89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe
Abstract
1. Introduction
2. Results
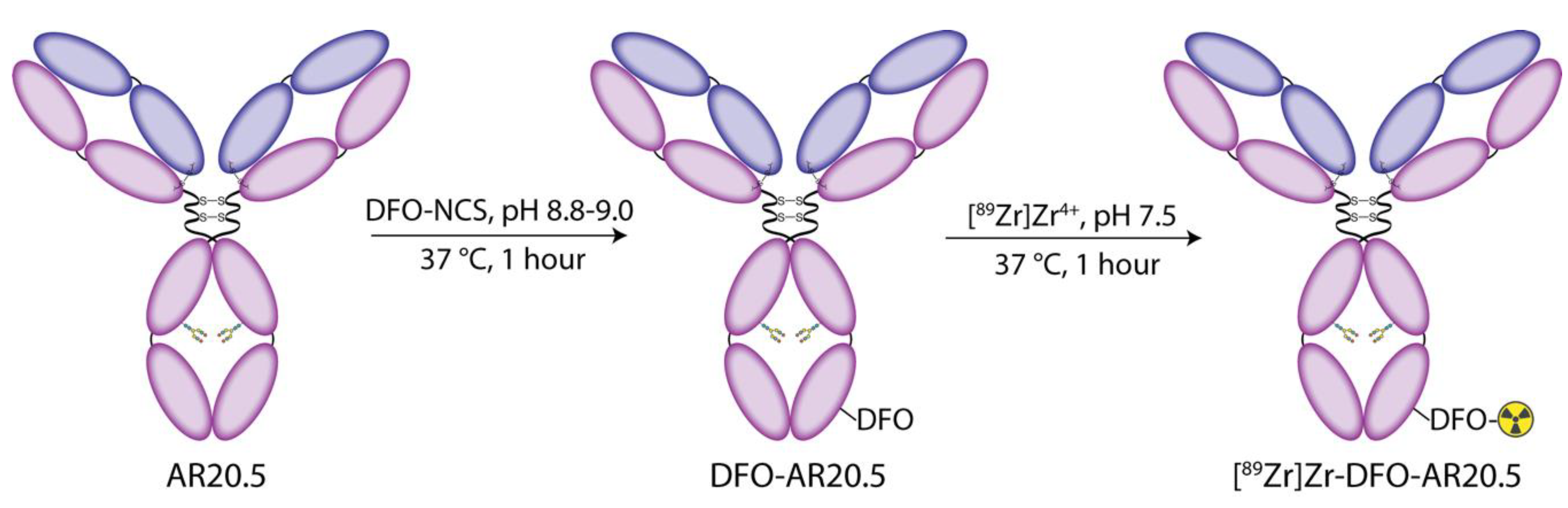
2.1. Modification of AR20.5 with DFO and Evaluation of the In Vitro Behavior of the Immunoconjugate in Human Ovarian Cancer Cells
2.2. Radiolabeling of DFO-AR20.5 with Zr-89
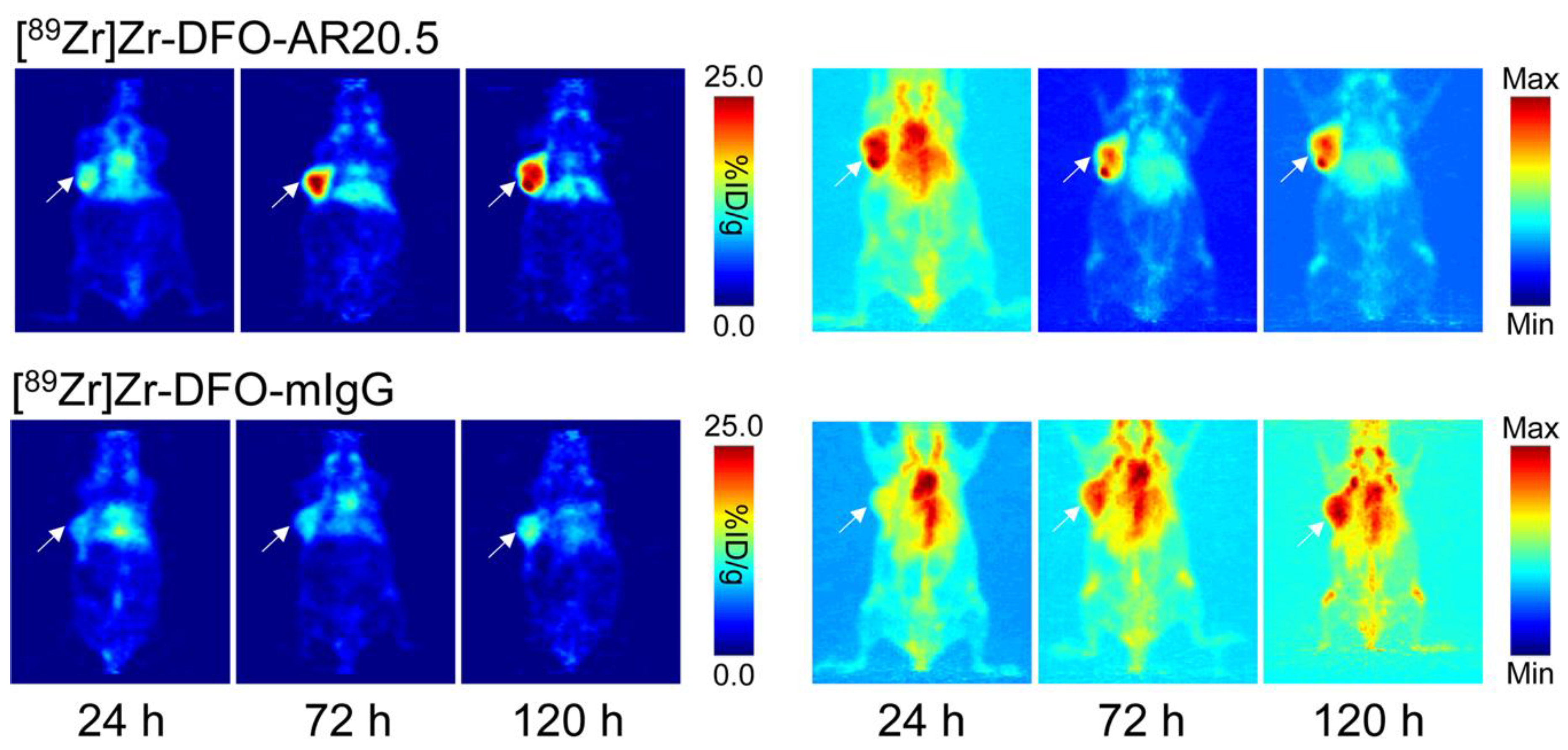
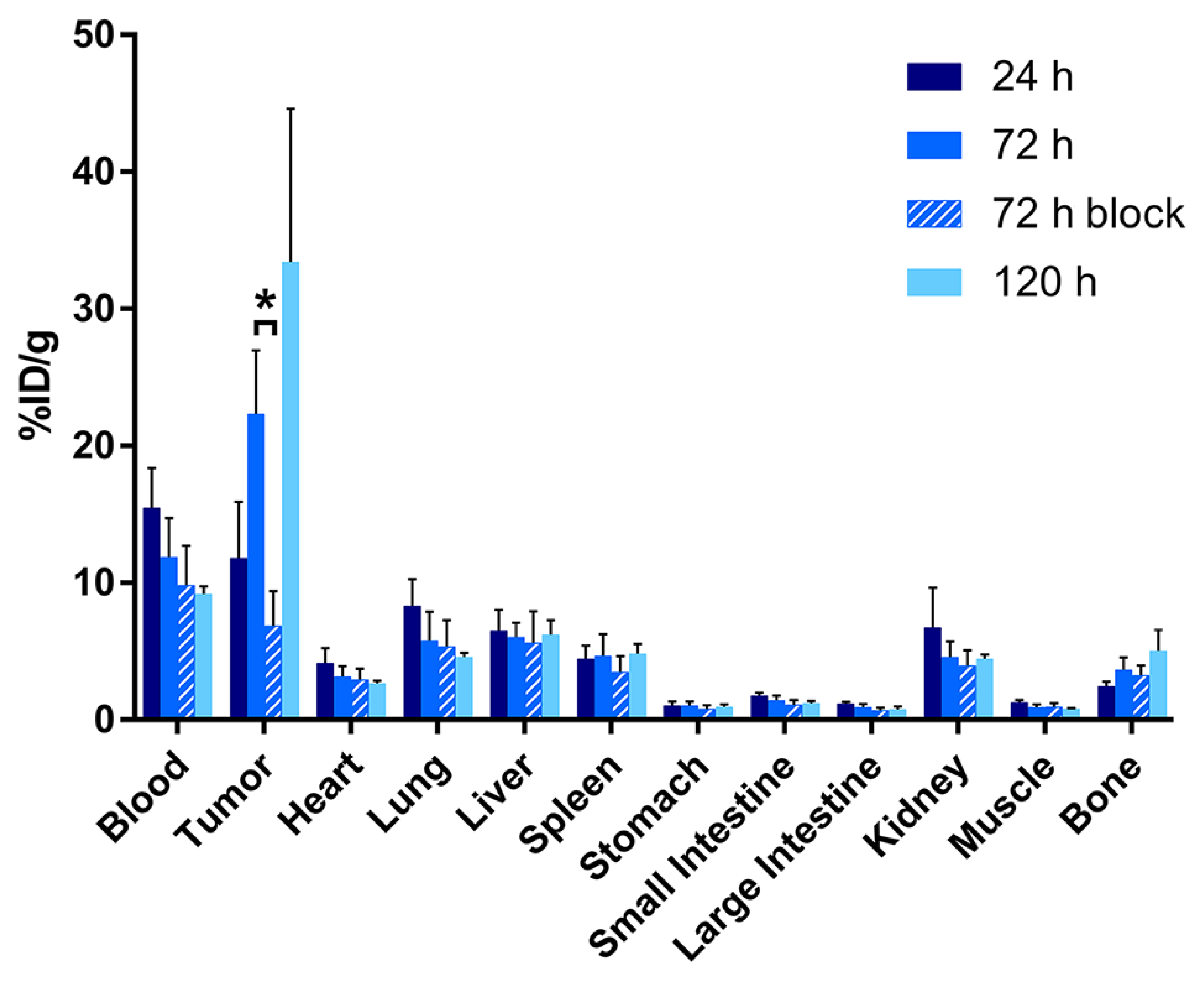
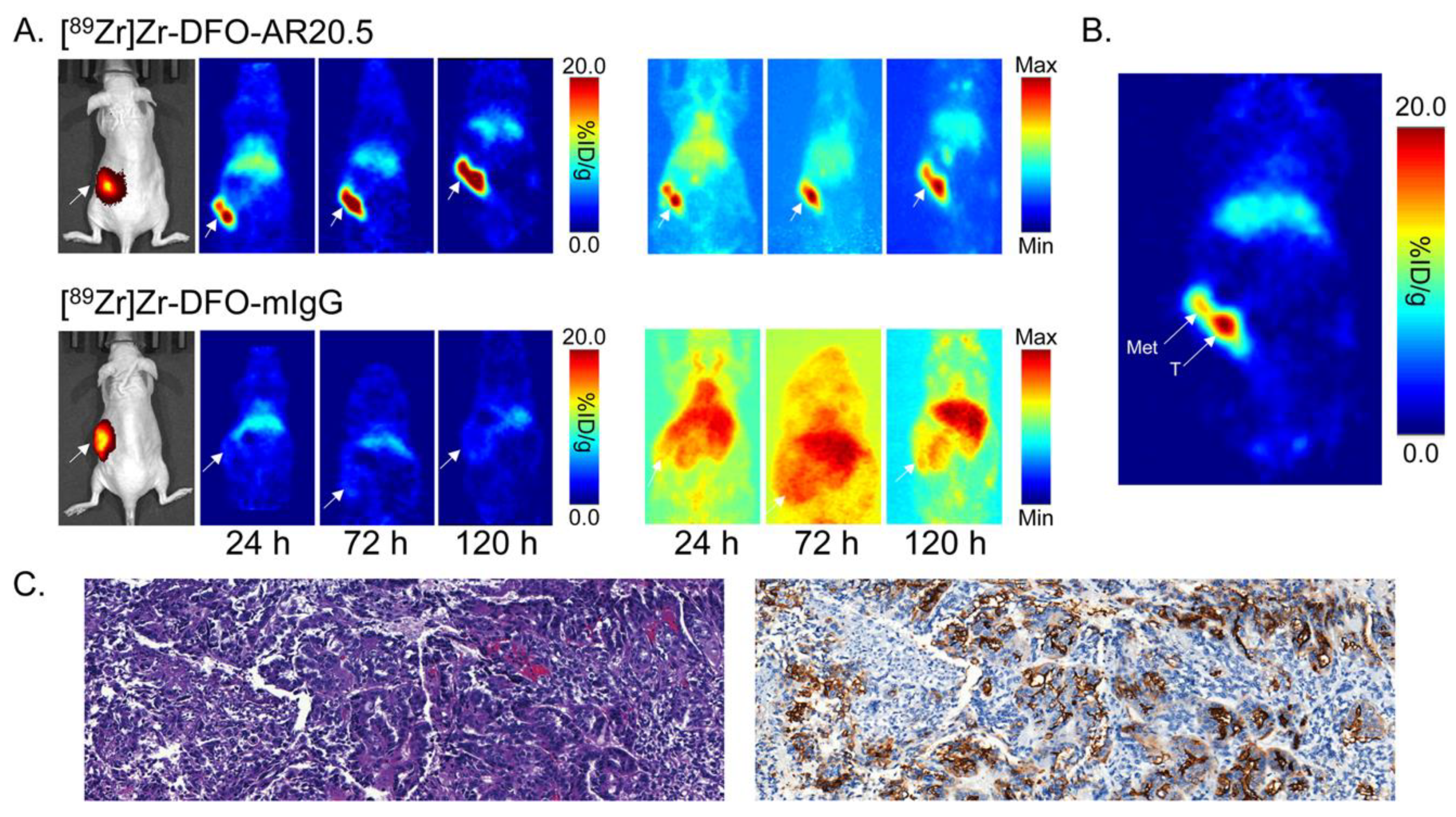
2.3. Evaluation of the In Vivo Behavior of [89Zr]Zr-DFO-AR20.5 in Mice Bearing Subcutaneous SKOV3 Xenografts
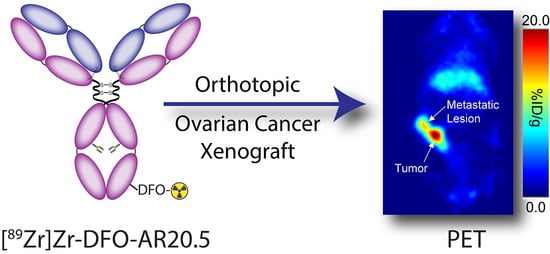
2.4. Evaluation of the In Vivo Behavior of [89Zr]Zr-DFO-AR20.5 in Mice Bearing Orthotopic SKOV3-Red-FLuc Xenografts and Histopathological Analysis of Mouse Tumors and Metastases
3. Discussion
4. Materials and Methods
4.1. Instrumentation
4.2. Modification of Antibodies with DFO
Degree of Labeling Determination via MALDI-ToF Mass Spectrometry
4.3. Radiolabeling with 89Zr
4.4. Cell Culture
4.5. Flow Cytometry
4.6. Radioimmunoconjugate Stability Assays
4.7. Xenograft Models
4.7.1. Subcutaneous Xenograft Model
4.7.2. Orthotopic Xenograft Model
4.8. PET Imaging
4.9. Acute Biodistribution
4.10. Histopathology
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gendler, S.J.; Lancaster, C.F.; Taylor-Papadimitriou, J.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Lalani, E.N.; Wilson, D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 1990, 265, 15286–15293. [Google Scholar]
- Finn, O.J.; Jerome, K.R.; Henderson, R.A.; Pecher, G.; Domenech, N.; Magarian-Blander, J.; Barratt-Boyes, S.M. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol. Rev. 1995, 145, 61–89. [Google Scholar] [CrossRef]
