Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents
Abstract
1. Introduction
2. Synthesis of Metal Oxides
2.1. Reactions in ILs
2.1.1. Titanium Oxide
2.1.2. Zinc Oxide
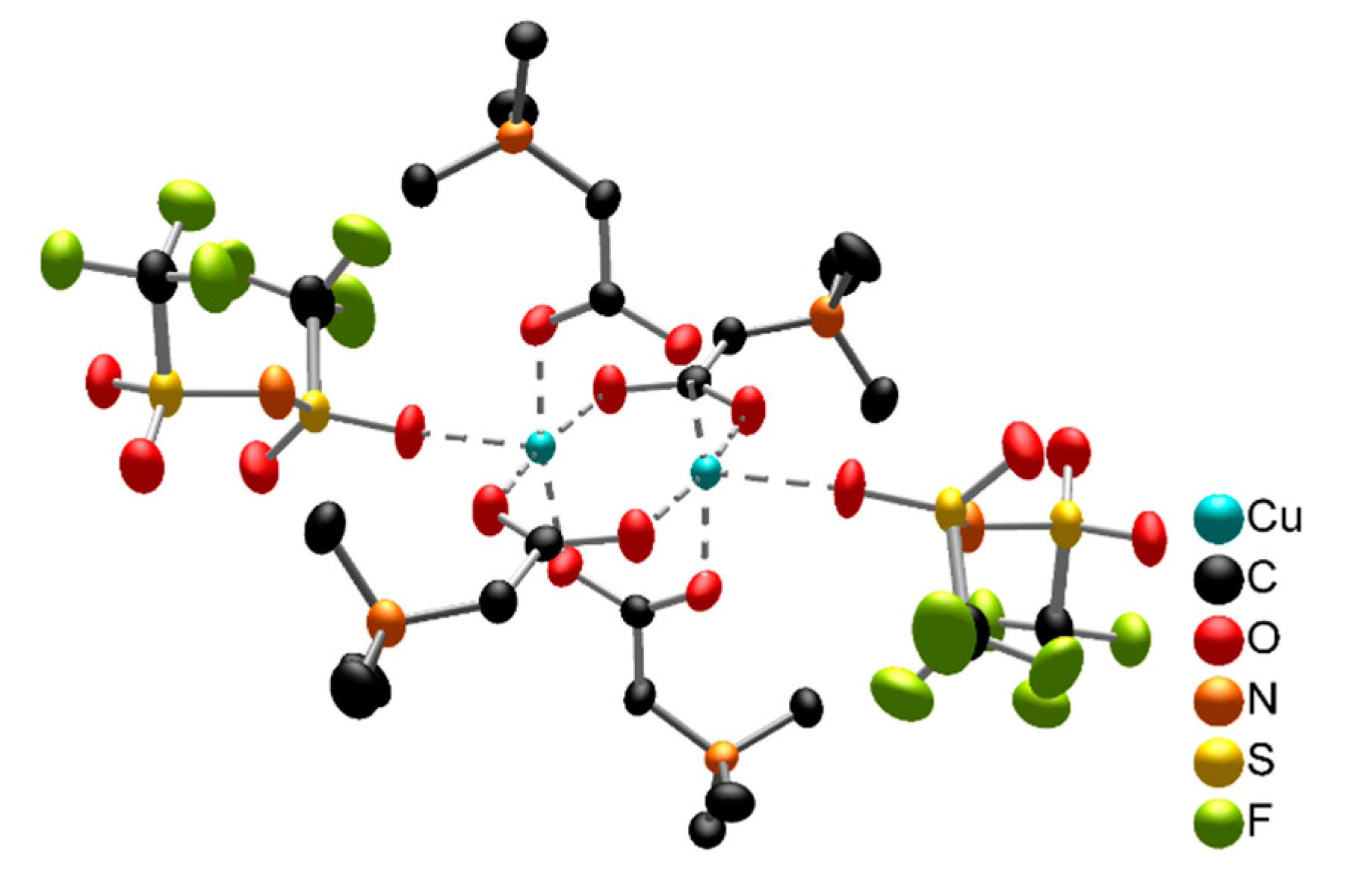
2.1.3. Copper Oxide
2.1.4. Iron Oxides
2.1.5. Cerium Oxide
2.1.6. Miscellaneous
2.1.7. Conclusion on IL-Based Metal Oxide Synthesis
2.2. Reactions in DESs
2.2.1. Zinc Oxide
2.2.2. Iron Oxides
2.2.3. Tin Oxides
2.2.4. Miscellaneous
2.2.5. Conclusion on DES-Based Metal Oxide Synthesis
2.3. Hydroxide Synthesis and Calcination
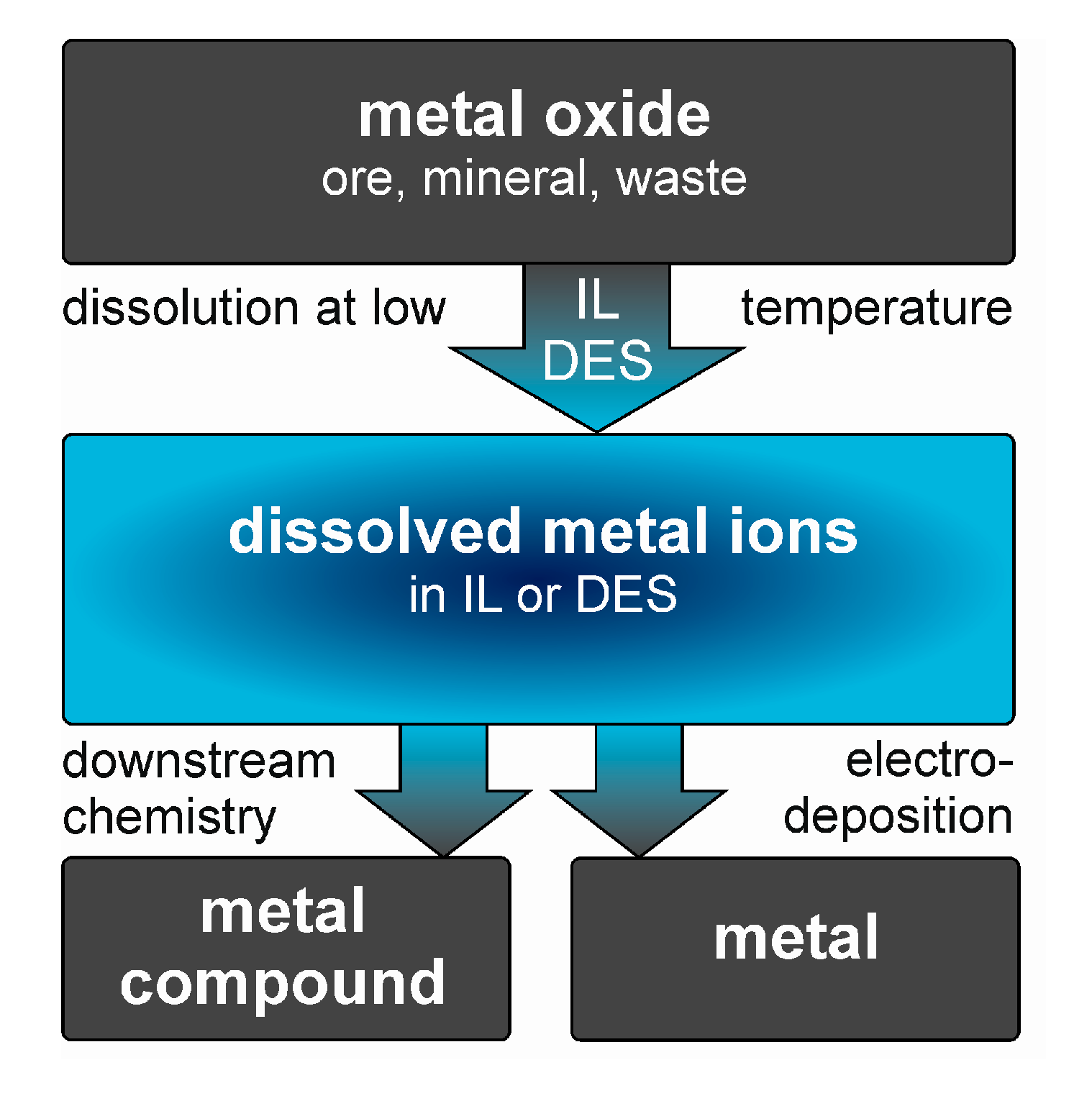
3. Dissolution of Metal Oxides
3.1. Dissolution in ILs
3.1.1. Chloridometalate ILs
3.1.2. Air- and Water-Stable ILs
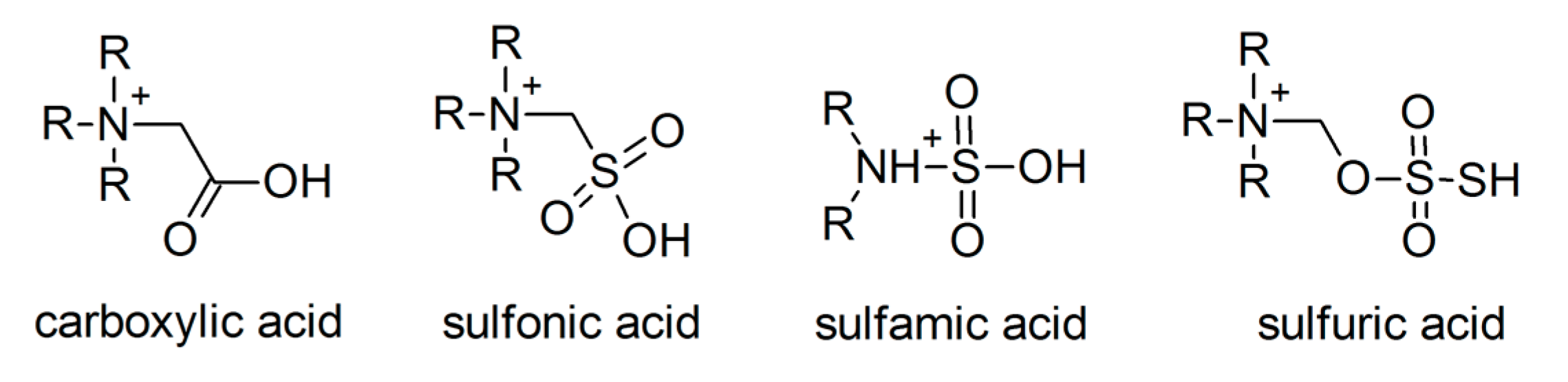
3.1.3. Task-Specific ILs
3.1.4. Conclusion on Metal Oxide Dissolution in ILs
3.2. Dissolution in DESs
3.2.1. Choline Chloride-Urea
3.2.2. Acidic DESs
3.2.3. Conclusion on Dissolution of Metal Oxides in DESs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jolivet, J.-P.; Henry, M.; Livage, J. Metal Oxide Chemistry and Synthesis: From Solution to Solid State; Wiley: Chichester, UK, 2000; ISBN 978-0-471-97056-9. [Google Scholar]
- Cao, H.; Xu, J.Y.; Zhang, D.Z.; Chang, S.-H.; Ho, S.T.; Seelig, E.W.; Liu, X.; Chang, R.P.H. Spatial Confinement of Laser Light in Active Random Media. Phys. Rev. Lett. 2000, 84, 5584–5587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, X.; Xu, J.; Niu, J.; Yang, D. Arrays of ZnO nanowires fabricated by a simple chemical solution route. Nanotechnology 2003, 14, 423–426. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Xie, Y. Selected-Control Synthesis of ZnO Nanowires and Nanorods via a PEG-Assisted Route. Inorg. Chem. 2003, 42, 8105–8109. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; He, R.; Pham, J.; Yang, P. Morphogenesis of One-Dimensional ZnO Nano- and Microcrystals. Adv. Mater. 2003, 15, 402–405. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Block Copolymer Templating Syntheses of Mesoporous Metal Oxides with Large Ordering Lengths and Semicrystalline Framework. Chem. Mater. 1999, 11, 2813–2826. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Xiao, G.; Zhang, H.; Qiu, X.; Li, H.; Chen, L. Mesoscale Organization of Nearly Monodisperse Flowerlike Ceria Microspheres. J. Phys. Chem. B 2006, 110, 13445–13452. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L. Controllable Synthesis of Shuttle-Shaped Ceria and Its Catalytic Properties for CO Oxidation. Eur. J. Inorg. Chem. 2009, 2009, 3883–3887. [Google Scholar] [CrossRef]
- Araújo, V.D.; Avansi, W.; de Carvalho, H.B.; Moreira, M.L.; Longo, E.; Ribeiro, C.; Bernardi, M.I.B. CeO2 nanoparticles synthesized by a microwave-assisted hydrothermal method: Evolution from nanospheres to nanorods. CrystEngComm 2012, 14, 1150–1154. [Google Scholar] [CrossRef]
- Wang, Y.; Mori, T.; Li, J.-G.; Ikegami, T. Low-Temperature Synthesis of Praseodymium-Doped Ceria Nanopowders. J. Am. Ceram. Soc. 2004, 85, 3105–3107. [Google Scholar] [CrossRef]
- Verma, A.; Karar, N.; Bakhshi, A.K.; Chander, H.; Shivaprasad, S.M.; Agnihotry, S.A. Structural, morphological and photoluminescence characteristics of sol-gel derived nano phase CeO2 films deposited using citric acid. J. Nanopart. Res. 2007, 9, 317–322. [Google Scholar] [CrossRef]
- Guillou, N.; Nistor, L.C.; Fuess, H.; Hahn, H. Microstructural studies of nanocrystalline CeO2 produced by gas condensation. Nanostruct. Mater. 1997, 8, 545–557. [Google Scholar] [CrossRef]
- U.S. Geological Survey Mineral Commodity Summaries. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/mcs/mcs2018.pdf (accessed on 3 September 2018).
- Holleman, A.F.; Wiberg, E.; Wiberg, N.; Fischer, G. Anorganische Chemie; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; ISBN 978-3-11-049585-0. [Google Scholar]
- Rabie, K.A. A group separation and purification of Sm, Eu and Gd from Egyptian beach monazite mineral using solvent extraction. Hydrometallurgy 2007, 85, 81–86. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Dissolution Behavior of La2O3, Pr2O3, Nd2O3, CaO and Al2O3 in Sulfuric Acid Solutions and Study of Cerium Recovery from Rare Earth Polishing Powder Waste via Two-Stage Sulfuric Acid Leaching. Mater. Trans. 2013, 54, 713–719. [Google Scholar] [CrossRef]
- Jayachandran, K.; Gupta, R.; Chandrakumar, K.R.S.; Goswami, D.; Noronha, D.M.; Paul, S.; Kannan, S. Remarkably enhanced direct dissolution of plutonium oxide in task-specific ionic liquid: Insights from electrochemical and theoretical investigations. Chem. Commun. 2019, 55, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Ionic Liquids in Synthesis; Wasserscheid, P., Ed.; Wiley-VCH: Weinheim, Germany, 2002; ISBN 978-3-527-31239-9. [Google Scholar]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Prechtl, M.H.G.; Campbell, P.S. Metal oxide and bimetallic nanoparticles in ionic liquids: Synthesis and application in multiphase catalysis. Nanotechnol. Rev. 2013, 2. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Hartley, J.; Ryder, K.S. Processing of metals and metal oxides using ionic liquids. Green Chem. 2011, 13, 471. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Metal complexation in ionic liquids. Annu. Rep. Prog. Chem. Sect. A Inorg. Chem. 2008, 104, 21. [Google Scholar] [CrossRef]
