Deep Blue Light Amplification from a Novel Triphenylamine Functionalized Fluorene Thin Film
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Molecule Synthesis
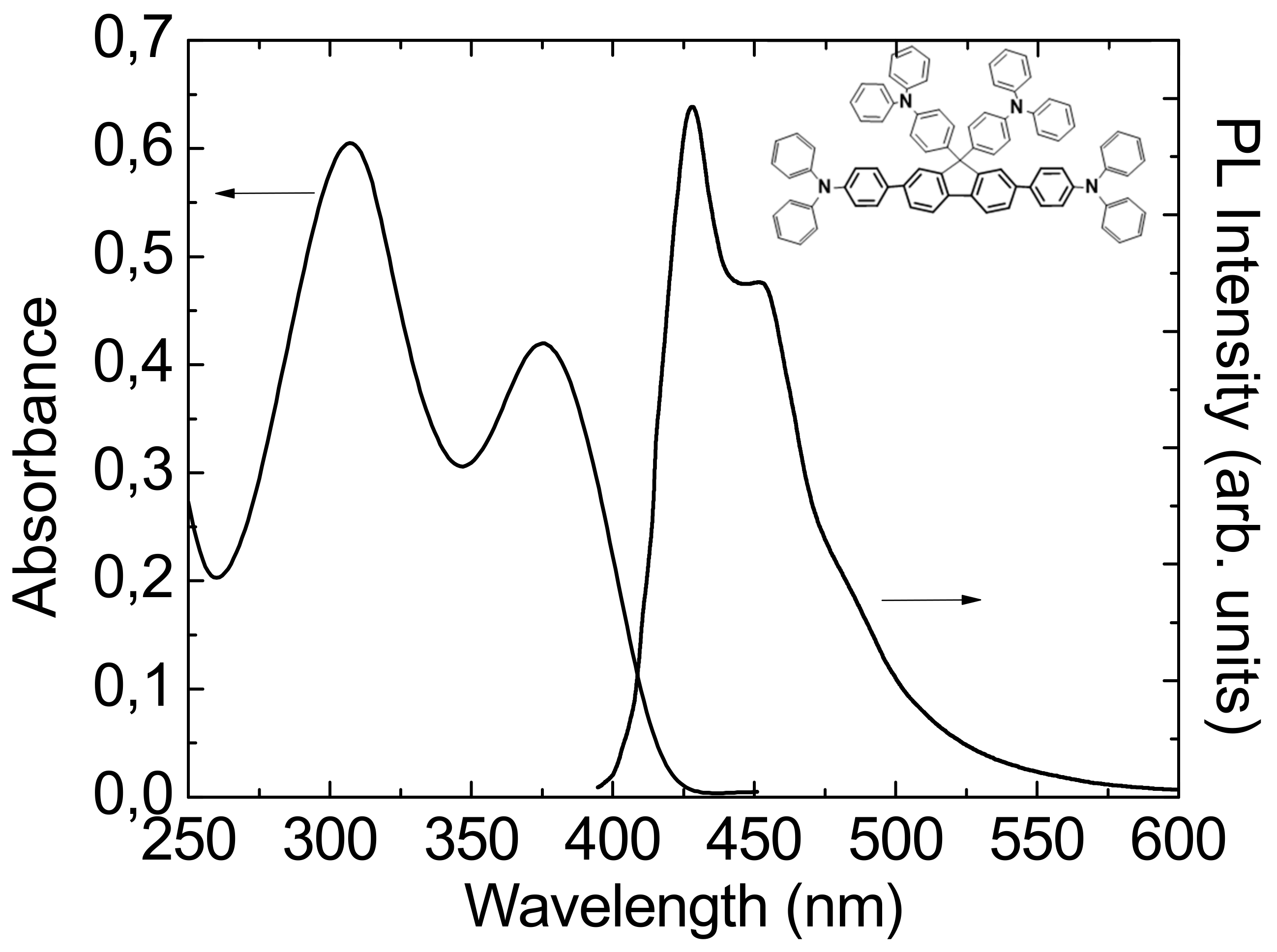
3.2. Steady State Absorption and Photoluminescence Spectra
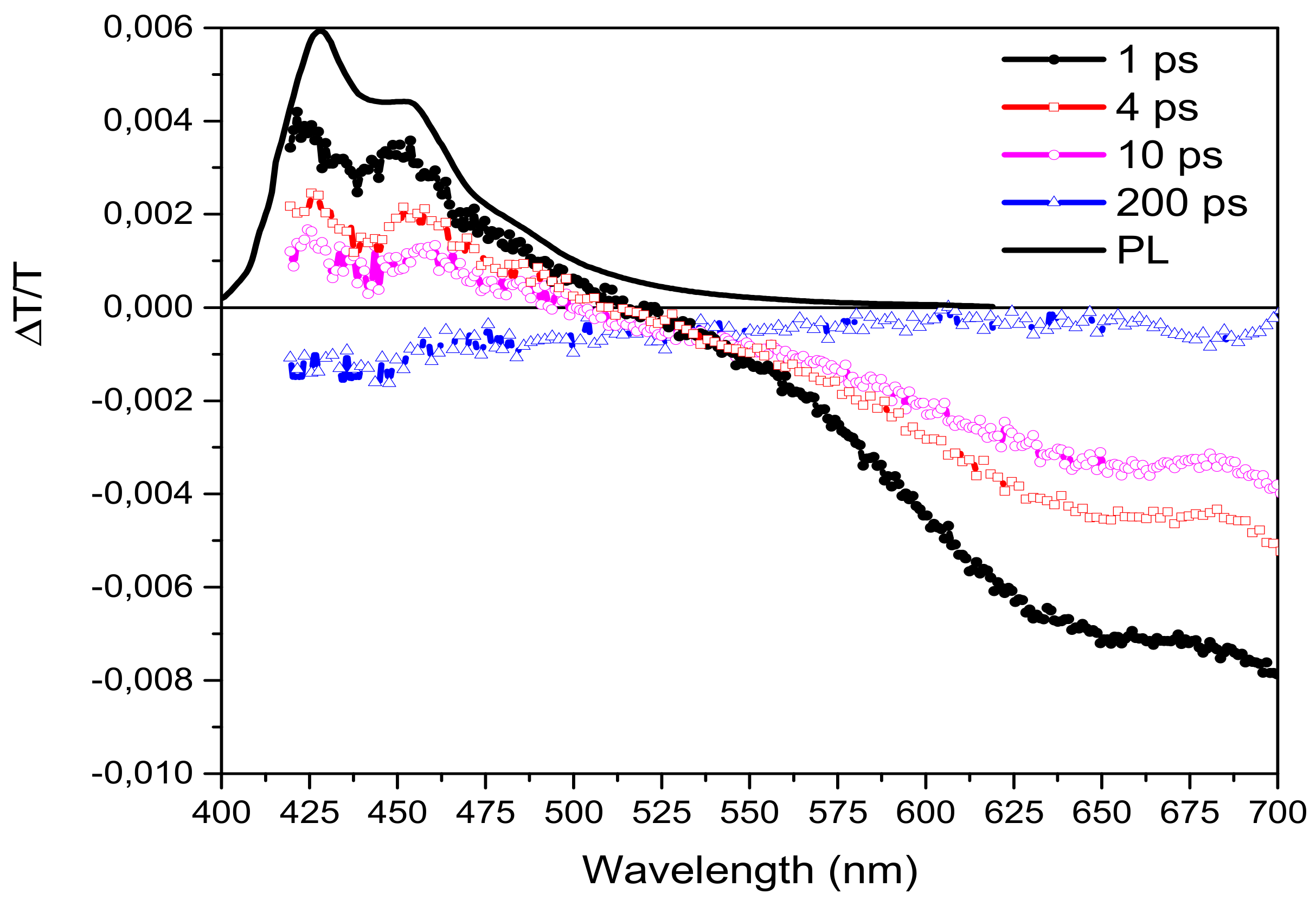
3.3. Ultrafast Spectroscopy
3.4. ASE Measurements with Femtosecond Excitation
3.5. ASE Measurements with Nanosecond Excitation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mei, J.; Diao, Y.; Appleton, A.L.; Fang, L.; Bao, Z. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent Advances in the Development of Semiconducting DPP-Containing Polymers for Transistor Applications. Adv. Mater. 2013, 25, 1859–1880. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Jiang, L.; Dong, H. Organic Micro- and Nano-Crystal Field-Effect Transistors. Prog. Chem. 2013, 25, 12–27. [Google Scholar]
- Vohra, V.; Giovanella, U.; Tubino, R.; Murata, H.; Botta, C. Electroluminescence from Conjugated Polymer Electrospun Nanofibers in Solution Processable Organic Light-Emitting Diodes. ACS Nano 2011, 5, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Tasch, S.; List, E.J.W.; Ekström, O.; Graupner, W.; Leising, G.; Schlichting, P.; Rohr, U.; Geerts, Y.; Scherf, U.; Müllen, K. Efficient white light-emitting diodes realized with new processable blends of conjugated polymers. Appl. Phys. Lett. 1997, 71, 2883–2885. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Morphology of polymer/fullerene bulk heterojunction solar cells. J. Mater. Chem. 2006, 16, 45–61. [Google Scholar] [CrossRef]