- de Bono, J.S.; Rha, S.Y.; Stephenson, J.; Schultes, B.C.; Monroe, P.; Eckhardt, G.S.; Hammond, L.A.; Whiteside, T.L.; Nicodemus, C.F.; Cermak, J.M.; et al. Phase I trial of a murine antibody to MUC1 in patients with metastatic cancer: Evidence for the activation of humoral and cellular antitumor immunity. Ann. Oncol. 2004, 15, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Papadimitriou, J.; Burchell, J.; Miles, D.W.; Dalziel, M. MUC1 and cancer. Biochim. Biophys. Acta 1999, 1455, 301–313. [Google Scholar] [CrossRef]
- Gendler, S.J. MUC1, the renaissance molecule. J. Mammary Gland Biol. Neoplasia 2001, 6, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Walsh, M.D.; Cummings, M.C.; Wright, R.G.; Khoo, S.K.; Parsons, P.G.; McGuckin, M.A. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumors. J. Pathol. 1997, 183, 311–317. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Liu, F.; Yu, Q.; Chu, G.; Perkins, A.C.; Li, Y. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol. Oncol. 2007, 105, 695–702. [Google Scholar] [CrossRef]
- Van Elssen, C.H.; Frings, P.W.; Bot, F.J.; Van de Vijver, K.K.; Huls, M.B.; Meek, B.; Hupperets, P.; Germeraad, W.T.; Bos, G.M. Expression of aberrantly glycosylated Mucin-1 in ovarian cancer. Histopathology 2010, 57, 597–606. [Google Scholar] [CrossRef]
- Qi, W.; Schultes, B.C.; Liu, D.; Kuzma, M.; Decker, W.; Madiyalakan, R. Characterization of an anti-MUC1 monoclonal antibody with potential as a cancer vaccine. Hybrid. Hybridomics 2001, 20, 313–324. [Google Scholar] [CrossRef]
