracted from the pulping process, usually have a large molecular weight and a three-dimensional, crossed-linked network structure, as described above, which makes them less reactive in applications such as, for example, chemical replacement of petroleum-based polyols in materials such as polyurethanes, due to steric hindrance [6].
It has previously been demonstrated that lignin degradation and/or de-polymerization approaches can be effective in enhancing lignin reactivity in bio-based material applications [7]. The major lignin degradation methods can be categorized according to the mechanism of lignin network depolymerization, namely, oxidative, solvolytic, hydrogenolytic or hydrolytic reactions, which are briefly reviewed below. Lignin structures were initially investigated using oxidation reactions. The types of linkages between the precursors were investigated more than 50 years ago [8]. Freudenberg et al. [9] confirmed lignin’s aromatic nature by oxidizing it with nitrobenzene in an alkaline medium at 160–180 °C for 2–3 h. In their experiments, benzaldehydes were recovered as a main product with minor amounts of benzoic acids. In this reaction, nitrobenzene was considered to act as a two-electron-accepting oxidant producing quinone methide intermediates from the phenolic units of lignin [9]. Several solvolytic depolymerization studies have been reported in the literature involving different solvent mixtures (e.g., glycerol, THF, methyl-THF and γ-valerolactone (GVL)) and different types of catalysts (e.g., oxalic acid, HCl, Lewis acids, metal chlorides and ammonia) [10,11]. Acid-catalyzed solvolytic treatment is one of the earliest techniques used to deconstruct wood components and separate lignin. Hewson et al. [12] conducted a series of treatments on maple wood meal using different combinations of acids and solvents, including HCl/ethanol and formic acid/ethylene glycol to separate lignin into water-soluble and water-insoluble components at a low temperature range. It was concluded that this approach was not sufficient to de-polymerize the complex lignin structure into monomeric/oligomeric compounds. Gasson et al. [13] investigated formic acid-catalyzed lignin depolymerization at higher temperatures in alcohol solvents. Different ratios of formic acid to ethanol were employed in a pressurized autoclave reactor. They found that methoxyphenol, catechol and phenol were obtained as the major components when the reaction temperature was raised to the 360 to 400 °C range. The primary reaction pathway for lignin degradation to form phenolic products was believed to take place via the cleavage of the β-O-4 bonds as catalyzed by acid [14]. Recently, Kristianto et al. [15] reported highly effective lignin depolymerization in ethanol solvent in the presence of formic acid and Ru/C. A higher formic acid content and prolonged reaction times at 350 °C in the presence of Ru/C increased the bio-oil (de-polymerized lignin) yield and reduced the oxygen content in the bio-oil owing to hydrotreatment effects of the hydrogen generated in-situ from formic acid decomposition.
In other work by the authors’ group, Mahmood et al. [16] achieved the depolymerization of kraft lignin via hydrolysis using aqueous NaOH as a catalyst. The process itself was very effective and achieved a high yield (70–90%) of depolymerized kraft lignin (DKL) with a weight-average molecular weight (Mw) of ~1500 g/mol at 250–350 °C for 2 h. However, it was a high-pressure process with the reactor pressure ranging from 5 MPa to 16 MPa. In contrast to the above-described research work, oxidative depolymerization of lignin is a low-temperature and pressure process, offering a simpler and more energy/cost-effective approach to depolymerization of lignin at low temperatures with high product yields. Various sophisticated strategies have been reported for oxidative depolymerization of lignin model compounds or lignin, including electrochemical and photocatalytic approaches as well as approaches employing heterogeneous catalysts or ionic liquids. Valuable monomers with high selectivity were produced from lignin oxidative degradation approaches. In fact, oxidative technologies have been used for a long time for bleaching purposes in the pulp and paper industry. Some of these oxidative technologies (e.g., using chlorine) are no longer in use due to more stringent environmental regulations relating to the production of chlorinated dioxins and furans [10].
Oxidative approaches to lignin depolymerization, especially utilizing molecular oxygen, hydrogen peroxide, peroxyacids or ozone, have a great potential to become important and economically viable lignin delignification technologies [17], as the reaction pathways can yield valuable chemicals such as simple aldehydes (vanillin, syringaldehyde, p-hydroxybenzaldehyde) or acids (vanillic acid and syringic acid) [18]. Depending on the extent of oxidation, various carboxylic acids having aromatic and non-aromatic structures with mono- or di-carboxyl functional groups can be produced [19]. Hydrogen peroxide, molecular oxygen and other oxidants in the presence or absence of a catalyst have been investigated extensively. Crestini et al. reported the degradation of kraft lignin and model compounds using H2O2 as an oxidant and methylrhenium trioxide (CH3ReO3) as a catalyst, producing degraded lignin compounds containing aliphatic-OH, syringyl-OH, guaiacyl-OH, p-hydroxy phenyl-OH and COOH groups at room temperature [20].
Oxidative lignin depolymerization is advantageous and environmentally friendly owing to its mild reaction conditions, but it is confronted with the challenge of maintaining sufficient selectivity while avoiding over-oxidation of the substrate to gaseous products (CO or CO2). Another advantage of lignin oxidation is that it can result in not only depolymerization but also functionalization of complex lignin, resulting in fine chemicals bearing alcohol, aldehyde or carboxylic/dicarboxylic acid functional groups. Consequently, this exclusive feature of oxidatively depolymerized lignin (low molecular weight and functionalized) could be utilized directly for industrial applications without further modification.
Sun and Argyropoulos [21] investigated the reactivity and the efficiency of several oxidants, namely chlorine dioxide, ozone, dimethyldioxirane and alkaline hydrogen peroxide with lignin using quantitative 31P-NMR. They found that guaiacyl phenolic units were the most vulnerable sites in all the oxidative treatments, and chlorine dioxide and ozone were the most efficient reagents towards the formation of carboxylic acids. The elimination of condensed phenolic structures at a given reagent charge was in the order of ozone > chlorine dioxide > alkaline hydrogen peroxide. However, while ozone and chlorine dioxide must be generated on-site, hydrogen peroxide is widely available from various suppliers and is significantly less expensive than the other two oxidants. Furthermore, hydrogen peroxide is a non-toxic chemical, which decomposes to molecular oxygen and water, if not properly stabilized [22,23].