- Neugebauer, H.; Loi, M.A.; Winder, C.; Sariciftci, N.S.; Cerullo, G.; Gouloumis, A.; Vázquez, P.; Torres, T. Photophysics and photovoltaic device properties of phthalocyanine–fullerene dyad:conjugated polymer mixtures. Sol. Energy Mater. Sol. Cells 2004, 83, 201–209. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic Semiconductor Lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef]
- Cookson, T.; Georgiou, K.; Zasedatelev, A.; Grant, R.T.; Virgili, T.; Cavazzini, M.; Galeotti, F.; Clark, C.; Berloff, N.G.; Lidzey, D.G.; et al. A Yellow Polariton Condensate in a Dye Filled Microcavity. Adv. Opt. Mater. 2017, 5, 1700203. [Google Scholar] [CrossRef]
- Anni, M. Polymer-II-VI Nanocrystals Blends: Basic Physics and Device Applications to Lasers and LEDs. Nanomaterials 2019, 9, 1036. [Google Scholar] [CrossRef]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Lu, T.; Lai, W.-Y.; Huang, W. Low-Threshold Non-Doped Deep Blue Lasing from Monodisperse Truxene-Cored Conjugated Starbursts with High Photostability. Chem. Asian J. 2019, 14, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Scherf, U.; List, E.J.W. Semiconducting Polyfluorenes—Towards Reliable Structure–Property Relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Leclerc, M. Polyfluorenes: Twenty years of progress. J. Polym. Sci. Part Polym. Chem. 2001, 39, 2867–2873. [Google Scholar] [CrossRef]
- Knaapila, M.; Stepanyan, R.; Lyons, B.P.; Torkkeli, M.; Monkman, A.P. Towards General Guidelines for Aligned, Nanoscale Assemblies of Hairy-Rod Polyfluorene. Adv. Funct. Mater. 2006, 16, 599–609. [Google Scholar] [CrossRef]
- Chen, P.; Yang, G.; Liu, T.; Li, T.; Wang, M.; Huang, W. Optimization of opto-electronic property and device efficiency of polyfluorenes by tuning structure and morphology. Polym. Int. 2006, 55, 473–490. [Google Scholar] [CrossRef]