- Horm, T.M.; Schroeder, J.A. MUC1 and metastatic cancer: Expression, function, and therapeutic targeting. Cell Adhes. Commun. 2013, 7, 187–198. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Budiu, R.A.; Mantia-Smaldone, G.; Elishaev, E.; Chu, T.; Thaller, J.; McCabe, K.; Lenzner, D.; Edwards, R.P.; Vlad, A.M. Soluble MUC1 and serum MUC1-specific antibodies are potential prognostic biomarkers for platinum-resistance ovarian cancer. Cancer Immunol. Immunother. 2011, 60, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, L.; Chen, H.; Li, L.; Ma, Y.; Ni, J.; Li, Y. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev. 2013, 32, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Bitler, B.G.; Menzl, I.; Huerta, C.L.; Sands, B.; Knowlton, W.; Chang, A.; Schroeder, J.A. Intracellular MUC1 peptides inhibit cancer progression. Clin. Cancer Res. 2009, 15, 100–109. [Google Scholar] [CrossRef]
- Bitler, B.G.; Schroeder, J.A. Anti-cancer therapies that utilize cell penetrating peptides. Recent Pat. Anti-Cancer Drug Discov. 2010, 5, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, A.; Nakayama, H.; Kawasaki, M.; Horie, I.; Miyata, T.; Isohama, Y.; Kim, K.C.; Katsuki, H. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem. Biophys. Res. Commun. 2011, 405, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Moreno, M.; Verheijen, R.H.; Sweep, F.C.; Thomas, C.M.; Massuger, L.F.; von Mendsorff-Pouilly, S. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int. J. Cancer 2008, 123, 1848–1853. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Drakaki, H.; Loveland, B.E.; Piddlesden, S.J.; Piebanski, M.; Pouniotis, D.S.; Alexis, M.N.; et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1. Breast Cancer Res. 2006, 8, R27. [Google Scholar] [CrossRef]
- Maher, J.; Wilkie, S.; Davies, D.M.; Arif, S.; Picco, G.; Julien, S.; Foster, J.; Burchell, J.; Taylor-Papadimitriou, J. Targeting of tumor-associated glycoforms of MUC1 with CAR T cells. Immunity 2016, 45, 945–946. [Google Scholar] [CrossRef]