Hydrogen peroxide (H2O2) is the simplest peroxide with an oxygen–oxygen single bond. It has been widely used as an oxidizer, bleaching agent and antiseptic reagent. Concentrated hydrogen peroxide is a potent oxidizing agent due to its unstable peroxide bond. H2O2 has been commercially used for many years as an efficient bleaching reagent in the pulp and paper industry. It can effectively remove chromophoric structures present in lignin, but it is incapable of degrading the lignin structure network. To degrade lignin, H2O2 must be used together with organic acids or mineral acids [22].
Moreover, the solutions of hydrogen peroxide in water are non-ideal, as the volume of a solution is less than the sum of the volumes of the components, and there is an appreciable heat effect upon mixing. Furthermore, the vapor pressures of the solutions do not follow Raoult’s law [24].
H2O2 is a very weak acid that remains undissociated at pH < 9.0. At higher pH levels, perhydroxyl anions (HO2−) appear, and they are commonly considered to be the reactive species in oxidation reactions under alkaline conditions [25]. As is well known, the chemical composition of softwoods (SW) is different from hardwoods (HW) with respect to cellulose, hemicellulose and lignin content [26]. The type of linkages predominant in these two types of wood are shown in Table 1.

Table 1.
General compositions of softwoods and hardwoods and their lignin linkages [27,28].
In addition, hardwoods contain mainly a mixture of syringyl (S) and guaiacyl (G) units, whereas softwoods contain mainly guaiacyl (G) units. SW and HW lignins also differ in the relative amounts of linkages between the phenylpropane units (Table 1) [29]. In the pulping delignification process, the G-based lignin structural network is more resistant to cleavage, thereby leading to a lower degree of lignin depolymerization during pulping. Essentially, the efficiency of pulping is directly proportional to the amount of syringyl (S) units in lignin [30].
Furthermore, guaiacyl (G) units have a free C-5 position, which makes them amenable to lignin re-polymerization or condensation reactions through the formation of carbon–carbon bonds [31]. As seen in Table 1, the linkage β-O-4 (arylglycerol-β-aryl ether) is clearly the most frequent linkage type, accounting for more than 50% of the linkages in lignin [28]. The dominant weak β-O-4 linkages in lignin provide opportunities for cleavage by many processes [32]. Other linkages are more resistant to chemical degradation [33].
In softwoods, the G-type lignins contain more resistant linkages with those involving the C5 of aromatic nuclei (e.g., β-5, 5–5′ and 4-O-5′ linkages) than S type lignins (hardwood lignins). This is the main reason for the higher frequency of C–C linkages between aromatic rings in softwood lignins than in hardwood lignins [28].
In contrast to what is reported by Kadla et al. [22], we showed that reaction with concentrated H2O2 can have a significant effect on lignin degradation in the absence of metal catalysts. Furthermore, the separation of the low molecular weight lignin product from the reaction mixture does not require the use of expensive purification processes. Hence, the process described here could set the stage for increased use of lignin in the depolymerized/oxidized form as required in various high-value applications.
2. Results
2.1. General Observations and Product Yields
In the present work, lignin was reacted with concentrated H2O2 (50%) at ambient temperature (25 °C). The reaction needed 120 h at room temperature at 25 °C to reach completion. Hence, we accelerated the reaction by increasing the temperature to 35 °C, which reduced the reaction time to about 80 h. Reaction temperatures above 35 °C should be avoided to prevent burning the reaction slurry. A homogenous, shiny reddish powder of de-polymerized lignin product at a very high yield (>95% ± 3%) was obtained in all reaction runs (see Figure 1).

Figure 1.
(a) SKL and HKL reaction mixtures during oxidative treatment with concentrated hydrogen peroxide and (b) depolymerized lignin sample after the treatment.
Furthermore, it was observed that during reaction of the hardwood lignin, the reaction mixture expanded and foamed, while the foaming was not significant during the softwood lignin treatment. The foam formation was likely due to the release of gases from the reaction system. The gas formation could be due to cleavage of the methoxy groups of the syringyl structures in hardwood lignin, producing methane (CH4). In addition, decomposition of unreacted or residual H2O2, forming O2 and water vapor, could also contribute to foam formation, in particular, in the presence of metal ion catalysts that might be present as impurities in lignin [34]. Homogenous powder products of de-polymerized lignins were obtained and the products had a high solubility in water and common organic solvents (e.g., acetone, ethanol, methanol, etc.).
The mass loss of the reaction mixture (lignin and H2O2) vs. treatment time was monitored during the treatment where 5 g on a dry basis (d.b.) of SKL or HKL and 10 g of 50 wt% H2O2 were used, as illustrated in Figure 2. As shown in this figure, the mass loss in the case of the HKL treatment was faster than that of the SKL treatment. After 5 days, however, almost the same degree of mass loss was achieved in both treatments with HKL or SKL. The faster mass loss with HKL might be due to the higher reactivity of HKL (containing more syringyl nuclei) compared to SKL (containing more guaiacyl nuclei) towards H2O2 [35].

Figure 2.
Mass of reaction mixture vs. treatment time. Note: in these tests, 5 g (d.b.) SKL or HKL and 10 g of 50 wt% H2O2 were used.
2.2. Elemental Analysis
Elemental analyses of the SKL, HKL and the depolymerized lignin products were conducted to investigate the changes in the composition of the lignins during the treatment. Table 2 presents the elemental contents (wt% on a dry basis) of C, H, N, S, ash and O of the original SKL, HKL and depolymerized lignin samples produced at various lignin/H2O2 mass ratios (1:1, 1:0.75 and 1:0.5). As seen here, the changes in H, N and S were negligible in all de-polymerization treatments with H2O2 at all mass ratios, while the carbon contents in both SKL and HKL were significantly reduced, accompanied by a marked increase in oxygen content in the depolymerized lignin products. This result suggests that the oxidative depolymerization of lignin is likely to lead to more oxygen-rich functional groups, e.g., COOH and –OH in the depolymerized lignin products, as expected. Additionally, the gases released may account for the reduction in carbon content (e.g., CO2, CH4).

Table 2.
Elemental compositions of the original SKL, HKL, and depolymerized lignin samples at various lignin/H2O2 mass ratios (1:1, 1:0.75 and 1:0.5).