- Mele, A.; Tran, C.D.; De Paoli Lacerda, S.H. The Structure of a Room-Temperature Ionic Liquid with and without Trace Amounts of Water: The Role of C–H···O and C–H···F Interactions in 1-n-Butyl-3-Methylimidazolium Tetrafluoroborate. Angew. Chem. Int. Ed. 2003, 42, 4364–4366. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Qin, Q.; Wang, Q.; Zheng, W. Ionic liquid-assisted solvothermal synthesis of oriented self-assembled Fe3O4 nanoparticles into monodisperse nanoflakes. CrystEngComm 2013, 15, 3284. [Google Scholar] [CrossRef]
- Morris, R.E. Ionothermal synthesis-ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Slowing, I.I.; Anderegg, J.; Mudring, A.-V. Ionic-Liquid-Assisted Microwave Synthesis of Solid Solutions of Sr1−xBaxSnO3 Perovskite for Photocatalytic Applications. ChemSusChem 2017, 10, 3387–3401. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Mudring, A.-V. Facile ultrasound-assisted synthesis of ZnO nanorods in an ionic liquid. Mater. Lett. 2009, 63, 732–735. [Google Scholar] [CrossRef]
- Alammar, T.; Birkner, A.; Mudring, A.-V. Ultrasound-Assisted Synthesis of CuO Nanorods in a Neat Room-Temperature Ionic Liquid. Eur. J. Inorg. Chem. 2009, 2009, 2765–2768. [Google Scholar] [CrossRef]
- Alammar, T.; Noei, H.; Wang, Y.; Grünert, W.; Mudring, A.-V. Ionic Liquid-Assisted Sonochemical Preparation of CeO2 Nanoparticles for CO Oxidation. ACS Sustain. Chem. Eng. 2015, 3, 42–54. [Google Scholar] [CrossRef]
- Alammar, T.; Birkner, A.; Shekhah, O.; Mudring, A.-V. Sonochemical preparation of TiO2 nanoparticles in the ionic liquid 1-(3-hydroxypropyl)-3-methylimidazolium-bis(trifluoromethylsulfonyl)amide. Mater. Chem. Phys. 2010, 120, 109–113. [Google Scholar] [CrossRef]
- Louisnard, O.; González-García, J. Acoustic Cavitation. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 13–64. ISBN 978-1-4419-7471-6. [Google Scholar]
- Merouani, S.; Hamdaoui, O.; Haddad, B. Acoustic cavitation in 1-butyl-3-methylimidazolium bis(triflluoromethyl-sulfonyl)imide based ionic liquid. Ultrason. Sonochem. 2018, 41, 143–155. [Google Scholar] [CrossRef]
- Voepel, P.; Smarsly, B.M. Synthesis of Titanium Oxide Nanostructures in Ionic Liquids. Z. Anorg. Allg. Chem. 2017, 643, 3–13. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phy. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; ISBN 978-0-07-049439-8. [Google Scholar]
- Kaper, H.; Endres, F.; Djerdj, I.; Antonietti, M.; Smarsly, B.M.; Maier, J.; Hu, Y.-S. Direct Low-Temperature Synthesis of Rutile Nanostructures in Ionic Liquids. Small 2007, 3, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Antonietti, M. Synthesis of Very Small TiO2 Nanocrystals in a Room-Temperature Ionic Liquid and Their Self-Assembly toward Mesoporous Spherical Aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Miao, Z.; Liu, Z.; Zhang, Z.; Han, B.; An, G.; Miao, S.; Xie, Y. Facile Synthesis of High Quality TiO2 Nanocrystals in Ionic Liquid via a Microwave-Assisted Process. J. Am. Chem. Soc. 2007, 129, 6362–6363. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Noei, H.; Wang, Y.; Mudring, A.-V. Mild yet phase-selective preparation of TiO2 nanoparticles from ionic liquids―A critical study. Nanoscale 2013, 5, 8045. [Google Scholar] [CrossRef]
- Nakashima, T.; Kimizuka, N. Interfacial Synthesis of Hollow TiO2 Microspheres in Ionic Liquids. J. Am. Chem. Soc. 2003, 125, 6386–6387. [Google Scholar] [CrossRef]
- Dylla, A.G.; Henkelman, G.; Stevenson, K.J. Lithium Insertion in Nanostructured TiO2(B) Architectures. Acc. Chem. Res. 2013, 46, 1104–1112. [Google Scholar] [CrossRef]
- Dylla, A.G.; Stevenson, K.J. Electrochemical and Raman spectroscopy identification of morphological and phase transformations in nanostructured TiO2(B). J. Mater. Chem. A 2014, 2, 20331–20337. [Google Scholar] [CrossRef]
- Etacheri, V.; Yourey, J.E.; Bartlett, B.M. Chemically Bonded TiO2-Bronze Nanosheet/Reduced Graphene Oxide Hybrid for High-Power Lithium Ion Batteries. ACS Nano 2014, 8, 1491–1499. [Google Scholar] [CrossRef]
- Yin, S.; Wu, J.; Aki, M.; Sato, T. Photocatalytic hydrogen evolution with fibrous titania prepared by the solvothermal reactions of protonic layered tetratitanate (H2Ti4O9). Int. J. Inorg. Mater. 2000, 2, 325–331. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.; Waclawik, E.R.; Ke, X.; Zhu, H. An Efficient Photocatalyst Structure: TiO2(B) Nanofibers with a Shell of Anatase Nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef]
- Kaper, H.; Sallard, S.; Djerdj, I.; Antonietti, M.; Smarsly, B.M. Toward a Low-Temperature Sol-Gel Synthesis of TiO2(B) Using Mixtures of Surfactants and Ionic Liquids. Chem. Mater. 2010, 22, 3502–3510. [Google Scholar] [CrossRef]
- Voepel, P.; Seitz, C.; Waack, J.M.; Zahn, S.; Leichtweiß, T.; Zaichenko, A.; Mollenhauer, D.; Amenitsch, H.; Voggenreiter, M.; Polarz, S.; et al. Peering into the Mechanism of Low-Temperature Synthesis of Bronze-type TiO2 in Ionic Liquids. Cryst. Growth Des. 2017, 17, 5586–5601. [Google Scholar] [CrossRef]
- Wessel, C.; Zhao, L.; Urban, S.; Ostermann, R.; Djerdj, I.; Smarsly, B.M.; Chen, L.; Hu, Y.-S.; Sallard, S. Ionic-Liquid Synthesis Route of TiO2(B) Nanoparticles for Functionalized Materials. Chem. Eur. J. 2011, 17, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldova, V.; Laskova, B.; Krysova, H.; Zukalova, M.; Kavan, L. Synthesis of nanostructured TiO2 (anatase) and TiO2(B) in ionic liquids. Catal. Today 2014, 230, 85–90. [Google Scholar] [CrossRef]
- Li, Z.; Geßner, A.; Richters, J.-P.; Kalden, J.; Voss, T.; Kübel, C.; Taubert, A. Hollow Zinc Oxide Mesocrystals from an Ionic Liquid Precursor (ILP). Adv. Mater. 2008, 20, 1279–1285. [Google Scholar] [CrossRef]