- Song, E.Y.; Qu, C.F.; Rizvi, S.M.A.; Raja, C.; Beretov, J.; Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F.; Perkins, A.; Allen, B.J. Bismuth-213 radioimmunotherapy with C595 anti-MUC1 monoclonal antibody in an ovarian cancer ascites model. Cancer Biol. Therapy 2008, 7, 76–80. [Google Scholar] [CrossRef]
- Salouti, M.; Babaei, M.H.; Rajabi, H.; Rasaee, M.J. Preparation and biological evaluation of 177Lu conjugation PR81 for radioimmunotherapy of breast cancer. Nucl. Med. Biol. 2011, 38, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Hughes, O.D.M.; Bishop, M.C.; Perkins, A.C.; Frier, M.; Price, M.R.; Denton, G.; Smith, A.; Rutherford, R.; Schubiger, P.A. Preclinical evaluation of copper-67 labelled anti-MUC1 mucin antibody C595 for therapeutic use in bladder cancer. Eur. J. Nucl. Med. 1997, 24, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.V.; Modrak, D.E.; Schutsky, K.; Cardillo, T.M. Combined 90yttrium-DOTA-labeled PAM4 antibody radioimmunotherapy and gemcitabine radiosensitization for the treatment of a human pancreatic cancer xenograft. Int. J. Cancer 2004, 109, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Rivalland, G.; Loveland, B.; Mitchell, P. Update on Mucin-1 immunotherapy in cancer: A clinical perspective. Expert Opin. Biol. Ther. 2015, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Syrkina, M.S.; Rubtsov, M.A. MUC1 in cancer immunotherapy: New hope or phantom menace? Biochemistry 2019, 84, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef]
- Heskamp, S.; Raave, R.; Boerman, O.; Ripkema, M.; Goncalves, V.; Denat, F. 89Zr-immuno-positron emission tomography in oncology: State-of-the-art 89Zr radiochemistry. Bioconj. Chem. 2017, 28, 2211–2223. [Google Scholar] [CrossRef]