- Pasini, M.; Giovanella, U.; Betti, P.; Bolognesi, A.; Botta, C.; Destri, S.; Porzio, W.; Vercelli, B.; Zotti, G. The Role of Triphenylamine in the Stabilization of Highly Efficient Polyfluorene-Based OLEDs: A Model Oligomers Study. ChemPhysChem 2009, 10, 2143–2149. [Google Scholar] [CrossRef]
- Neher, D. Polyfluorene Homopolymers: Conjugated Liquid-Crystalline Polymers for Bright Blue Emission and Polarized Electroluminescence. Macromol. Rapid Commun. 2001, 22, 1365–1385. [Google Scholar] [CrossRef]
- Nakamura, T.; Sharma, D.K.; Hirata, S.; Vacha, M. Intrachain Aggregates as the Origin of Green Emission in Polyfluorene Studied on Ensemble and Single-Chain Level. J. Phys. Chem. C 2018, 122, 8137–8146. [Google Scholar] [CrossRef]
- Jen, T.-H.; Wang, K.-K.; Chen, S.-A. Effect of thermal stability on performance of β-phase poly(9,9-di-n-octylfluorene) in deep blue electroluminescence. Polymer 2012, 53, 5850–5855. [Google Scholar] [CrossRef]
- Setayesh, S.; Grimsdale, A.C.; Weil, T.; Enkelmann, V.; Müllen, K.; Meghdadi, F.; List, E.J.W.; Leising, G. Polyfluorenes with Polyphenylene Dendron Side Chains: Toward Non-Aggregating, Light-Emitting Polymers. J. Am. Chem. Soc. 2001, 123, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Jenekhe, S.A. Blue Light-Emitting Diodes with Good Spectral Stability Based on Blends of Poly(9,9-dioctylfluorene): Interplay between Morphology, Photophysics, and Device Performance. Macromolecules 2003, 36, 5285–5296. [Google Scholar] [CrossRef]