2.3. FTIR Analysis
Figure 3 presents FTIR spectra of the original SKL (Figure 3A) and HKL (Figure 3B), as well as the depolymerized lignin products generated at different lignin/H2O2 mass ratios (1:1, 1:0.75 and 1:0.5). As shown in these figures, there were five major differences in the spectra of the depolymerized lignin products when compared with those of the original lignins (SKL or HKL), as displayed at wavenumbers 1727, 1600, 1515, 1460 and 1272 cm−1. The IR absorbance at wavenumber 1727 (cm−1) could be attributed to the non-conjugated C=O stretching vibration for carboxyl groups [36]. The intensity of this IR peak increased rapidly when increasing the amount of H2O2 in the treatment. This implies that the oxidation of lignin by H2O2 generated more C=O functional groups such as –COOH in the depolymerized lignin products.

Figure 3.
FTIR spectra of the original SKL (A), HKL (B), and depolymerized lignin products produced at various lignin/H2O2 mass ratios (1:1, 1:0.75 and 1:0.5).
The peaks at 1600 cm−1 and 1515 cm−1 could be ascribed to C=C in aromatic structures [37]. In the case of HKL, these peaks had reduced intensity in the depolymerized lignin products compared to those in the original lignin, indicating that the oxidative treatment reduced the aromatic structure content of lignin. In the case of SKL, specifically at 1600 cm−1, the peak was weak and broad without any significant trends being demonstrated at different lignin/H2O2 mass ratios. The signal reduction at 1600 cm−1 and 1515 cm−1 could be seen as suggestive evidence for lignin depolymerization as a result of oxidative ring opening. The band at 1460 cm−1 could be ascribed to C–H bending in methylene groups [36]. As seen in this figure, the peaks at 1460 cm−1 were of approximately similar intensities for the SKL controls and the de-polymerized lignin samples of SKL at the lignin/H2O2 mass ratio of 1:0.5. However, this peak was not observed in the de-polymerized SKL samples produced at the lignin/H2O2 mass ratios of 1:0.75 and 1:1. For HKL, these peaks almost disappeared for the depolymerized lignin samples, indicating that under severe oxidation conditions, the generation of electrophilic hydroxonium ions, HO+, could lead to the breaking of double bonds, yielding various aldehyde or ketone groups.
The band at 1270 cm−1 was assigned to guaiacyl C–O stretching. The peak intensity decreased in the two depolymerized lignin products (SKL) generated from the oxidative treatments at the lignin/H2O2 mass ratios of 1:0.75 and 1:1 ratio, suggesting that the oxidative depolymerization of lignin would lead to the cleavage of methoxyl groups, resulting in a reduction in the oxygen content of the de-polymerized lignin products, as suggested previously in Table 3. This peak was not observed for HKL samples.

Table 3.
Py–GC/MS analysis results for the DKL product from oxidative de-polymerization of SKL at room temperature at a 1:1 H2O2/SKL mass ratio.
As shown in Figure 3B, in the HKL sample, syringyl ring breathing was represented by the wavenumber 1330 cm−1 [3]. This peak disappeared during the oxidation treatment in all depolymerized lignin samples produced at different lignin to peroxide ratios, suggesting oxidative cleavage of methoxyl groups. Furthermore, the aromatic C–H in-plane deformation in syringyl structures observed at wavenumber 1118 cm−1 was observed in the HKL sample, but it was not detected for any of the treated hardwood lignins, again indicating cleavage of methoxyl groups during the oxidative treatment [38].
2.4. Molecular Weight Distribution by GPC
The molecular weights and distributions of the original lignins (SKL and HKL) and depolymerized lignin products, after acetobromination, were measured by GPC–UV using a method as described in [39]. Figure 4 displays the weight average molecular weight (Mw) of SKL and HKL, and the de-polymerized kraft lignin (DKL) products. As clearly shown, the molecular weight of hardwood kraft lignin (Mw = 2718 Da) was much lower than that of softwood kraft lignin (Mw = 6041 Da), as commonly reported in the literature [3,40]. The error bars indicate the relative standard deviations = 5%.
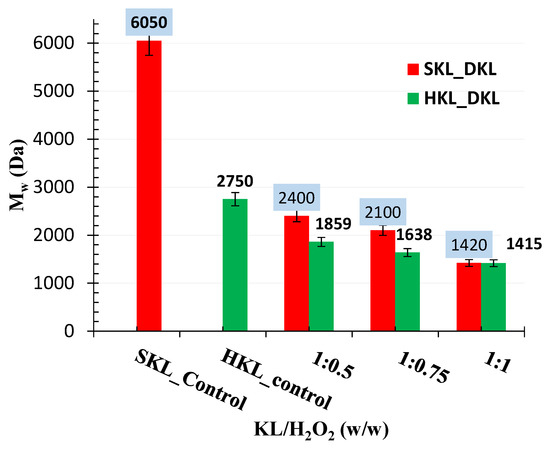
Figure 4.
Weight average molecular weight (Mw) of SKL and HKL, and the de-polymerized kraft lignin (DKL) products at different lignin to peroxide ratios.
As also clearly shown in Figure 4, an increase in the H2O2/KL mass ratio in the oxidative treatment led to a dramatic decrease in the molecular weight, especially for softwood kraft lignin. For instance, for SKL, the Mw dropped from 6041 Da to 1400 Da for the DKL from the treatment at a 1:1 H2O2/KL mass ratio at room temperature for 120 h, while, for HKL, the Mw was reduced from 2718 Da to 1415 Da for the DKL from treatment under the same conditions. Despite the slightly higher β-O-4 linkage content in hardwood lignin compared to softwood lignin, the rate of cleavage of benzyl ether bonds of syringyl nuclei (rich in hardwood lignin) was found to be slower than that of guaiacyl nuclei (more prevalent in softwood lignin) in acidic media [35].
Thus, in the case of both SKL and HKL, the lignin depolymerization products were of a lower molecular weight compared to the control lignins. Furthermore, their solubility in water at a low pH was remarkably high. As discussed previously, lignin oxidative depolymerization using concentrated H2O2 could involve the production of phenolic oligomers by cleavage of ether bonds and demethoxylation of syringyl and guaiacyl nuclei. Extensive oxidation could produce ring opening products (e.g., muconic acids and their derivatives) [17,41].
2.5. Functional Group Analysis Using 31P-NMR
One of the most important functional groups in lignin is the hydroxyl group, especially the free phenolic group, as the physical and chemical properties of lignin and depolymerized lignin are affected by the content of such groups. 31P-NMR spectroscopy is a unique tool in the measurement of lignin hydroxyl groups, providing quantitative information for various types of major hydroxyl groups. After sufficient phosphitylation with phosphorous-based reagents, different hydroxyl groups in lignin belonging to aliphatic, carboxylic, guaiacyl, syringyl, p-hydroxyphenyl, catechol as well as guaiacyl groups with carbon substituents at the C5 position, could be readily quantified with 31P-NMR spectroscopy [42].
Figure 5A,B presents the contents of different hydroxyl functional groups as a function of Mw for SKL and HKL. The carboxylic acid group is typically associated with extensive oxidation; hence, among all different types of hydroxyl groups, the carboxylic hydroxyl group content dramatically increased with decreasing Mw at a higher H2O2/lignin ratio. For example, while carboxylic-OH groups existed in both the SKL and HKL controls at relatively low levels (i.e., 0.3–0.4 mmol/g), these levels increased to 2 mmol/g in DKL derived from either SKL or HKL after treatment at the 1:1 H2O2/lignin mass ratio. This could be attributed to comprehensive oxidation of lignin by H2O2.
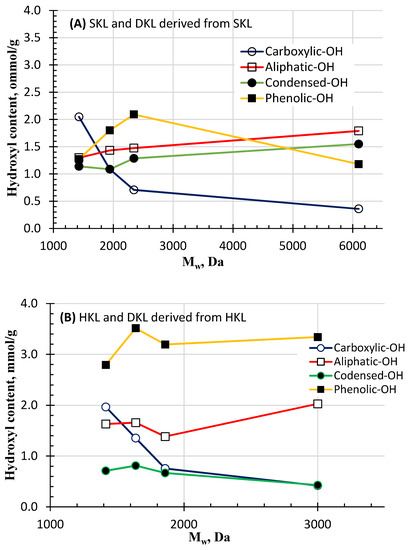
Figure 5.
Contents of different hydroxyl functional groups vs. Mw for SKL and DKL derived from SKL (A) and HKL and DKL derived from HKL (B).
As shown in Figure 5A, the aliphatic hydroxyl group is the dominant hydroxyl group in softwood kraft lignin due to the presence of phenylpropane units in the lignin structure, represented by either the primary-OH (in γ-C atom of the side chain) or the secondary-OH (in α-C atom) groups. Lignin oxidative depolymerization with permanganate and ionic liquid, resulted in a decrease in aliphatic OH and an increase in phenolic hydroxyl groups [43,44].
The reduction in aliphatic OH could be attributed to dehydration of the primary and/or secondary-OH groups [45]. As shown in Figure 5A, the aliphatic OH group content in SKL decreased slightly with increasing oxidation intensity, dropping from 1.5 mmol/g in the original lignin to 1.27 mmol/g in the DKL at an Mw of 1420 Da produced at the 1:1 H2O2/SKL mass ratio. A similar trend was observed with oxidative de-polymerization of hardwood kraft lignin for which the aliphatic OH content dropped from 2 mmol/g for the HKL to 1.6 mmol/g in the DKL at a Mw of 1415 Da produced at the 1:1 H2O2/HKL mass ratio (Figure 5B). The condensed hydroxyl structures in lignin comprise diphenylmethanes, diphenyl ethers and 5,5′-biphenolic moieties [46]. As shown in Figure 5A,B, softwood lignins, which are characterized with a high content of guaiacyl (G) units, have a more condensed and cross-linked structure than hardwood lignins because the C5 position in hardwood lignin is occupied by a methoxyl group. As shown in Figure 5A, for SKL, the total condensed OH groups decreased gradually from 1.55 mmol/g in the original softwood kraft lignin to 1.14 mmol/g in the DKL from the oxidative treatment of SKL at 1:1 H2O2/SKL mass ratio. This decrease could be explained by the scission of β-O-4 linkages that led to non-condensed moieties. On the contrary, the total content of condensed hydroxyl groups in hardwood kraft lignin increased from 0.43 mmol/g in the original HKL to 0.711 mmol/g in the DKL from the oxidative treatment of HKL at a 1:1 H2O2/SKL mass ratio (Figure 5B). This suggests that during oxidative depolymerization, demethoxylation might have occurred, converting syringyl (S) to guaiacyl (G) structures. Subsequently, repolymerization of the G structure at the C5 position could have taken place, accounting for the increase in the condensed OH structures in DKL. Non-condensed phenolic OH groups in lignin consist of syringyl phenolics, guaiacyl phenolics and p-hydroxy phenyl. In softwood lignin, the major non-condensed phenolic hydroxyls are guaiacyl phenolics with a minor amount of p-hydroxy phenyl. The total content of phenolic OH groups increased from 1.18 mmol/g in the original SKL to 2.09 mmol/g in the DKL at a 0.5:1 H2O2/SKL mass ratio, which was likely due to the reduction in condensed OH content (Figure 5A), but it decreased back to 1.27 mmol/g in the DKL at a 1:1 H2O2/SKL mass ratio. In contrast, the total content of phenolic OH groups in HKL (3.34 mmol/g) was much higher than that of SKL (1.18 mmol/g), as hardwood lignins contain more syringyl phenolics than softwood lignins. The total content of non-condensed phenolic OH groups in HKL dropped to 2.79 mmol/g in the DKL at a 1:1 H2O2/HKL mass ratio, likely due to oxidative ring cleavage, which was in good agreement with the reduced aromatic structure in the DKLs as shown by the FTIR results (Figure 3).
2.6. TGA Analysis
The thermal stability of the original kraft lignins and the depolymerized kraft lignins (DKL) were investigated using thermogravimetric analysis (TGA) plots and the derivative of the TG curves (DTG) under a nitrogen environment, as illustrated in Figure 6 (SKL) and Figure 7 (HKL). The thermal degradation of lignin is a complex process because the structure contains various oxygen functional groups with different decomposition pathways, including competitive and/or consecutive reactions. As shown in Figure 6 and Figure 7, generally both the SKL and HKL lignins started to decompose at 200–300 °C, while the maximum decomposition rate occurred around 350–400 °C. Various aromatic hydrocarbons can form from lignin decomposition, such as phenolics, hydroxyphenolics and guaiacyl-/syringyl-type compounds, most products having phenolic –OH groups [47,48]. The weight loss at temperatures < 100 °C for all samples in both figures could be ascribed to the loss of water contained in the KL or DKL samples [49] as well as volatile sulphur compounds. The lignin decomposition temperatures depend on its molecular structure. Lignins with higher molecular weights incorporating a significant number of intramolecular β-O-4 and C–C bonds have a high thermal stability and, as a result, higher decomposition temperatures [50]. Depolymerization of kraft lignins using a low and a high H2O2/lignin mass ratio produced DKL products with reduced Mw (Figure 4). Consequently, the DKL products from either SKL (Figure 6) or HKL (Figure 7) had a much lower decomposition temperature than that of the original lignins. For instance, the initial decomposition temperature and the temperature of maximum decomposition rate of the SKL control with an Mw of 6050 Da were 280 and 370 °C, respectively, while for the DKL (Mw = 1420 Da) obtained at a H2O2/lignin mass ratio of 1:1 (Figure 6), these parameters were 173 and 270 °C, respectively. The same conclusions can be drawn when comparing the thermal decomposition temperatures of HKL and DKL (Figure 7). This decrease in decomposition temperatures can be attributed to the greatly reduced Mw [51].

Figure 6.
TGA curves (A) and DTG plots (B) of SKL and its de-polymerized products.
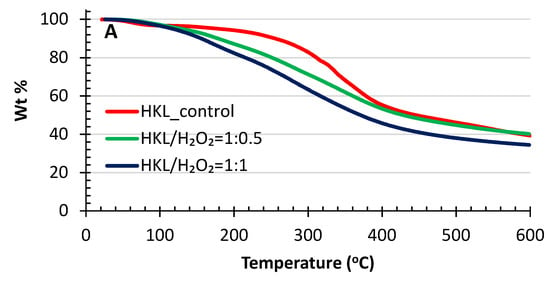
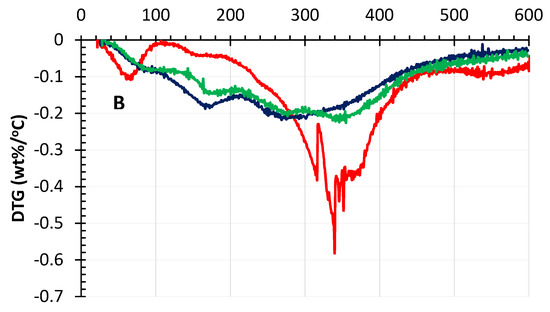
Figure 7.
TGA curves (A) and DTG plots (B) of HKL and its de-polymerized products.
2.7. Py–GC/MS Analysis
Py–GC/MS analysis was conducted for the DKL products from oxidative de-polymerization of SKL (Table 3) and HKL (Table 4) at room temperature at a 1:1 H2O2/KL mass ratio. The results of Py–GC/MS analysis could reveal key compounds of the volatile fraction of the DKL samples at 350 °C, identified by their mass spectra by comparison with those appearing in the NIST library. This technique could also provide an idea of the structural components of lignin, which break down at high temperatures to more volatile chemical compounds. It should be noted that the small peaks with an area less than 1.5% of the total area were not included in the Tables. As shown in both Tables, the detectable compounds were mainly aromatic and aliphatic compounds such as guaiacol-type, phenol-type, catechol-type and other oxygen-rich derivatives (carboxylic acids, esters and alcohols). Various types of phenolic compounds can be generated via the cleavage of β-aryl etheric bonds present in both softwood and hardwood samples [52]. Meanwhile, some aliphatic compounds (e.g., 1-butene, 3,3-dimethyl- and adipic acid, di (oct-4-yl ester)) can form from the demethoxylation reaction especially in hardwood samples [53]. The demethoxylation cleavage reaction was confirmed in this work by FTIR analysis (Figure 3).

Table 4.
Py–GC/MS analysis results for the DKL product from oxidative de-polymerization of HKL at room temperature and 1:1 H2O2/HKL mass ratio.
Moreover, the Py–GC/MS analysis results also confirmed that the SKL and HKL were extensively oxidatively depolymerized by H2O2 at ambient temperature, producing low-molecular aromatic/phenolic compounds that can be utilized for bio-based phenolic resins, polyurethane materials and dispersants [6,54].
3. Discussion
As previously mentioned, the SKL and HKL lignins used in this work were both in the H-form (protonated form) since they were washed with acid, giving a pH value of around 4.0 once suspended in pure water. Thus, in this work, the oxidation reactions were carried out under acidic conditions. Hydrogen peroxide is also a weak acid. Hence, the low pH of the reaction medium is expected to have affected the lignin degradation mechanism in hydrogen peroxide [25]. H2O2 can act as a nucleophilic or an electrophilic agent depending on the pH of the reaction medium. Under acidic conditions, H2O2 can form a hydroxonium ion, HO+, which is a strongly electrophilic oxidant [55] formed by the reaction of either a mineral acid (e.g., sulfuric acid) or carboxylic acids (e.g., acetic acid, formic acid) with H2O2 as follows [17,56]:
H2O2 + H+ ⇌ HO+ + H2O
The electrophilic HO+ ion was previously found to be highly reactive towards electron-rich sites such as olefinic, carbonyl and aromatic ring structures (e.g., as found in lignin) [57,58] leading to lignin demethoxylation and β-ether cleavage. As such, depolymerization of lignin can be achieved using an acidic system of hydrogen peroxide via HO+ ions. As shown in Equation (1), the formation of HO+ ions from H2O2 is a reversible reaction; therefore, one would expect that high concentrations of the reactant (H2O2) would favor the formation of HO+ ions thereby promoting lignin depolymerization at room temperature [59].
Although the exact mechanism of lignin degradation via concentrated H2O2 has not been reported, the reaction mechanism for the room-temperature H2O2 depolymerization of acidic kraft lignin may resemble the reaction scheme for the reaction of model compounds with HO+ at low pH [55], as illustrated in Scheme 1, involving mainly HO+ oxidative ring opening (1) and cleavage of β-aryl ether bonds (2).

Scheme 1.
Oxidative ring opening and de-polymerization pathways [55].
4. Materials and Methods
4.1. Materials
Two types of kraft lignin (KL), softwood kraft lignin (SKL) from a western Canadian mill (West Fraser, Hinton, Alberta, Canada) and a hardwood kraft lignin (HKL) from a central Canadian mill (Resolute, Thunder Bay, ON, Canada) were used in this study. Both kraft lignins were separated from kraft black liquor using the LignoForceTM process previously developed by FPInnovations (Pointe Claire, Quebec, Canada). More specifically, the black liquor was oxidized with molecular oxygen at 80 °C, and then the pH was reduced using CO2 and H2SO4 followed by filtration and washing with dilute H2SO4. As a result, both lignins produced were in the H-form (protonated lignin), and both substrates, SKL and HKL, when suspended in pure water, had a pH value of around 4.0. The low pH of the reaction medium is expected to affect lignin degradation in hydrogen peroxide [25]. Hydrogen peroxide can act as a nucleophilic or an electrophilic agent depending on the pH of the solution (it is relatively stable under acidic conditions). Thus, in this work, the oxidation reactions were carried out under acidic conditions. Table 5 displays the elemental analysis and the physical properties of SKL and HKL. All other chemicals used in the study, such as 50% hydrogen peroxide, were ACS reagent-grade chemicals purchased from Sigma-Aldrich (Oakville, ON, Canada) and used as received.

Table 5.
Elemental analysis and the physical properties of softwood kraft lignin (SKL) and hardwood kraft lignin (HKL).
4.2. Oxidative Treatment of SKL and HKL
The oxidative treatment of kraft lignins, SKL and HKL (dry solid powder), was conducted in a 150-mL beaker under ambient conditions. In a typical run, 6 g (or 4.5 g or 3 g) of 50% H2O2 was added slowly to 3 g of SKL or HKL on a dry basis, with the ratio of H2O2/KL being kept at 1:1 w/w (or 1:0.75 w/w or 1:0.5 w/w, respectively). The mixture was then uniformly mixed (manual mixing with a spatula) at ambient temperature to obtain a homogenous slurry. The mixture was then kept inside a fume hood at ambient temperature without a cover, or in an oven under controlled temperature (not exceeding 35 °C) if a shorter reaction time was desired. The mass was monitored over time until a final dry product was obtained. The recovered modified KL was air dried for 24 h to determine the yield. The yield was determined based on the percentage of the dry mass of the modified KL to the dry mass of the initial KL.
4.3. Product Characterization
4.3.1. Elemental analysis
The elemental composition of the samples, i.e., CHNS (carbon, hydrogen, nitrogen and sulfur), was determined using a CHNS-O flash elemental analyzer 1112 series (Thermo, Waltham, MA, USA), using 2,5-bis (5-tert-butyl-benzoxazol-2-yl) thiophene (BBOT) as the calibration standard. The oxygen content (%) was calculated by difference (=100% − C% − H% − N% − S% − Ash%).
4.3.2. GPC Analysis
The average molecular weights and molecular weight distributions of SKL and HKL, and the depolymerized lignin products were measured using a Waters GPC–UV (Waters, Mississauga, ON, Canada) (gel permeation chromatographic system with an on-line UV detector at 270 nm) equipped with a Waters Styragel HR1 column at 40 °C, using THF as the eluent at 1 mL min−1 and linear polystyrene standards for molecular weight calibration. All the samples were derivatized by acetobromination before injecting into GPC.
4.3.3. FTIR Analysis
Fourier-transform infrared spectroscopy (FTIR) was employed to investigate the functional group structures of the dry samples of SKL (control) and HKL (control) and the depolymerized lignin products and their changes during the de-polymerization process. The FTIR analysis was performed on a Nicolet-6700 FTIR with a universal ATR accessory (Perkin-Elmer, Waltham, MA, USA).
4.3.4. 31P-NMR
The hydroxyl group content of the lignin and modified lignin samples was measured using quantitative 31P-NMR spectroscopy (Varian Inova, Palo Alto, CA, USA). The samples were derivatized with 100 μL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP). Derivatized samples (30–40 mg) were dissolved in 500 μL of anhydrous pyridine and deuterated chloroform (1.6:1, v/v) and mixed with 100 L of a solution of N-hydroxy-5-norbornene-2,3-dicarboxylic acid imide (10 mg mL−1) and chromium (III) acetylacetonate (5 mg mL−1) as internal standard and relaxation agent. The solution was thoroughly mixed and transferred to a sealed 5 mm NMR tube. All NMR experiments were carried out at 298 K on a Varian Inova 500 NMR Spectrometer [60]. 31P-NMR spectra were acquired using an inverse-gated decoupling pulse sequence with a 90-pulse angle, 25 s relaxation delay, and 256 scans.
4.3.5. TGA Analysis
Thermogravimetric analysis (TGA) of the lignin and depolymerized lignin products was performed using a PerkinElmer Pyris 1,1 TGA in a nitrogen atmosphere (PerkinElmer, Waltham, MA, USA). The dry samples weighing approximately 10 mg were heated in an N2 flow at 20 mL/min from 25 °C to 600 °C at 10 °C/min. The weight loss, percent and the rate of weight loss (DTG) of the samples were recorded.
4.3.6. Py–GC/MS
Pyrolysis–gas chromatography–mass spectroscopic analysis was conducted on an Agilent 6890B GC coupled with a 5973A MSD using 30 m × 0.25 mm × 0.25 µm DB-5 columns with temperature programming as follows: 1 min hold at an initial temperature of 50 °C followed by a 30 °C min−1 ramp to the final temperature of 350 °C with a 1 min hold. Py–GC/MS analysis was done using the double-shot mode. Pyrolysis of samples (∼100 μg) was performed with a py2020i microfurnace pyrolyzer/AS1020 autosampler (Frontier Laboratories, Koriyama, Fukushima, Japan). The compounds were identified by comparing their mass spectra with those in the NIST library.
5. Conclusions
This work successfully demonstrated a cost-effective approach for converting kraft lignin into low molecular weight compounds at close to 100% yield by oxidative degradation of lignin slurries with concentrated H2O2 at ambient temperature under acidic conditions. The obtained depolymerized kraft lignin (DKL) products were comprehensively characterized by GPC–UV, FTIR, 31P-NMR, TGA, Py–GC/MS and elemental analysis. The characterization results suggest effective depolymerization of softwood and hardwood lignins. The DKL products have a Mw of around 1400 Da with a high phenolic and carboxylic group content when produced at a 1:1 H2O2/KL mass ratio. The Py–GC/MS results disclosed the presence of highly oxygenated fragments with functional groups such as phenols, carboxylic acids, esters, ketones and aldehydes in the DKL products. The novelty of this oxidative lignin de-polymerization approach is that it can be conducted at room temperature in the absence of a catalyst when acid-washed kraft lignins are used as the substrate. Furthermore, the proposed process can readily be scaled-up to the industrial level, and the DKL produced can be used directly in applications without any further purification.
Author Contributions
Conceptualization, Z.A., W.W.A.D., M.P. and C.X.; Formal analysis, Z.A., W.W.A.D., M.P. and C.X.; Funding acquisition, C.X.; Investigation, Z.A.; Methodology, Z.A., W.W.A.D., M.P. and C.X.; Resources, M.P. and C.X.; Supervision, M.P. and C.X.; Writing—original draft, Z.A.; Writing—review and editing, W.W.A.D., M.P. and C.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSERC Biomaterials and Chemicals Strategic Research Network (Lignoworks) partnered with FPInnovations, Lignol and Weyerhaeuser, as well as by an NSERC Discovery Grant, the NSERC/FPInnovations Industrial Research Chair program and an ORF-RE grant in Forest Biorefinery. The APC was funded by MDPI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A.; Isac-García, J.; Martín-Martínez, F.J. Lignin and Lignans as Renewable Raw Materials: Chemistry, Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 64–80. [Google Scholar]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerization strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Heitner, C.; Dimmel, D.; Schmidt, J. Lignin and Lignans: Advances in Chemistry; CRC press: Boca Raton, FL, USA, 2016; pp. 5–7. [Google Scholar]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Wang, G.; Sui, W.; Tao, Z. Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis. Ind. Crop. Prod. 2019, 128, 177–185. [Google Scholar] [CrossRef]
- Morohoshi, N.; Glasser, W.G. The structure of lignins in pulps. 4. Comparative evaluation of five lignin depolymerization techniques. Wood Sci. Technol. 1979, 13, 165–178. [Google Scholar] [CrossRef]
- Freudenberg, K.; Chen, C.L.; Harkin, J.M.; Nimz, H.; Renner, H. Observations on lignin. Chem. Commun. (London) 1965, 224–225. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Rößiger, B.; Röver, R.; Unkelbach, G.; Pufky-Heinrich, D. Production of bio-phenols for industrial application: Scale-up of the base-catalyzed depolymerization of lignin. Green Sustain. Chem. 2017, 7, 193–202. [Google Scholar] [CrossRef]
- Hewson, W.B.; Hibbert, H. Studies on Lignin and Related Compounds. LXV. Re-ethanolysis of Isolated Lignins. J. Am. Chem. Soc. 1943, 65, 1173–1176. [Google Scholar] [CrossRef]
- Gasson, J.R.; Forchheim, D.; Sutter, T.; Hornung, U.; Kruse, A.; Barth, T. Modeling the lignin degradation kinetics in an ethanol/formic acid solvolysis approach. Part 1. Kinetic model development. Ind. Eng. Chem. Res. 2012, 51, 10595–10606. [Google Scholar] [CrossRef]
- Forchheim, D.; Gasson, J.R.; Hornung, U.; Kruse, A.; Barth, T. Modeling the lignin degradation kinetics in a ethanol/formic acid solvolysis approach. Part 2. Validation and transfer to variable conditions. Ind. Eng. Chem. Res. 2012, 51, 15053–15063. [Google Scholar] [CrossRef]
- Kristianto, I.; Limarta, S.O.; Lee, H.; Ha, J.M.; Suh, D.J.; Jae, J. Effective depolymerization of concentrated acid hydrolysis lignin using a carbon-supported ruthenium catalyst in ethanol/formic acid media. Bioresour. Technol. 2017, 234, 424–431. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Production of polyols via direct hydrolysis of kraft lignin: Effect of process parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef]
- Das, L.; Kolar, P.; Sharma-Shivappa, R.; Classen, J.; Osborne, J. Oxidative depolymerization of lignin using supported niobium catalysts. Chem. Eng. 2017, 1, 17. [Google Scholar] [CrossRef]
- Kang, J.; Irmak, S.; Wilkins, M. Conversion of lignin into renewable carboxylic acid compounds by advanced oxidation processes. Renew. Energy. 2019, 135, 951–962. [Google Scholar] [CrossRef]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.S.; Saladino, R. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorganic Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef]
- Sun, Y.; Argyropoulos, D.S. A comparison of the reactivity and efficiency of ozone, chlorine dioxide, dimethyldioxirane and hydrogen peroxide with residual kraft lignin. Holzforschung-Int. J. Biol. Chem. Phys. Technol. Wood 1996, 50, 175–182. [Google Scholar] [CrossRef]
- Kadla, J.F.; Chang, H.M. The reactions of peroxides with lignin and lignin model compounds. In Oxidative Delignification Chemistry; ACS symposium series: Washington, DC, USA, 2001; Volume 785, pp. 108–129. [Google Scholar]
- Sales, F.G.; Maranhão, L.C.; Lima Filho, N.M.; Abreu, C.A. Kinetic evaluation and modeling of lignin catalytic wet oxidation to selective production of aromatic aldehydes. Ind. Eng. Chem. Res. 2006, 45, 6627–6631. [Google Scholar] [CrossRef]
- Robinette, H. Hydrogen Peroxide; Schumb, W.C., Satterfield, C.N., Wentworth, R.L., Eds.; Reinhold Publishing Corp.: New York, NY, USA, 1956; p. 195. [Google Scholar]
- Xiang, Q.; Lee, Y.Y. Oxidative cracking of precipitated hardwood lignin by hydrogen peroxide. Appl. Biochem. Biotechnol. 2000, 84, 153–162. [Google Scholar] [CrossRef]
- Lourenco, A.; Gominho, J.; Marques, A.V.; Pereira, H. Reactivity of syringyl and guaiacyl lignin units and delignification kinetics in the kraft pulping of Eucalyptus globulus wood using Py-GC–MS/FID. Bioresour. Technol. 2012, 123, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Doelle, K.; Bajrami, B. Sodium Hydroxide and Calcium Hydroxide Hybrid Oxygen Bleaching with System. In IOP Conference Series: Materials Science and Engineering; IOP publishing: Bristol, UK, 2018; p. 012136. [Google Scholar]
- Chinnappan, B.; Baskar, S.; Dhillon, R. Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science; Springer Science & Business Media: New York, NY, USA, 2012; pp. 383–400. [Google Scholar]
- Sixta, H. Pulp properties and applications. In Handbook of Pulp; Wiley-VCH: Weinheim, Germany, 2006; p. 197. [Google Scholar]
- Gonzalez-Vila, F.J.; Almendros, G.; Del Río, J.C.; Martın, F.; Gutiérrez, A.; Romero, J. Ease of delignification assessment of wood from different Eucalyptus species by pyrolysis (TMAH)-GC/MS and CP/MAS 13C-NMR spectrometry. J. Anal. Appl. Pyrolysis 1999, 49, 295–305. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rodríguez, I.M.; del Río, J.C. Chemical characterization of lignin and lipid fractions in industrial hemp bast fibers used for manufacturing high-quality paper pulps. J. Agric. Food Chem. 2006, 54, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Adler, E. Lignin chemistry-past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Marzzacco, C.J. The enthalpy of decomposition of hydrogen peroxide: A general chemistry calorimetry experiment. J. Chem. Educ. 1999, 76, 1517. [Google Scholar] [CrossRef]
- Shimada, K.; Hosoya, S.; Ikeda, T. Condensation reactions of softwood and hardwood lignin model compounds under organic acid cooking conditions. J. Wood Chem. Technol. 1997, 1, 57–72. [Google Scholar] [CrossRef]
- Kline, L.M.; Hayes, D.G.; Womac, A.R.; Labbe, N. Simplified determination of lignin content in hard and soft woods via UV-spectrophotometric analysis of biomass dissolved in ionic liquids. BioResources 2010, 5, 1366–1383. [Google Scholar]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibers. In Proceedings of the Fourier Transform-materials Analysis, InTech Janeza Trdine, Rijeka, Croatia, 23 May 2012; pp. 45–68. [Google Scholar]
- Kubo, S.; Kadla, J.F. Hydrogen bonding in lignin: A Fourier transform infrared model compound study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef]
- Baumberger, S.; Abaecherli, A.; Fasching, M.; Gellerstedt, G.; Gosselink, R.; Hortling, B.; Li, J.; Saake, B.; de Jong, E. Molar mass determination of lignins by size-exclusion chromatography: Towards standardisation of the method. Holzforschung 2007, 61, 459–468. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Ludwig, C.H. Lignins. Occurrence, formation, structure, and reactions; Wiley-Inter-science: New York, NY, USA, 1971; pp. 433–479. [Google Scholar]
- Chen, C.; Jin, D.; Ouyang, X.; Zhao, L.; Qiu, X.; Wang, F. Effect of structural characteristics on the depolymerization of lignin into phenolic monomers. Fuel 2018, 223, 366–372. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Zakis, G.F. Functional Analysis of Lignins and Their Derivatives; Tappi Press: Atlanta, GA, USA, 1994; pp. 30–102. [Google Scholar]
- Lucia, L.A.; Goodell, M.M.; Chakar, F.S.; Ragauskas, A.J. Breaking the Oxygen Delignification Barrier: Lignin Reactivity and Inactivity; ACS Symposium Series: Atlanta, GA, USA, 2000; pp. 92–107. [Google Scholar]
- Wen, J.L.; Yuan, T.Q.; Sun, S.L.; Xu, F.; Sun, R.C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin-A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Wittkowski, R.; Ruther, J.; Drinda, H.; Rafiei-Taghanaki, F. Formation of Smoke Flavor Compounds by Thermal Lignin Degradation; ACS symposium series: Washington, DC, USA, 1992; pp. 232–490. [Google Scholar]
- Domínguez, J.C.; Oliet, M.; Alonso, M.V.; Gilarranz, M.A.; Rodríguez, F. Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crop. Prod. 2008, 27, 150–156. [Google Scholar] [CrossRef]
- El-Saied, H.; Nada, A.A.M. The thermal behaviour of lignins from wasted black pulping liquors. Polym. Degrad. Stab. 1993, 40, 417–421. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, M.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Fast fractionation of technical lignins by organic cosolvents. ACS Sustain. Chem. Eng. 2018, 6, 6064–6072. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Ház, A.; Jablonský, M.; Orságová, A.; Šurina, I. Characterization of lignins by py-GC/MS. In Proceedings of the 4th International Scientific Conference, Renewable Energy Sources, Tatranské Matliare, Slovakia, 21–23 May 2013; pp. 55–59. [Google Scholar]
- Siddiqui, H.; Mahmood, N.; Yuan, Z.; Crapulli, F.; Dessbesell, L.; Rizkalla, A.; Ray, A.; Xu, C.C. Sustainable bio-based phenol-formaldehyde resoles using hydrolytically depolymerized kraft lignin. Molecules 2017, 22, 1850. [Google Scholar] [CrossRef] [PubMed]
- Gierer, J. Chemistry of delignification. Wood Sci. Technol. 1986, 20, 1–33. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Zhu, W.; Wang, S.Q. Delignification of maize stems by peroxy mono-sulfuric acid, peroxy formic acid, peracetic acid, and hydrogen peroxide. 1. Physicochemical and structural characterization of the solubilized lignins. J. Agric. Food Chem. 2000, 48, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C. Lignin reactions in delignification with peroxyacetic acid. In Chemistry of Delignification with Oxygen, Ozone and Peroxides; Gratzl, J., Nakano, J., Singh, R.P., Eds.; Uni Publishers Co., Ltd.: Tokyo, Japan, 1980; pp. 217–228. [Google Scholar]
- Oki, T.; Ishikawa, H.; Okubo, K. Oxidative degradation of dihydrodehydrodiisoeugenol and its methyl derivative [of lignin] by peroxide and oxygen alkali methods. J. Jpn. Wood Res. Soc. 1980, 26, 463–470. [Google Scholar]
- Evstigneev, E.I. Oxidation of hydrolysis lignin with hydrogen peroxide in acid solutions. Russ. J. Appl. Chem. 2013, 86, 258–265. [Google Scholar] [CrossRef]
- Hosseinaei, O.; Harper, D.; Bozell, J.; Rials, T. Improving processing and performance of pure lignin carbon fibers through hardwood and herbaceous lignin blends. Int. J. Mol. Sci. 2017, 18, 1410. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).