- Carmon, K.S.; Azhdarinia, A. Application of immuno-PET in antibody-drug conjugate development. Mol. Imaging 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.M.; Lewis, J.S.; O’Donoghue, J.A. First-in-human human epidermal growth factor receptor 2-targeted imaging using 89Zr-pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef]
- McKnight, B.; Viola-Villegas, N. 89Zr-immunoPET companion diagnostics and their impact in clinical drug development. J. Label. Compd. Radiopharm. 2018, 61, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Movahedin, M.; Brooks, T.M.; Supekar, N.T.; Gokanapudi, N.; Boons, G.-J.; Brooks, C.L. Glycosylation of MUC1 influences the binding of a therapeutic antibody by altering the conformational equilibrium of the antigen. Glycobiology 2017, 27, 677–687. [Google Scholar] [CrossRef]
- Mehla, K.; Tremayne, J.; Grunkemeyer, J.A.; O’Connell, K.A.; Steele, M.M.; Caffrey, T.C.; Zhu, X.; Yu, F.; Singh, P.K.; Schultes, B.C.; et al. Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors. Cancer Immunol. Immunother. 2018, 67, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Alirezapour, B.; Rasaee, J.M.; Jalilian, A.R.; Rajabifar, S.; Mohammadnejad, J.; Paknejad, M.; Maadi, E.; Moradkhani, S. Development of [64Cu]-DOTA-PR81 radioimmunoconjugate for MUC-1 positive PET imaging. Nucl. Med. Biol. 2016, 43, 73–80. [Google Scholar] [CrossRef]
- Garkavij, M.; Samarzija, M.; Ewers, S.B.; Jakopovic, M.; Tezak, S.; Tennvall, J. Concurrent radiotherapy and tumor targeting with 111In-HMFG1-F(ab’)2 in patients with MUC1-positive non-small cell lung cancer. Anticancer Res. 2005, 25, 4663–4672. [Google Scholar]
- Goldenberg, D.M.; Nabi, H.A. Breast cancer imaging with radiolabeled antibodies. Semin. Nucl. Med. 1999, 39, 41–48. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Rossi, E.A.; Sharkey, R.M.; McBride, W.J.; Chang, C.-H. Multifunctional antibodies by the dock-and-lock method for improved cancer imaging and therapy by pretargeting. J. Nucl. Med. 2008, 49, 158–163. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Kaul, S.; Klivenyi, G.; Junkermann, H.; Magener, A.; Henze, M.; Doll, J.; Haberkorn, U.; Amelung, F.; Baster, G. Immunoscintigraphy with positron emission tomography: Gallium-68 chelate imaging of breast cancer pretargeted with bispecific anti-MUC1/anti-Ga chelate antibodies. Cancer Res. 2001, 61, 3712–3717. [Google Scholar]
- Stergiou, N.; Nagel, J.; Pektor, S.; Haimes, A.-S.; Jäkel, J.; Brenner, W.; Schmidt, M.; Miederer, M.; Kunz, H.; Roesch, F.; et al. Evaluation of a novel monoclonal antibody against tumor-associated MUC1 for diagnosis and prognosis of breast cancer. Int. J. Med. Sci. 2019, 16, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J. Vis. Exp. 2015, 96, 52521. [Google Scholar] [CrossRef]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sevak, K.K.; Monette, S.; Carlin, S.D.; Knight, J.C.; Wuest, F.R.; Sala, E.; Zeglis, B.M.; Lewis, J.S. Preclinical 89Zr immuno-PET of high-grade serous ovarian cancer and lymph node metastasis. J. Nucl. Med. 2016, 57, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, C.; O’Reilly, E.M.; O’Donoghue, J.A.; Pandit-Taskar, N.; Carrasquillo, J.A.; Lyashchenko, S.K.; Ruan, S.; Teng, R.; Scholz, W.W.; Maffuid, P.W.; et al. Retooling a blood-based biomarker: Phase I assessment of the high-affinity CA19-9 antibody HuMab-5B1 for immunoPET. Clin. Cancer Res. 2019, 25, 7014–7023. [Google