- Li, Z.; Shkilnyy, A.; Taubert, A. Room Temperature ZnO Mesocrystal Formation in the Hydrated Ionic Liquid Precursor (ILP) Tetrabutylammonium Hydroxide. Cryst. Growth Des. 2008, 8, 4526–4532. [Google Scholar] [CrossRef]
- Taubert, A.; Kübel, C.; Martin, D.C. Polymer-Induced Microstructure Variation in Zinc Oxide Crystals Precipitated from Aqueous Solution. J. Phys. Chem. B 2003, 107, 2660–2666. [Google Scholar] [CrossRef]
- Taubert, A.; Glasser, G.; Palms, D. Kinetics and Particle Formation Mechanism of Zinc Oxide Particles in Polymer-Controlled Precipitation from Aqueous Solution. Langmuir 2002, 18, 4488–4494. [Google Scholar] [CrossRef]
- Taubert, A.; Palms, D.; Weiss, Ö.; Piccini, M.-T.; Batchelder, D.N. Polymer-Assisted Control of Particle Morphology and Particle Size of Zinc Oxide Precipitated from Aqueous Solution. Chem. Mater. 2002, 14, 2594–2601. [Google Scholar] [CrossRef]
- Edwards, D.A.; Hayward, R.N. Transition metal acetates. Can. J. Chem. 1968, 46, 3443–3446. [Google Scholar] [CrossRef]
- Wang, L.; Chang, L.; Zhao, B.; Yuan, Z.; Shao, G.; Zheng, W. Systematic Investigation on Morphologies, Forming Mechanism, Photocatalytic and Photoluminescent Properties of ZnO Nanostructures Constructed in Ionic Liquids. Inorg. Chem. 2008, 47, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.R.; Jia, D.Z.; Yu, J.Q.; Xin, X.Q.; Xue, Z.L. One-Step Solid-State Reactions at Ambient Temperatures–A Novel Approach to Nanocrystal Synthesis. Adv. Mater 1999, 11, 941–942. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, J.-F.; Pan, Z.; Dai, S. Ionothermal Synthesis of Hierarchical ZnO Nanostructures from Ionic-Liquid Precursors. Chem. Mater. 2006, 18, 4473–4477. [Google Scholar] [CrossRef]
- Taubert, A.; Uhlmann, A.; Hedderich, A.; Kirchhoff, K. CuO Particles from Ionic Liquid/Water Mixtures: Evidence for Growth via Cu(OH)2 Nanorod Assembly and Fusion. Inorg. Chem. 2008, 47, 10758–10764. [Google Scholar] [CrossRef]
- Li, Z.; Rabu, P.; Strauch, P.; Mantion, A.; Taubert, A. Uniform Metal (Hydr)Oxide Particles from Water/Ionic Liquid Precursor (ILP) Mixtures. Chem. Eur. J. 2008, 14, 8409–8417. [Google Scholar] [CrossRef]
- Ma, J.; Wang, T.; Duan, X.; Lian, J.; Liu, Z.; Zheng, W. Ionothermal synthesis of aggregated α-Fe2O3 nanoplates and their magnetic properties. Nanoscale 2011, 3, 4372. [Google Scholar] [CrossRef]
- Wang, Y.; Maksimuk, S.; Shen, R.; Yang, H. Synthesis of iron oxide nanoparticles using a freshly-made or recycled imidazolium-based ionic liquid. Green Chem. 2007, 9, 1051. [Google Scholar] [CrossRef]
- Jacob, D.S.; Bitton, L.; Grinblat, J.; Felner, I.; Koltypin, Y.; Gedanken, A. Are Ionic Liquids Really a Boon for the Synthesis of Inorganic Materials? A General Method for the Fabrication of Nanosized Metal Fluorides. Chem. Mater. 2006, 18, 3162–3168. [Google Scholar] [CrossRef]
- Van Dao, D.; Nguyen, T.T.D.; Majhi, S.M.; Adilbish, G.; Lee, H.-J.; Yu, Y.-T.; Lee, I.-H. Ionic liquid-supported synthesis of CeO2 nanoparticles and its enhanced ethanol gas sensing properties. Mater. Chem. Phys. 2019, 231, 1–8. [Google Scholar] [CrossRef]
- Li, Z.-X.; Li, L.-L.; Yuan, Q.; Feng, W.; Xu, J.; Sun, L.-D.; Song, W.-G.; Yan, C.-H. Sustainable and Facile Route to Nearly Monodisperse Spherical Aggregates of CeO2 Nanocrystals with Ionic Liquids and Their Catalytic Activities for CO Oxidation. J. Phys. Chem. C 2008, 112, 18405–18411. [Google Scholar] [CrossRef]
- Alammar, T.; Chow, Y.-K.; Mudring, A.-V. Energy efficient microwave synthesis of mesoporous Ce0.5M0.5O2 (Ti, Zr, Hf) nanoparticles for low temperature CO oxidation in an ionic liquid—A comparative study. New J. Chem. 2015, 39, 1339–1347. [Google Scholar] [CrossRef]
- Lian, J.; Ma, J.; Duan, X.; Kim, T.; Li, H.; Zheng, W. One-step ionothermal synthesis of γ-Al2O3 mesoporous nanoflakes at low temperature. Chem. Commun. 2010, 46, 2650. [Google Scholar] [CrossRef] [PubMed]
- Bonne, M.; Gaudin, P.; Gu, Y.; Jérôme, F.; Pouilloux, Y.; Duprez, D.; Royer, S. Ionic Liquids Mediated Ionothermal Process for the One-Step Synthesis of High Surface Area Alumina Supported Noble Metals. Mod. Res. Catal. 2013, 02, 28–35. [Google Scholar] [CrossRef][Green Version]
- Kinoshita, K.; Minami, H.; Tarutani, Y.; Tajima, K.; Okubo, M.; Yanagimoto, H. Preparations of Polystyrene/Aluminum Hydroxide and Polystyrene/Alumina Composite Particles in an Ionic Liquid. Langmuir 2011, 27, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tang, S.; Gu, L.; Liu, T.; Zhang, X. Synthesis of rod-like mesoporous γ-Al2O3 by an ionic liquid-assisted sol-gel method. Mater. Lett. 2015, 151, 20–23. [Google Scholar] [CrossRef]
- Park, H.; Yang, S.H.; Jun, Y.S.; Hong, W.H.; Kang, J.K. Facile Route to Synthesize Large-Mesoporous γ-Alumina by Room Temperature Ionic Liquids. Chem. Mater. 2007, 19, 535–542. [Google Scholar] [CrossRef]
- Li Juan, C.; Sheng Mao, Z.; Zhi Shen, W.; Zhi Jun, Z.; Hong Xin, D. Preparation of PbS-type PbO nanocrystals in a room-temperature ionic liquid. Mater. Lett. 2005, 59, 3119–3121. [Google Scholar] [CrossRef]
- Bühler, G.; Thölmann, D.; Feldmann, C. One-Pot Synthesis of Highly Conductive Indium Tin Oxide Nanocrystals. Adv. Mater. 2007, 19, 2224–2227. [Google Scholar] [CrossRef]
- Zhang, T.; Doert, T.; Ruck, M. Solvothermal synthesis and enhanced photo-electrochemical performance of hierarchically structured strontium titanate micro-particles. Dalton Trans. 2017, 46, 14219–14225. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 2010–2011. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xie, S.; Zhang, J.; Liu, R. Synthesis of spherical Fe3O4 magnetic nanoparticles by co-precipitation in choline chloride/urea deep eutectic solvent. Mater. Lett. 2013, 112, 177–179. [Google Scholar] [CrossRef]