- Uckert, F.; Tak, Y.-H.; Müllen, K.; Bässler, H. 2,7-Poly(9-fluorenone): A Trap-Free Electron-Injection Material with a High Charge Carrier Mobility for Use in Light-Emitting Diodes. Adv. Mater. 2000, 12, 905–908. [Google Scholar] [CrossRef]
- Weinfurtner, K.-H.; Fujikawa, H.; Tokito, S.; Taga, Y. Highly efficient pure blue electroluminescence from polyfluorene: Influence of the molecular weight distribution on the aggregation tendency. Appl. Phys. Lett. 2000, 76, 2502–2504. [Google Scholar] [CrossRef]
- Blanchard, P.; Malacrida, C.; Cabanetos, C.; Roncali, J.; Ludwigs, S. Triphenylamine and some of its derivatives as versatile building blocks for organic electronic applications. Polym. Int. 2019, 68, 589–606. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Design and preparation of triphenylamine-based polymeric materials towards emergent optoelectronic applications. Prog. Polym. Sci. 2019, 89, 250–287. [Google Scholar] [CrossRef]
- Kadashchuk, A.; Schmechel, R.; von Seggern, H.; Scherf, U.; Vakhnin, A. Charge-carrier trapping in polyfluorene-type conjugated polymers. J. Appl. Phys. 2005, 98, 024101. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H.; Ishida, M.; Kakimoto, M.; Imai, Y. Synthesis and characterization of novel soluble triphenylamine-containing aromatic polyamides based on N,N′-bis(4-aminophenyl)-N,N′-diphenyl-1,4-phenylenediamine. J. Polym. Sci. A Polym. Chem. 2002, 40, 2810–2818. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H. Synthesis and properties of new soluble triphenylamine-based aromatic poly(amine amide)s derived from N,N′-bis(4-carboxyphenyl)-N,N′-diphenyl-1,4-phenylenediamine. J. Polym. Sci. A Polym. Chem. 2003, 41, 94–105. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, C.-W.; