Broussoflavonol B from Broussonetia kazinoki Siebold Exerts Anti-Pancreatic Cancer Activity through Downregulating FoxM1
Abstract
1. Introduction
2. Results
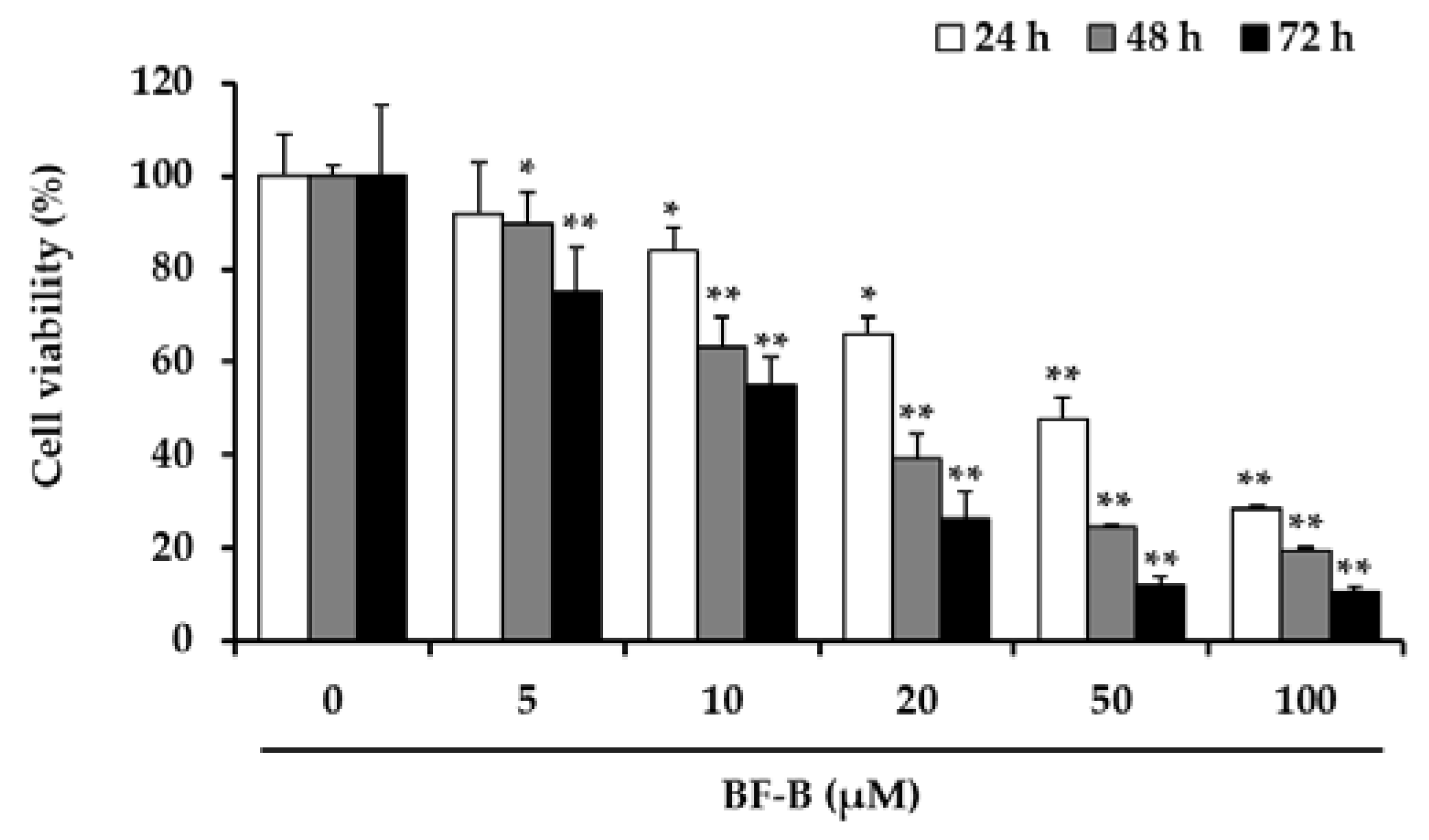
2.1. BF-B Reduces Viability of Human Pancreatic Cancer PANC-1 Cells
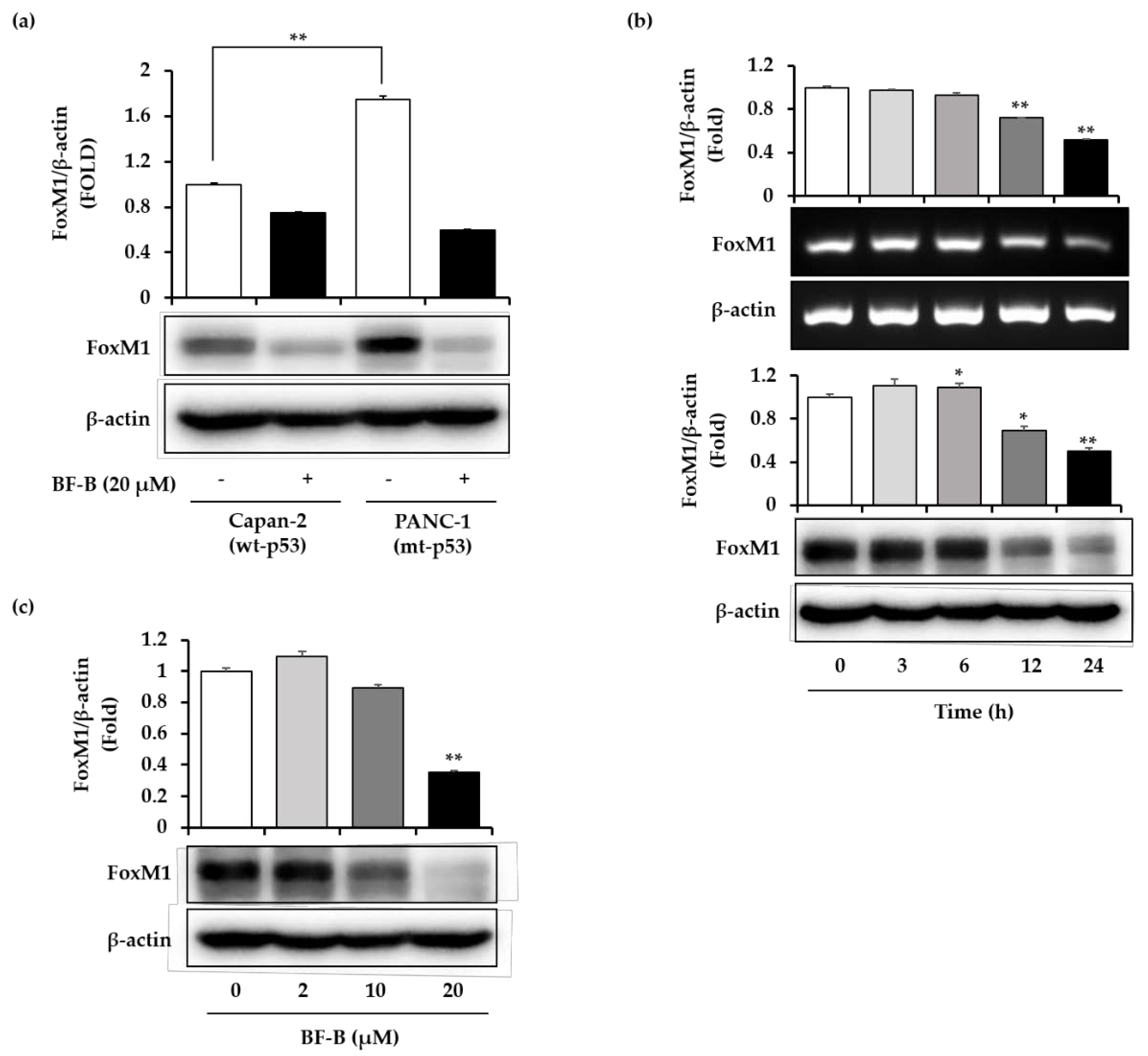
2.2. BF-B Inhibits FoxM1 Expression
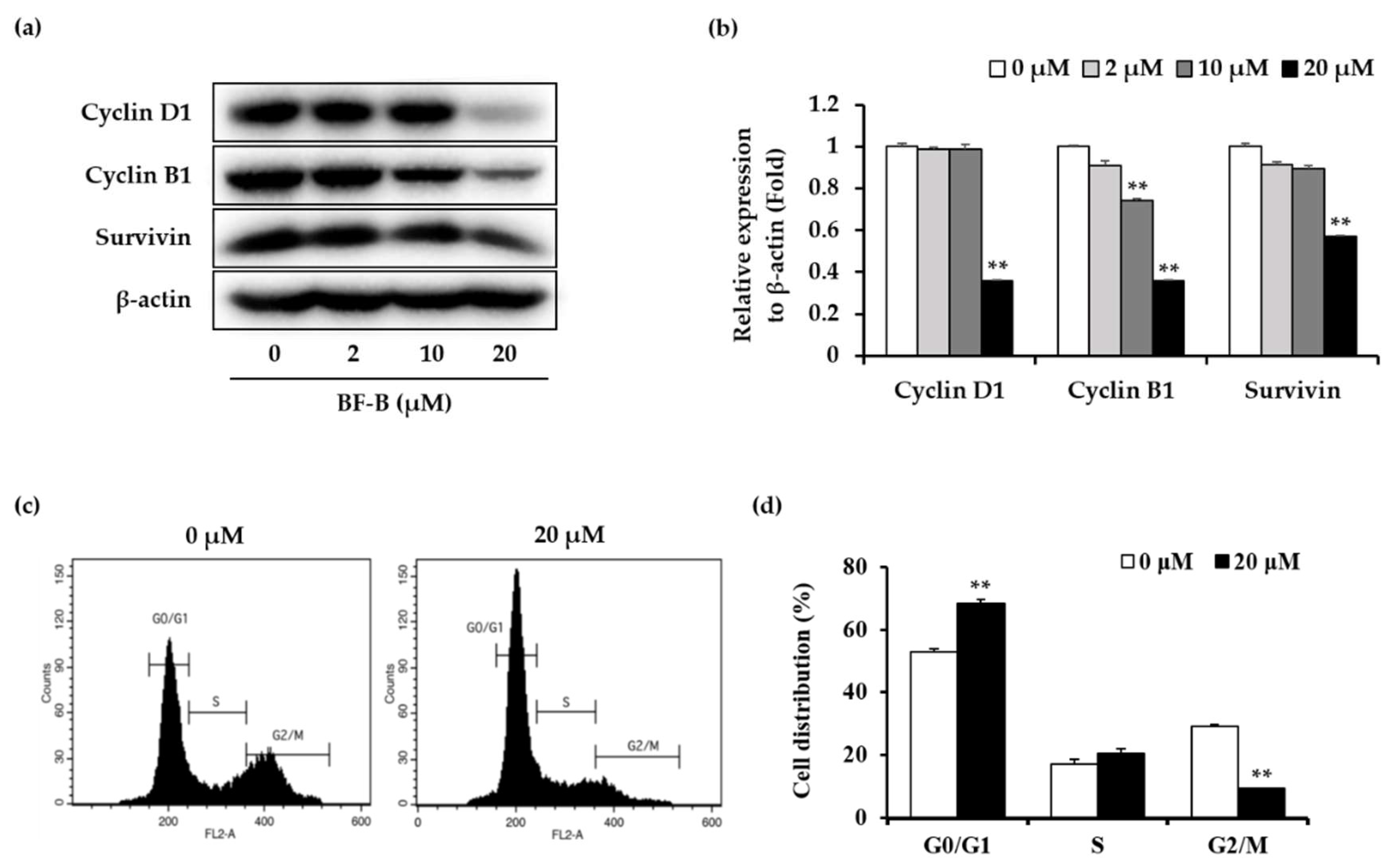
2.3. BF-B Induces G0/G1 Arrest through Down-Regulating FoxM1 Target Genes
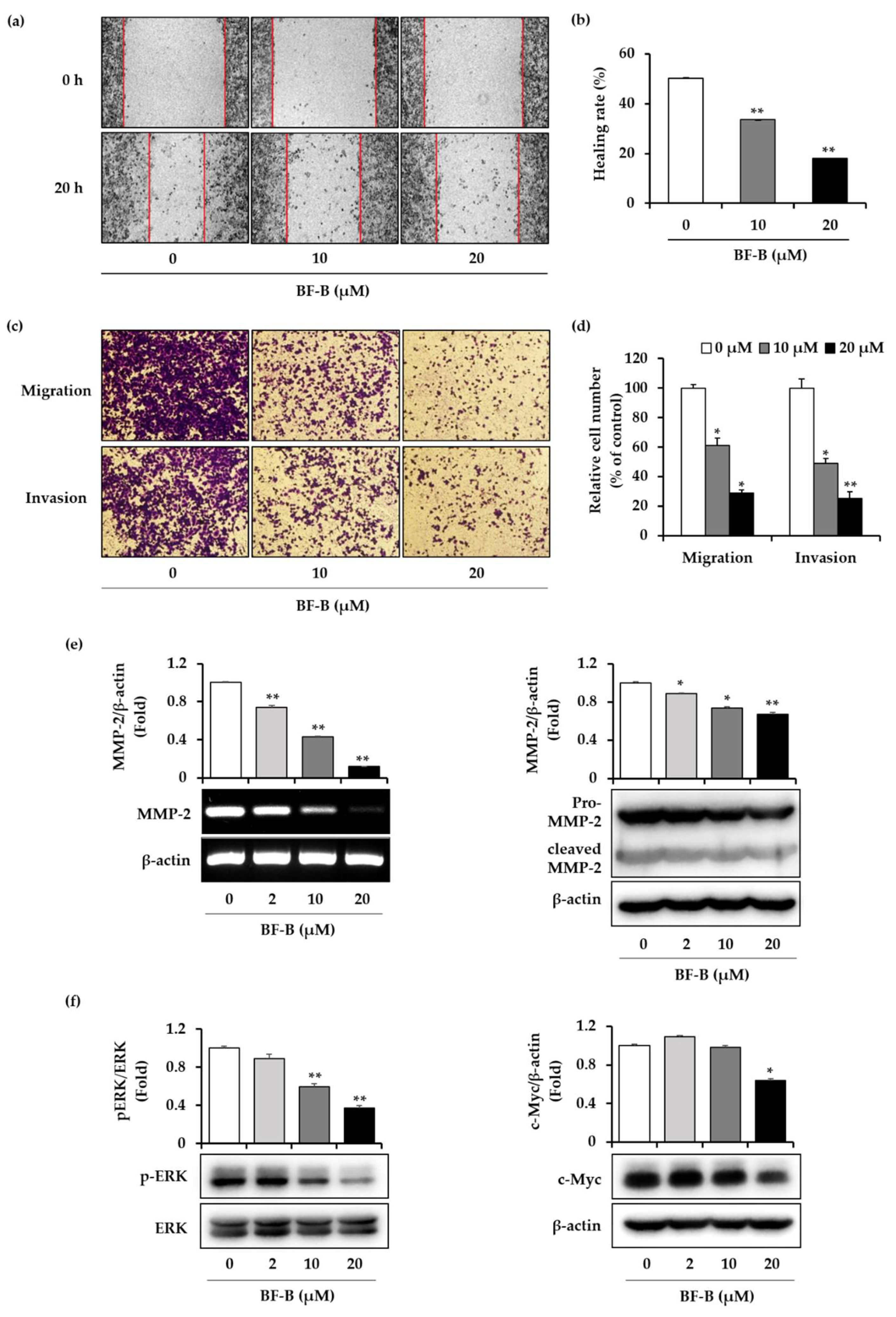
2.4. BF-B Inhibits Cell Migration and Invasion of Pancreatic Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation
4.2. Cell Culture
4.3. MTT Assay
4.4. RT-PCR
4.5. Western Blot
4.6. Cell Cycle Analysis
4.7. Wound Healing Assay
4.8. Transwell Migration and Invasion Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Gao, G.; Zou, G.; Yu, H.; Zheng, X. Natural Products as Adjunctive Treatment for Pancreatic Cancer: Recent Trends and Advancements. BioMed Res. Int. 2017, 2017, 8412508. [Google Scholar] [CrossRef] [PubMed]
- Rozenblum, E.; Schutte, M.; Goggins, M.; Hahn, S.A.; Panzer, S.; Zahurak, M.; Goodman, S.N.; Sohn, T.A.; Hruban, R.H.; Yeo, C.J.; et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997, 57, 1731–1734. [Google Scholar] [PubMed]
- Scarpa, A.; Capelli, P.; Mukai, K.; Zamboni, G.; Oda, T.; Iacono, C.; Hirohashi, S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am. J. Pathol. 1993, 142, 1534–1543. [Google Scholar] [PubMed]
- Kato, S.; Han, S.Y.; Liu, W.; Otsuka, K.; Shibata, H.; Kanamaru, R.; Ishioka, C. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc. Natl. Acad. Sci. USA 2003, 100, 8424–8429. [Google Scholar] [CrossRef]