- Gu, C.D.; Huang, M.L.; Ge, X.; Zheng, H.; Wang, X.L.; Tu, J.P. NiO electrode for methanol electro-oxidation: Mesoporous vs. nanoparticulate. Int. J. Hydrogen Energy 2014, 39, 10892–10901. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Wang, X.; Tu, J. One-pot synthesis of SnO2/reduced graphene oxide nanocomposite in ionic liquid-based solution and its application for lithium ion batteries. Mater. Res. Bull. 2013, 48, 4112–4117. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Lu, Y.; Wang, X.L.; Tu, J.P. A versatile protocol for the ionothermal synthesis of nanostructured nickel compounds as energy storage materials from a choline chloride-based ionic liquid. J. Mater. Chem. A 2013, 1, 13454–13461. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Correlation between Microstructure and Electrochemical Behavior of the Mesoporous Co3O4 Sheet and Its Ionothermal Synthesized Hydrotalcite-like α-Co(OH)2 Precursor. J. Phys. Chem. C 2014, 118, 911–923. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, Z.-X.; Jiang, Z.-Y.; Kuang, Q.; Zhang, S.-H.; Xu, T.; Huang, R.-B.; Zheng, L.-S. Formation of ZnO hexagonal micro-pyramids: A successful control of the exposed polar surfaces with the assistance of an ionic liquid. Chem. Commun. 2005, 5572–5574. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Tu, J.P.; Ge, X.; Wang, X.L.; Gu, C.D. One-step synthesis of hematite nanospindles from choline chloride/urea deep eutectic solvent with highly powerful storage versus lithium. J. Power Sources 2015, 274, 1–7. [Google Scholar] [CrossRef]
- Hammond, O.S.; Eslava, S.; Smith, A.J.; Zhang, J.; Edler, K.J. Microwave-assisted deep eutectic-solvothermal preparation of iron oxide nanoparticles for photoelectrochemical solar water splitting. J. Mater. Chem. A 2017, 5, 16189–16199. [Google Scholar] [CrossRef]
- Zheng, H.; Gu, C.-D.; Wang, X.-L.; Tu, J.-P. Fast synthesis and optical property of SnO nanoparticles from choline chloride-based ionic liquid. J. Nanopart. Res. 2014, 16, 2288. [Google Scholar] [CrossRef]
- Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 2009, 20, 455602. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.D.; Mai, Y.J.; Zhou, J.P.; Tu, J.P. SnO2 nanocrystallite: Novel synthetic route from deep eutectic solvent and lithium storage performance. Funct. Mater. Lett. 2011, 04, 377–381. [Google Scholar] [CrossRef]
- Oderinde, O.; Kang, M.; Kalulu, M.; Yao, F.; Fu, G. Facile synthesis and study of the photochromic properties of deep eutectic solvent-templated cuboctahedral-WO3/MoO3 nanocomposites. Superlattices Microstruct. 2019, 125, 103–112. [Google Scholar] [CrossRef]
- Minami, H.; Kinoshita, K.; Tsuji, T.; Yanagimoto, H. Preparation of Highly Crystalline Magnesium Oxide and Polystyrene/Magnesium Hydroxide Composite Particles by Sol-gel Processes in an Ionic Liquid. J. Phys. Chem. C 2012, 116, 14568–14574. [Google Scholar] [CrossRef]
- Alammar, T.; Hamm, I.; Grasmik, V.; Wark, M.; Mudring, A.-V. Microwave-Assisted Synthesis of Perovskite SrSnO3 Nanocrystals in Ionic Liquids for Photocatalytic Applications. Inorg. Chem. 2017, 56, 6920–6932. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Chen, Z.; He, C.; Su, J.; Wen, Y. A mild process for the synthesis of Na2Ti3O7 as an anode material for sodium-ion batteries in deep eutectic solvent. J. Mater. Sci. Mater. Electron. 2019, 30, 8422–8427. [Google Scholar] [CrossRef]
- Thorat, G.M.; Jadhav, H.S.; Roy, A.; Chung, W.-J.; Seo, J.G. Dual Role of Deep Eutectic Solvent as a Solvent and Template for the Synthesis of Octahedral Cobalt Vanadate for an Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 16255–16266. [Google Scholar] [CrossRef]
- Boston, R.; Foeller, P.Y.; Sinclair, D.C.; Reaney, I.M. Synthesis of Barium Titanate Using Deep Eutectic Solvents. Inorg. Chem. 2017, 56, 542–547. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, P.; Wei, F.; Li, W.; Zhou, Y.; Zheng, W.; Zhang, G. Porous Ir-Sn Binary Oxide Nanorod Assembly as an Efficient Electrocatalyst for Water Oxidation. Int. J. Electrochem. Sci. 2018, 13, 3235–3245. [Google Scholar] [CrossRef]
- Söldner, A.; Zach, J.; Iwanow, M.; Gärtner, T.; Schlosser, M.; Pfitzner, A.; König, B. Preparation of Magnesium, Cobalt and Nickel Ferrite Nanoparticles from Metal Oxides using Deep Eutectic Solvents. Chem. Eur. J. 2016, 22, 13108–13113. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Kandwal, P.; Iqbal, M.; Huskens, J.; Murali, M.S.; Verboom, W. A novel CMPO-functionalized task specific ionic liquid: Synthesis, extraction and spectroscopic investigations of actinide and lanthanide complexes. Dalton Trans. 2013, 42, 4343. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.; Jin, L.; Nockemann, P.; Robertson, P.K.J.; Stella, L.; Ruhela, R.; Seddon, K.R.; Gunaratne, H.Q.N. Ionic liquids tethered to a preorganised 1,2-diamide motif for extraction of lanthanides. Green Chem. 2019, 21, 2583–2588. [Google Scholar] [CrossRef]
- Mehdi, H.; Binnemans, K.; Van Hecke, K.; Van Meervelt, L.; Nockemann, P. Hydrophobic ionic liquids with strongly coordinating anions. Chem. Commun. 2010, 46, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.L. Ionic Liquids as Extraction Solvents: Where do We Stand? Sep. Sci. Technol. 2006, 41, 2047–2063. [Google Scholar] [CrossRef]
- Wellens, S.; Brooks, N.R.; Thijs, B.; Meervelt, L.V.; Binnemans, K. Carbene formation upon reactive dissolution of metal oxides in imidazolium ionic liquids. Dalton Trans. 2014, 43, 3443–3452. [Google Scholar] [CrossRef]
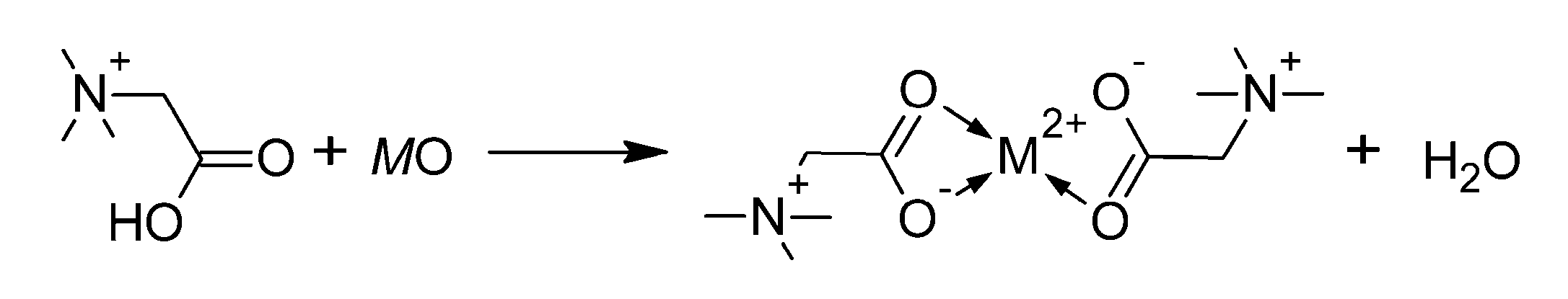
- Nockemann, P.; Thijs, B.; Pittois, S.; Thoen, J.; Glorieux, C.; Van Hecke, K.; Van Meervelt, L.; Kirchner, B.; Binnemans, K. Task-Specific Ionic Liquid for Solubilizing Metal Oxides. J. Phys. Chem. B 2006, 110, 20978–20992. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Hecke, K.V.; Meervelt, L.V.; Binnemans, K. Polynuclear Metal Complexes Obtained from the Task-Specific Ionic Liquid Betainium Bistriflimide. Cryst. Growth Des. 2008, 8, 1353–1363. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Parac-Vogt, T.N.; Van Hecke, K.; Van Meervelt, L.; Tinant, B.; Hartenbach, I.; Schleid, T.; Ngan, V.T.; Nguyen, M.T.; et al. Carboxyl-Functionalized Task-Specific Ionic Liquids for Solubilizing Metal Oxides. Inorg. Chem. 2008, 47, 9987–9999. [Google Scholar] [CrossRef]
- Dupont, D.; Raiguel, S.; Binnemans, K. Sulfonic acid functionalized ionic liquids for dissolution of metal oxides and solvent extraction of metal ions. Chem. Commun. 2015, 51, 9006–9009. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Dissolution of metal oxides in task-specific ionic liquid. RSC Adv. 2019, 9, 29699–29710. [Google Scholar] [CrossRef]
- Mahjoor, P.; Latturner, S.E. Synthesis and Structural Characterization of [bpyr]4[V4O4Cl12] and [bpyr]4[Bi4Cl16] grown in Ionic Liquid [bpyr][AlCl4] (bpyr = 1-Butylpyridinium). Cryst. Growth Des. 2009, 9, 1385–1389. [Google Scholar] [CrossRef]
- Wellens, S.; Vander Hoogerstraete, T.; Möller, C.; Thijs, B.; Luyten, J.; Binnemans, K. Dissolution of metal oxides in an acid-saturated ionic liquid solution and investigation of the back-extraction behaviour to the aqueous phase. Hydrometallurgy 2014, 144–145, 27–33. [Google Scholar] [CrossRef]
- Dupont, D.; Renders, E.; Binnemans, K. Alkylsulfuric acid ionic liquids: A promising class of strongly acidic room-temperature ionic liquids. Chem. Commun. 2016, 52, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Renders, E.; Raiguel, S.; Binnemans, K. New metal extractants and super-acidic ionic liquids derived from sulfamic acid. Chem. Commun. 2016, 52, 7032–7035. [Google Scholar] [CrossRef] [PubMed]
- Billard, I.; Gaillard, C.; Hennig, C. Dissolution of UO2, UO3 and of some lanthanide oxides in BumimTf2N: Effect of acid and water and formation of UO2(NO3)3–. Dalton Trans. 2007, 4214–4221. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, Y.; Shi, Z.; Xu, J.; Wang, Z. Direct Electrochemical Deposition of Lithium from Lithium Oxide in a Highly Stable Aluminium-Containing Solvate Ionic Liquid. ChemElectroChem 2018, 5, 3368–3372. [Google Scholar] [CrossRef]
- Yeh, H.-W.; Tang, Y.-H.; Chen, P.-Y. Electrochemical study and extraction of Pb metal from Pb oxides and Pb sulfate using hydrophobic Brønsted acidic amide-type ionic liquid: A feasibility demonstration. J. Electrochem. Soc. 2018, 811, 68–77. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Lunstroot, K.; Parac-Vogt, T.N.; Görller-Walrand, C.; Binnemans, K.; Van Hecke, K.; Van Meervelt, L.; Nikitenko, S.; Daniels, J.; et al. Speciation of Rare-Earth Metal Complexes in Ionic Liquids: A Multiple-Technique Approach. Chem. Eur. J. 2009, 15, 1449–1461. [Google Scholar] [CrossRef]
- Yao, A.; Chu, T. Fe-containing ionic liquids as effective and recoverable oxidants for dissolution of UO2 in the presence of imidazolium chlorides. Dalton Trans. 2013, 42, 8413. [Google Scholar] [CrossRef]
- Zarzana, C.A.; Groenewold, G.S.; Benson, M.T.; Delmore, J.E.; Tsuda, T.; Hagiwara, R. Production of Gas-Phase Uranium Fluoroanions Via Solubilization of Uranium Oxides in the [1-Ethyl-3-Methylimidazolium]+[F(HF)2.3]- Ionic Liquid. J. Am. Soc. Mass Spectrom. 2018, 29, 1963–1970. [Google Scholar] [CrossRef]
- Rao, C.J.; Venkatesan, K.A.; Nagarajan, K.; Srinivasan, T.G. Dissolution of uranium oxides and electrochemical behavior of U(VI) in task specific ionic liquid. Radiochim. Acta 2008, 96. [Google Scholar] [CrossRef]
- Dai, S.; Shin, Y.S.; Toth, L.M.; Barnes, C.E. Comparative UV−Vis Studies of Uranyl Chloride Complex in Two Basic Ambient-Temperature Melt Systems: The Observation of Spectral and Thermodynamic Variations Induced via Hydrogen Bonding. Inorg. Chem. 1997, 36, 4900–4902. [Google Scholar] [CrossRef] [PubMed]
- Wanigasekara, E.; Freiderich, J.W.; Sun, X.-G.; Meisner, R.A.; Luo, H.; Delmau, L.H.; Dai, S.; Moyer, B.A. Tandem dissolution of UO3 in amide-based acidic ionic liquid and in situ electrodeposition of UO2 with regeneration of the ionic liquid: A closed cycle. Dalton Trans. 2016, 45, 10151–10154. [Google Scholar] [CrossRef] [PubMed]
- Nockemann, P.; Van Deun, R.; Thijs, B.; Huys, D.; Vanecht, E.; Van Hecke, K.; Van Meervelt, L.; Binnemans, K. Uranyl Complexes of Carboxyl-Functionalized Ionic Liquids. Inorg. Chem. 2010, 49, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.C.; Castleman, A.W.; Thorn, D.L. Vanadium Oxide Complexes in Room-Temperature Chloroaluminate Molten Salts. Inorg. Chem. 1999, 38, 5709–5715. [Google Scholar] [CrossRef]
- Liu, Z.; El Abedin, S.Z.; Endres, F. Dissolution of zinc oxide in a protic ionic liquid with the 1-methylimidazolium cation and electrodeposition of zinc from ZnO/ionic liquid and ZnO/ionic liquid–water mixtures. Electrochem. Commun. 2015, 58, 46–50. [Google Scholar] [CrossRef]
- Chaumont, A.; Wipff, G. Solvation of Uranyl(II), Europium(III) and Europium(II) Cations in “Basic” Room-Temperature Ionic Liquids: A Theoretical Study. Chem. Eur. J. 2004, 10, 3919–3930. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]. Green Chem. 2015, 17, 2150–2163. [Google Scholar] [CrossRef]
- Fan, F.-L.; Qin, Z.; Cao, S.-W.; Tan, C.-M.; Huang, Q.-G.; Chen, D.-S.; Wang, J.-R.; Yin, X.-J.; Xu, C.; Feng, X.-G. Highly Efficient and Selective Dissolution Separation of Fission Products by an Ionic Liquid [Hbet][Tf2N]: A New Approach to Spent Nuclear Fuel Recycling. Inorg. Chem. 2019, 58, 603–609. [Google Scholar] [CrossRef]
- Davris, P.; Marinos, D.; Balomenos, E.; Alexandri, A.; Gregou, M.; Panias, D.; Paspaliaris, I. Leaching of rare earth elements from ‘Rödberg’ ore of Fen carbonatite complex deposit, using the ionic liquid HbetTf2N. Hydrometallurgy 2018, 175, 20–27. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Developing New Process for Selective Extraction of Rare Earth Elements from Bauxite Residue Based on Functionalized Ionic Liquids. In Light Metals 2018; Martin, O., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–156. ISBN 978-3-319-72283-2. [Google Scholar]
- Li, H.; Shao, H.; Wang, Y.; Qin, D.; Liu, B.; Zhang, W.; Yan, W. Soft material with intense photoluminescence obtained by dissolving Eu2O3 and organic ligand into a task-specific ionic liquid. Chem. Commun. 2008, 5209–5211. [Google Scholar] [CrossRef] [PubMed]
- Mawire, G.; van Dyk, L. Extraction of Scandium (Sc) Using a Task-Specific Ionic Liquid Protonated Betaine Bis(Trifluoromethylsulfonyl)Imide [Hbet][Tf2N]. In Extraction 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 2723–2734. ISBN 978-3-319-95021-1. [Google Scholar]
- Schaeffer, N.; Grimes, S.; Cheeseman, C. Interactions between trivalent rare earth oxides and mixed [Hbet][Tf2N]:H2O systems in the development of a one-step process for the separation of light from heavy rare earth elements. Inorg. Chim. Acta 2016, 439, 55–60. [Google Scholar] [CrossRef][Green Version]
- Dupont, D.; Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef]
- Orefice, M.; Binnemans, K.; Vander Hoogerstraete, T. Metal coordination in the high-temperature leaching of roasted NdFeB magnets with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide. RSC Adv. 2018, 8, 9299–9310. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; Machiels, L.; Binnemans, K. p-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Solubilizing Metal Oxides. ACS Sustain. Chem. Eng. 2019, 7, 3940–3948. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Shikotra, P. Selective Extraction of Metals from Mixed Oxide Matrixes Using Choline-Based Ionic Liquids. Inorg. Chem. 2005, 44, 6497–6499. [Google Scholar] [CrossRef]
- Tsuda, T.; Boyd, L.E.; Kuwabata, S.; Hussey, C.L. Electrochemistry of Copper(I) Oxide in the 66.7–33.3 mol % Urea–Choline Chloride Room-Temperature Eutectic Melt. J. Electrochem. Soc. 2010, 157, F96. [Google Scholar] [CrossRef]
- Xie, X.; Zou, X.; Lu, X.; Xu, Q.; Lu, C.; Chen, C.; Zhou, Z. Electrodeposition behavior and characterization of copper–zinc alloy in deep eutectic solvent. J. Appl. Electrochem. 2017, 47, 679–689. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, J.; Lan, X.; Zhao, X.; Mou, H.; Mu, T. A strategy for the dissolution and separation of rare earth oxides by novel Brønsted acidic deep eutectic solvents. Green Chem. 2019, 21, 4748–4756. [Google Scholar] [CrossRef]
- Shuwa, S.M.; Al-Hajri, R.S.; Jibril, B.Y.; Al-Waheibi, Y.M. Novel Deep Eutectic Solvent-Dissolved Molybdenum Oxide Catalyst for the Upgrading of Heavy Crude Oil. Ind. Eng. Chem. Res. 2015, 54, 3589–3601. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y. Electrodeposition of Nickel Coating in Choline Chloride-Urea Deep Eutectic Solvent. Int. J. Electrochem. Sci. 2018, 13, 10798–10808. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Wang, D.; Qi, C.; Jie, Y. Morphology-controlled preparation of lead powders by electrodeposition from different PbO-containing choline chloride-urea deep eutectic solvent. Appl. Surf. Sci. 2015, 335, 153–159. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Wang, D.; Qi, C.; Gong, K. Electrochemistry of Pb(II)/Pb during preparation of lead wires from PbO in choline chloride—Urea deep eutectic solvent. Russ. J. Electrochem. 2015, 51, 773–781. [Google Scholar] [CrossRef]
- Yang, H.; Reddy, R.G. Fundamental Studies on Electrochemical Deposition of Lead from Lead Oxide in 2:1 Urea/Choline Chloride Ionic Liquids. J. Electrochem. Soc. 2014, 161, D586–D592. [Google Scholar] [CrossRef]
- Billik, P.; Antal, P.; Gyepes, R. Product of dissolution of V2O5 in the choline chloride–urea deep eutectic solvent. Inorg. Chem. 2015, 60, 37–40. [Google Scholar]
- Yang, H.; Reddy, R.G. Electrochemical Kinetics of Reduction of Zinc Oxide to Zinc Using 2:1 Urea/ChCl Ionic Liquid. Electrochim. Acta 2015, 178, 617–623. [Google Scholar] [CrossRef]
- Yang, H.; Reddy, R.G. Electrochemical deposition of zinc from zinc oxide in 2:1 urea/choline chloride ionic liquid. Electrochim. Acta 2014, 147, 513–519. [Google Scholar] [CrossRef]
- Liu, A.; Shi, Z.; Reddy, R.G. Electrodeposition of Zinc from Zinc Oxide in 2:1 Urea/1-Butyl-3-methylimidazolium Chloride Ionic Liquid. J. Electrochem. Soc. 2017, 164, D666–D673. [Google Scholar] [CrossRef]
- Zheng, Y.; Dong, K.; Wang, Q.; Zhang, S.; Zhang, Q.; Lu, X. Electrodeposition of zinc coatings from the solutions of zinc oxide in imidazolium chloride/urea mixtures. Sci. China Chem. 2012, 55, 1587–1597. [Google Scholar] [CrossRef]
- He, W.; Shen, L.; Shi, Z.; Gao, B.; Hu, X.; Xu, J.; Wang, Z. Zinc Electrodeposition from Zinc Oxide in the Urea/1-ethyl-3-methylimidazolium Chloride at 353 K. Electrochemistry 2016, 84, 872–877. [Google Scholar] [CrossRef]
- Rimsza, J.M.; Corrales, L.R. Adsorption complexes of copper and copper oxide in the deep eutectic solvent 2:1 urea–choline chloride. Comput. Theor. Chem. 2012, 987, 57–61. [Google Scholar] [CrossRef]
- Riaño, S.; Petranikova, M.; Onghena, B.; Vander Hoogerstraete, T.; Banerjee, D.; Foreman, M.R.S.; Ekberg, C.; Binnemans, K. Separation of rare earths and other valuable metals from deep-eutectic solvents: A new alternative for the recycling of used NdFeB magnets. RSC Adv. 2017, 7, 32100–32113. [Google Scholar] [CrossRef]
- Tran, M.K.; Rodrigues, M.-T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
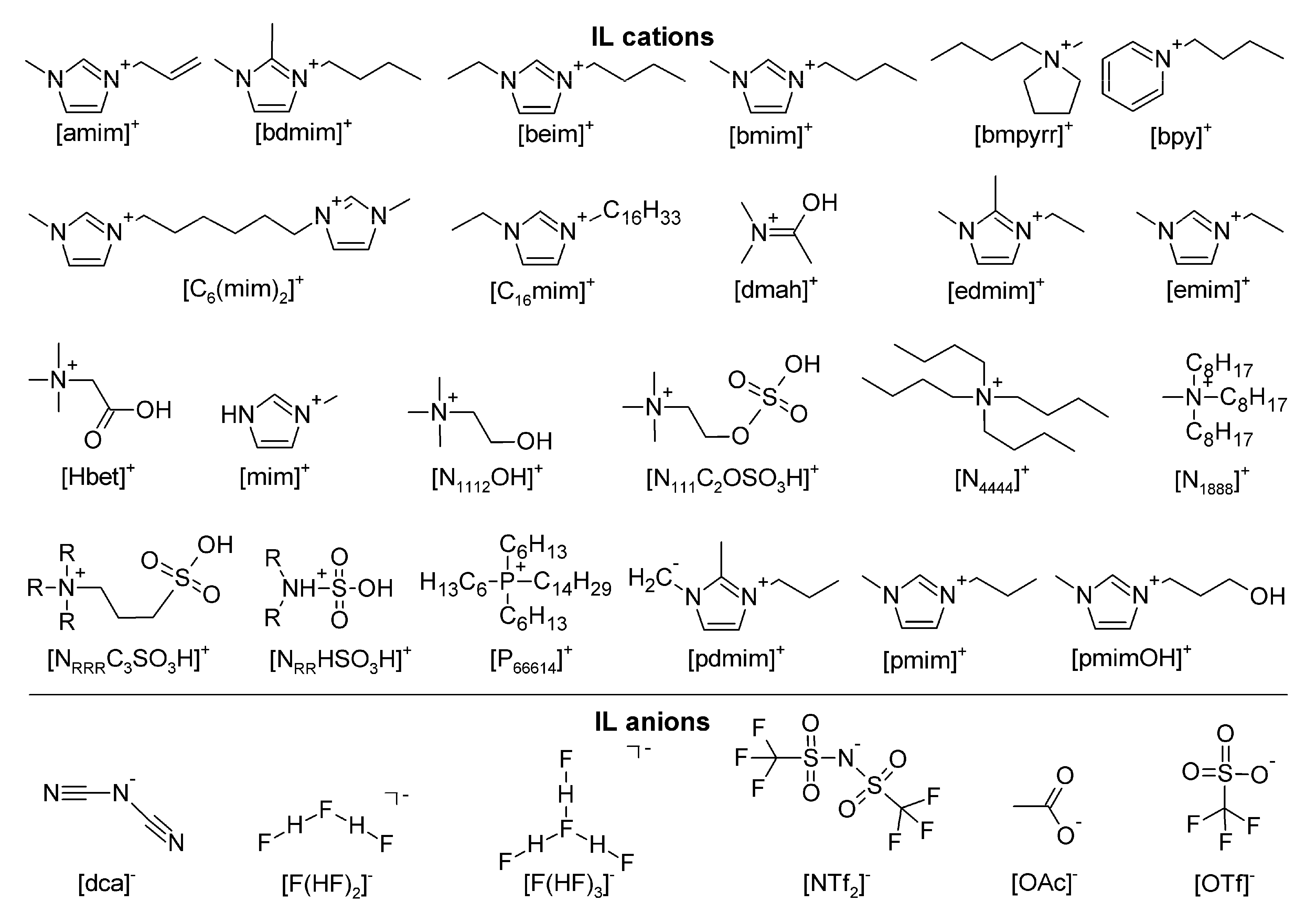
| Abbreviation | Full Name |
|---|---|
| [amim]+ | 1-allyl-3-methylimidazolium cation |
| [bdmim]+ | 1-butyl-2,3-dimethylimidazolium cation |
| [beim]+ | 1-butyl-3-ethylimidazolium cation |
| bet | betaine |
| [bmim]+ | 1-butyl-3-methylimidazolium cation |
| [bmpyrr]+ | 1-butyl-1-methylpyrrolidinium cation |
| [bpy]+ | 1-butylpyridinium cation |
| [C6(mim)2]2+ | 6-bis(3-methylimidazolium-1-yl)hexane cation |
| [C16mim]+ | 1-hexadecyl-3-methylimidazolium cation |
| [dca]− | dicyanamide anion |
| [dmah]+ | N,N-dimethylacetamidium cation |
| [edmim]+ | 1-ethyl-2,3-dimethylimidazolium cation |
| [emim]+ | 1-ethyl-3-methylimidazolium cation |
| [F(HF)n]− | fluorohydrogenate anion |
| [Hbet]+ | betainium cation |
| [mim]+ | 1-methylimidazolium cation |
| [N1112OH]+ | 2-hydroxyethyltrimethylammonium cation |
| [N111C2OSO3H]+ | trimethylammoniumethane hydrogen sulfate cation |
| [N4444]+ | tetrabutylammonium cation |
| [N1888]+ | methyltrioctylammonium cation |
| [NRRRC3SO3H]+ | trialkylammoniumpropanesulfonic acid cation |
| [NRRHSO3H]+ | dialkylsulfamic acid cation |
| [NTf2]− | bis(trifluoromethylsulfonyl)imide anion |
| [OAc]− | acetate anion |
| OBu− | butoxide |
| OiPr− | isopropoxide |
| [OTf]− | trifluoromethanesulfonate anion |
| [P66614]+ | trihexyltetradecylphosphonium cation |
| [PRRRC3SO3H]+ | trialkylphosphoniumpropanesulfonic acid cation |
| [pdmim]+ | 1-propyl-2,3-dimethylimidazolium cation |
| [pmim]+ | 1-propyl-3-methylimidazolium cation |
| [pmimOH]+ | 1-(3-hydroxypropyl)-3-methylimdazolium cation |
| Metal Oxide | Solvent | Reference |
|---|---|---|
| Ag2O | [bmim]Cl | [101] |
| [emim][SCN] | [101] | |
| [emim][dca] | [101] | |
| [emim][OAc] | [101] | |
| [Hbet][NTf2]/H2O and derivates | [102,103,104] | |
| Al2O3 | [NRRRC3SO3H][NTf2]/H2O | [105] |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| BaO | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| Bi2O3 | [bpyr]Cl/AlCl3 | [107] |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| CaO | [P66614]Cl/aq. HCl | [108] |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| CdO | [Hbet][NTf2]/H2O and derivates | [102,104] |
| CoO | [P66614]Cl/aq. HCl | [108] |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| Co3O4 | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| [NRRRSO3H][NTf2]/H2O | [105] | |
| [PRRRSO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| Cr2O3 | [NRRRC3SO3H][NTf2]/H2O | [105] |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| Cu2O | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| CuO | [P66614]Cl/aq. HCl | [108] |
| [emim]Cl | [101] | |
| [emim][OAc] | [101] | |
| [Hbet][NTf2]/H2O and derivates | [102,104] | |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| Dy2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| Er2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| Eu2O3 | [bmim][NTf2]/aq. HNO3 | [111] |
| [Hbet][NTf2]/H2O and derivates | [102,104] | |
| Fe2O3 | [P66614]Cl/aq. HCl | [108] |
| [Hbet]2[NTf2]Cl | [106] | |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| Gd2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| HgO | [Hbet][NTf2]/H2O and derivates | [102,104] |
| Ho2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| La2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| Li2O | Ethylene carbonate/AlCl3 | [112] |
| Lu2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| MgO | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| MnO | [P66614]Cl/aq. HCl | [108] |
| [Hbet][NTf2]/H2O | [102,103] | |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| MnO2 | [Hbet]2[NTf2]Cl | [106] |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| MoO3 | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| Nd2O3 | [bmim][NTf2]/aq. HNO3 | [111] |
| [Hbet][NTf2]/H2O and derivates | [102,104] | |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| NiO | [P66614]Cl/aq. HCl | [108] |
| [emim]Cl | [101] | |
| [emim][OAc] | [101] | |
| [Hbet][NTf2]/H2O and derivates | [102,103,104] | |
| [Hbet]2[NTf2]Cl | [106] | |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] | |
| [N111C2OSO3H][NTf2]/H2O | [109] | |
| PbO | [Hbet][NTf2]/H2O and derivates | [103,104,113] |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| PbO2 | [Hbet][NTf2]/H2O | [113] |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| PdO | [Hbet][NTf2]/H2O and derivates | [102,104] |
| Pr6O11 | [bmim][NTf2]/aq. HNO3 | [111] |
| [Hbet][NTf2]/H2O and derivates | [104,114] | |
| PuO2 | [Hbet][NTf2]/H2O | [18] |
| Sc2O3 | Derivates of [Hbet][NTf2]/H2O | [104] |
| Sm2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| SnO | [Hbet]2[NTf2]Cl | [106] |
| SrO | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| Tb4O7 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| ThO2 | [Hbet]2[NTf2]Cl | [106] |
| TiO2 | [NRRRC3SO3H][NTf2]/H2O | [105] |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| Tm2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| UO2 | [emim]Cl/FeCl3 | [115] |
| [bmim]Cl/FeCl3 | [115] | |
| [bdmim]Cl/FeCl3 | [115] | |
| [bmim][NTf2]/aq. HNO3 | [111] | |
| [emim][F(HF)n] (n = 2, 3) | [116] | |
| [Hbet][NTf2]/H2O | [117] | |
| UO3 | [emim]Cl/AlCl3 | [118] |
| [pdmim]Cl/AlCl3 | [118] | |
| [bmim][NTf2]/aq. HNO3 | [111] | |
| [dmah][NTf2] | [119] | |
| [emim][F(HF)n] (n = 2, 3) | [116] | |
| [Hbet][NTf2]/H2O and derivates | [102,104,117,120] | |
| V2O3 | [Hbet][NTf2] | [106] |
| [Hbet]2[NTf2]Cl | [106] | |
| V2O5 | [emim]Cl/AlCl3 | [121] |
| [bmim]Cl/AlCl3 | [121] | |
| [bpyr]Cl/AlCl3 | [107] | |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| Y2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| Yb2O3 | [Hbet][NTf2]/H2O and derivates | [102,104] |
| WO3 | [NRRRC3SO3H][NTf2]/H2O | [105] |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| ZnO | [P66614]Cl/aq. HCl | [108] |
| [emim]Cl | [101] | |
| [emim][OAc] | [101] | |
| [omim][OTf] | [122] | |
| [Hbet][NTf2]/H2O and derivates | [102,103,104] | |
| [Hbet][NTf2] | [106] | |
| [Hbet]2[NTf2]Cl | [106] | |
| [NRRRC3SO3H][NTf2]/H2O | [105] | |
| [PRRRC3SO3H][NTf2]/H2O | [105] | |
| [NRRH–SO3H][NTf2]/[emim]Cl | [110] |
| Metal Oxide | Solvent | Reference |
|---|---|---|
| CoO | Choline chloride-malonic acid (1:1) | [133] |
| Co3O4 | Choline chloride-malonic acid (1:1) | [133] |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| CrO3 | Choline chloride-urea (1:2) | [133] |
| Choline chloride-malonic acid (1:1) | [133] | |
| Cu2O | Choline chloride-urea (1:2) | [133,135,136] |
| Choline chloride-malonic acid (1:1) | [133] | |
| Choline chloride-ethylene glycol (1:2) | [133] | |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| CuO | Choline chloride-urea (1:2) | [135,137] |
| Choline chloride-malonic acid (1:1) | [133,138] | |
| Choline chloride-oxalic acid (1:1) | [138] | |
| Choline chloride-phenylpropionic acid (1:2) | [138] | |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| Eu2O3 | Ethylene glycol-maleic acid (1:1; 2:1; 4:1; 6:1) | [139] |
| Ethylene glycol-citric acid (4:1) | [139] | |
| 1,2-Propanediol-maleic acid (4:1) | [139] | |
| Glycerol-maleic acid (4:1) | [139] | |
| 1,4-Butanediol-maleic acid (4:1) | [139] | |
| FeO | Choline chloride-malonic acid (1:1) | [133] |
| Fe2O3 | Choline chloride-malonic acid (1:1) | [133] |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| Fe3O4 | Choline chloride-malonic acid (1:1) | [133,138] |
| Choline chloride-oxalic acid (1:1) | [138] | |
| Choline chloride-phenylpropionic acid (1:2) | [138] | |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| Gd2O3 | Ethylene glycol-maleic acid (4:1) | [139] |
| In2O3 | Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] |
| La2O3 | Ethylene glycol-maleic acid (1:1; 2:1; 4:1; 6:1) | [139] |
| Ethylene glycol-citric acid (4:1) | [139] | |
| 1,2-Propanediol-maleic acid (4:1) | [139] | |
| Glycerol-maleic acid (4:1) | [139] | |
| MnO | Choline chloride-malonic acid (1:1) | [133] |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| Mn2O3 | Choline chloride-malonic acid (1:1) | [133] |
| MnO2 | Choline chloride-urea (1:2) | [135] |
| Choline chloride-malonic acid (1:1) | [133] | |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| MoO3 | Choline chloride-urea (1:2) | [140] |
| Nd2O3 | Ethylene glycol-maleic acid (4:1) | [139] |
| NiO | Choline chloride-urea (1:2) | [135] |
| Choline chloride-malonic acid (1:1) | [133] | |
| Ni2O3 | Choline chloride-urea (1:2) | [141] |
| PbO | Choline chloride-urea (1:2) | [142,143,144] |
| PbO2 | Choline chloride-urea (1:2) | [135] |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| Pr6O11 | Ethylene glycol-maleic acid (4:1) | [139] |
| Sm2O3 | Ethylene glycol-maleic acid (4:1) | [139] |
| V2O3 | Choline chloride-urea (1:2) | [133] |
| Choline chloride-malonic acid (1:1) | [133] | |
| Choline chloride-ethylene glycol (1:2) | [133] | |
| V2O5 | Choline chloride-urea (1:2) | [133,145] |
| Choline chloride-malonic acid (1:1) | [133] | |
| Choline chloride-ethylene glycol (1:2) | [133] | |
| ZnO | Choline chloride-urea (1:2) | [133,135,137,146,147] |
| Choline chloride-malonic acid (1:1) | [133,138] | |
| Choline chloride-oxalic acid (1:1) | [138] | |
| Choline chloride-phenylpropionic acid (1:2) | [138] | |
| Choline chloride-ethylene glycol (1:2) | [133] | |
| Choline chloride-p-toluenesulfonic acid (1:2; 1:2; 2:1) | [134] | |
| [bmim]Cl-urea (1:1; 1:2) | [148,149] | |
| [emim]Cl-urea (1:1; 1:2) | [149,150] | |
| [amim]Cl-urea (1:1) | [149] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, J.; Ruck, M. Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents. Molecules 2020, 25, 78. https://doi.org/10.3390/molecules25010078
Richter J, Ruck M. Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents. Molecules. 2020; 25(1):78. https://doi.org/10.3390/molecules25010078
Chicago/Turabian StyleRichter, Janine, and Michael Ruck. 2020. "Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents" Molecules 25, no. 1: 78. https://doi.org/10.3390/molecules25010078
APA StyleRichter, J., & Ruck, M. (2020). Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents. Molecules, 25(1), 78. https://doi.org/10.3390/molecules25010078