Scholar]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Aggeler, R.; Sawada, R.; Agnew, B.J.; Scholz, W.W.; Lewis, J.S. Site-specifically labeled CA19.9-labeled immunoconjugate for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. PNAS 2015, 112, 15850. [Google Scholar] [CrossRef]
- Vivier, D.; Sharma, S.K.; Adumeau, P.; Rodriguez, C.; Fung, K.; Zeglis, B.M. The impact of Fc[gamma]RI binding on immunoPET. J. Nucl. Med. 2019, 60, 1174–1182. [Google Scholar] [CrossRef]
- Vivier, D.; Fung, K.; Rodirguez, C.; Adumeau, P.; Ulaner, G.A.; Lewis, J.S.; Sharma, S.K.; Zeglis, B.M. The influence of glycans-specific bioconjugation on the Fc[gamma]RI binding and in vivo performance of 89Zr-DFO-pertuzumab. Theranostics 2020, 10, 1746–1757. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound DFO-AR20.5 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, K.; Vivier, D.; Keinänen, O.; Sarbisheh, E.K.; Price, E.W.; Zeglis, B.M. 89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe. Molecules 2020, 25, 2315. https://doi.org/10.3390/molecules25102315
Fung K, Vivier D, Keinänen O, Sarbisheh EK, Price EW, Zeglis BM. 89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe. Molecules. 2020; 25(10):2315. https://doi.org/10.3390/molecules25102315
Chicago/Turabian StyleFung, Kimberly, Delphine Vivier, Outi Keinänen, Elaheh Khozeimeh Sarbisheh, Eric W. Price, and Brian M. Zeglis. 2020. "89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe" Molecules 25, no. 10: 2315. https://doi.org/10.3390/molecules25102315
APA StyleFung, K., Vivier, D., Keinänen, O., Sarbisheh, E. K., Price, E. W., & Zeglis, B. M. (2020). 89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe. Molecules, 25(10), 2315. https://doi.org/10.3390/molecules25102315