- Muller, P.A.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Pandit, B.; Halasi, M.; Gartel, A.L. p53 negatively regulates expression of FoxM1. Cell Cycle 2009, 8, 3425–3427. [Google Scholar] [CrossRef]
- Barsotti, A.M.; Prives, C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene 2009, 28, 4295–4305. [Google Scholar] [CrossRef]
- Huang, C.; Du, J.; Xie, K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim. Biophys. Acta 2014, 1845, 104–116. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, X.; Jiang, L.; Zhang, L.; Xiang, M.; Ren, H. FoxM1 Induced Paclitaxel Resistance via Activation of the FoxM1/PHB1/RAF-MEK-ERK Pathway and Enhancement of the ABCA2 Transporter. Mol. Ther. Oncolytics 2019, 14, 196–212. [Google Scholar] [CrossRef]
- Kalin, T.V.; Ustiyan, V.; Kalinichenko, V.V. Multiple faces of FoxM1 transcription factor: Lessons from transgenic mouse models. Cell Cycle 2011, 10, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dai, B.; Kang, S.; Ban, K.; Huang, F.; Lang, F.F.; Aldape, K.D.; Xie, T.; Pelloski, C.E.; Xie, K.; et al. FoxM1B Is Overexpressed in Human Glioblastomas and Critically Regulates the Tumorigenicity of Glioma Cells. Cancer Res. 2006, 66, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Madureira, P.A.; Varshochi, R.; Constantinidou, D.; Francis, R.E.; Coombes, R.C.; Yao, K.; Lam, E.W. The Forkhead Box M1 Protein Regulates the Transcription of the Estrogen Receptor α in Breast Cancer Cells. J. Biol. Chem. 2006, 281, 25167–25176. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Y.; Zhu, Z.M.; Chen, L.B.; Wang, J.H.; Su, Q.S.; Yang, J.R.; Lin, Y.; Xue, L.J.; Liu, X.B.; Mo, X.B. FOXM1 expression correlates with tumor invasion and a poor prognosis of colorectal cancer. Acta Histochem. 2012, 114, 755–762. [Google Scholar] [CrossRef]
- Wang, G.W.; Huang, B.K.; Qin, L.P. The genus Broussonetia: A review of its phytochemistry and pharmacology. Phytother. Res. 2012, 26, 1–10. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, H.J.; Ryu, J.H. Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production. Molecules 2018, 23, 639. [Google Scholar] [CrossRef]
- Kim, H.S.; Lim, J.; Lee, D.Y.; Ryu, J.H.; Lim, J.S. Kazinol C from Broussonetia kazinoki activates AMP-activated protein kinase to induce antitumorigenic effects in HT-29 colon cancer cells. Oncol. Rep. 2015, 33, 223–229. [Google Scholar] [CrossRef][Green Version]
- Lim, J.; Nam, S.; Jeong, J.H.; Kim, M.J.; Yang, Y.; Lee, M.S.; Lee, H.G.; Ryu, J.H.; Lim, J.S. Kazinol U inhibits melanogenesis through the inhibition of tyrosinase-related proteins via AMP kinase activation. Br. J. Pharm. 2019, 176, 737–750. [Google Scholar] [CrossRef]
- Ryu, H.W.; Park, M.H.; Kwon, O.; Kim, D.; Hwang, J.; Jo, Y.H.; Ahn, K.S.; Hwang, B.Y.; Oh, S. Anti-inflammatory flavonoids from root bark of Broussonetia papyrifera in LPS-stimulated RAW264.7 cells. Bioorganic Chem. 2019, 92, 103233. [Google Scholar] [CrossRef]
- Guo, F.; Feng, L.; Huang, C.; Ding, H.; Zhang, X.; Wang, Z.; Li, Y. Prenylflavone derivatives from Broussonetia papyrifera, inhibit the growth of breast cancer cells in vitro and in vivo. Phytochem. Lett. 2013, 6, 331–336. [Google Scholar] [CrossRef]
- Guo, M.X.; Wang, M.L.; Deng, H.; Zhang, X.T.; Wang, Z.Y. A novel anticancer agent Broussoflavonol B downregulates estrogen receptor (ER)-α36 expression and inhibits growth of ER-negative breast cancer MDA-MB-231 cells. Eur. J. Pharm. 2013, 714, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Curtis-Long, M.J.; Jung, S.; Jeong, I.Y.; Kim, D.S.; Kang, K.Y.; Park, K.H. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. 2012, 132, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, T.; Du, J.; Zhang, B.; Xiang, D.; Li, W. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 2018, 40, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, J.; Xiong, X.; Yuan, W.; Qin, S.; Cao, W.; Dai, L.; Xie, F.; Li, A.; Liu, Z. Icariin suppresses cell cycle transition and cell migration in ovarian cancer cells. Oncol. Rep. 2019, 41, 2321–2328. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Valiveti, C.K.; Kumar, D.R.; Van Slambrouck, S.; Kesharwani, S.S.; Seefeldt, T.; Scaria, J.; Tummala, H.; Bhat, G.J. The Flavonoid Metabolite 2,4,6-Trihydroxybenzoic Acid Is a CDK Inhibitor and an Anti-Proliferative Agent: A Potential Role in Cancer Prevention. Cancers 2019, 11, 427. [Google Scholar] [CrossRef]
- Parikh, N.; Hilsenbeck, S.; Creighton, C.J.; Dayaram, T.; Shuck, R.; Shinbrot, E.; Xi, L.; Gibbs, R.A.; Wheeler, D.A.; Donehower, L.A. Effects of TP53 mutational status on gene expression patterns across 10 human cancer types. J. Pathol. 2014, 232, 522–533. [Google Scholar] [CrossRef]
- Wang, Z.; Banerjee, S.; Kong, D.; Li, Y.; Sarkar, F.H. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007, 67, 8293–8300. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmad, A.; Banerjee, S.; Azmi, A.; Kong, D.; Li, Y.; Sarkar, F.H. FoxM1 is a novel target of a natural agent in pancreatic cancer. Pharm. Res. 2010, 27, 1159–1168. [Google Scholar] [CrossRef]
- Wang, I.C.; Chen, Y.J.; Hughes, D.; Petrovic, V.; Major, M.L.; Park, H.J.; Tan, Y.; Ackerson, T.; Costa, R.H. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell. Biol. 2005, 25, 10875–10894. [Google Scholar] [CrossRef]
- Radhakrishnan, S.K.; Bhat, U.G.; Hughes, D.E.; Wang, I.C.; Costa, R.H.; Gartel, A.L. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006, 66, 9731–9735. [Google Scholar] [CrossRef]
- Dong, W.; Li, H.; Zhang, Y.; Yang, H.; Guo, M.; Li, L.; Liu, T. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim. Biophys. Sin. 2011, 43, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xu, X.; Li, G.; Lin, B.; Cao, J.; Teng, L. ER-α36: A novel biomarker and potential therapeutic target in breast cancer. Oncotargets Ther. 2014, 7, 1525–1533. [Google Scholar] [CrossRef][Green Version]
- Hwang, R.F.; Gordon, E.M.; Anderson, W.F.; Parekh, D. Gene therapy for primary and metastatic pancreatic cancer with intraperitoneal retroviral vector bearing the wild-type p53 gene. Surgery 1998, 124, 143–151. [Google Scholar] [CrossRef]
- Kern, S.E.; Pietenpol, J.A.; Thiagalingam, S.; Seymour, A.; Kinzler, K.W.; Vogelstein, B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 1992, 256, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qiu, Z.; Wang, L.; Peng, Z.; Jia, Z.; Logsdon, C.D.; Le, X.; Wei, D.; Huang, S.; Xie, K. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012, 72, 655–665. [Google Scholar] [CrossRef]
- Lieber, M.; Mazzetta, J.; Nolson-Rees, W.; Kaplan, M.; Todaro, G. Establishment of a continuous tumor-cell line (PANC-1) from a human carcinoma of the exocrine pancreas. Int. J. Cancer 1975, 15, 741–747. [Google Scholar] [CrossRef]
- Kyriazis, A.A.; Kyriazis, A.P.; Sternverg, C.N.; Sloane, N.H.; Loveless, J.D. Morphological, Biological, Biochemical, and Karyotypic Characteristics of Human Pancreatic Ductal Adenocarcinoma Capan-2 in Tissue Culture and the Nude Mouse. Cancer Res. 1986, 46, 5810–5815. [Google Scholar]
- Brunner, T.B.; Cengel, K.A.; Hahn, S.M.; Wu, J.; Fraker, D.L.; Mckenna, W.H.; Bernhard, E.J. Pancreatic Cancer Cell Radiation Survival and Prenyltransferase Inhibition: The Role of K-Ras. Cancer Res. 2005, 65, 8433–8441. [Google Scholar] [CrossRef]
- Dong, G.Z.; Jeong, J.H.; Lee, Y.I.; Lee, S.Y.; Zhao, H.Y.; Jeon, R.; Lee, H.J.; Ryu, J.H. Diarylheptanoids suppress proliferation of pancreatic cancer PANC-1 cells through modulating shh-Gli-FoxM1 pathway. Arch. Pharm. Res. 2017, 40, 509–517. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, J.H.; Ryu, J.H. Anti-pancreatic cancer activity of Z-ajoene from garlic: An inhibitor of the Hedgehog/Gli/FoxM1 axis. J. Funct. Foods 2019, 56, 102–109. [Google Scholar] [CrossRef]
- Dai, B.; Kang, S.H.; Gong, W.; Liu, M.; Aldape, K.D.; Sawaya, R.; Huang, S. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene 2007, 26, 6212–6219. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, B.; Miao, L.; Mei, Y.; Varshochi, R. Mutant p53-R273H gains new function in sustained activation of EGFR signaling via suppressing miR-27a expression. Cell Death Dis. 2013, 4, e574. [Google Scholar] [CrossRef] [PubMed]
- Lok, G.T.; Chan, D.W.; Liu, V.W.; Hui, W.W.; Leung, T.H.; Yao, K.M.; Ngan, H.Y. Aberrant activation of ERK/FOXM1 signaling cascade triggers the cell migration/invasion in ovarian cancer cells. Plos ONE 2011, 6, e23790. [Google Scholar] [CrossRef]
- Pan, H.; Zhu, Y.; Wei, W.; Shao, S.; Rui, X. Transcription factor FoxM1 is the downstream target of c-Myc and contributes to the development of prostate cancer. World J. Surg. Oncol. 2018, 16, 59. [Google Scholar] [CrossRef]
- Matsumoto, J.; Fujimoto, T.; Takino, C.; Saitoh, M.; Hano, Y.; Fukai, T.; Nomura, T. Components of Broussonetia papyrifera (L.) VENT. I. Structures of Two New Isoprenylated Flavonols and Two Chalcone Derivatives. Chem. Pharm. Bull. 1985, 33, 3250–3256. [Google Scholar] [CrossRef]
- Khan, M.; Bajpai, V.K.; Kang, S.C. Visual experiment MTT assay to evaluate the cytotoxicity potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Q.; Zheng, W.; Li, L.; Mei, W. Microwave-assisted synthesis of polypyridyl ruthenium(ii) complexes as potential tumor-targeting inhibitors against the migration and invasion of Hela cells through G2/M phase arrest. RSC Adv. 2017, 7, 26625–26632. [Google Scholar] [CrossRef]
- Su, J.; Zhou, X.; Wang, L.; Yin, X.; Wang, Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am. J. Cancer Res. 2016, 6, 1949–1962. [Google Scholar]
- Gu, X.D.; Xu, L.L.; Zhao, H.; Gu, J.Z.; Xie, X.H. Cantharidin suppressed breast cancer MDA-MB-231 cell growth and migration by inhibiting MAPK signaling pathway. Braz. J. Med. Biol. Res. 2017, 50, e5920. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Ryu, J.-H. Broussoflavonol B from Broussonetia kazinoki Siebold Exerts Anti-Pancreatic Cancer Activity through Downregulating FoxM1. Molecules 2020, 25, 2328. https://doi.org/10.3390/molecules25102328
Jeong JH, Ryu J-H. Broussoflavonol B from Broussonetia kazinoki Siebold Exerts Anti-Pancreatic Cancer Activity through Downregulating FoxM1. Molecules. 2020; 25(10):2328. https://doi.org/10.3390/molecules25102328
Chicago/Turabian StyleJeong, Ji Hye, and Jae-Ha Ryu. 2020. "Broussoflavonol B from Broussonetia kazinoki Siebold Exerts Anti-Pancreatic Cancer Activity through Downregulating FoxM1" Molecules 25, no. 10: 2328. https://doi.org/10.3390/molecules25102328
APA StyleJeong, J. H., & Ryu, J.-H. (2020). Broussoflavonol B from Broussonetia kazinoki Siebold Exerts Anti-Pancreatic Cancer Activity through Downregulating FoxM1. Molecules, 25(10), 2328. https://doi.org/10.3390/molecules25102328





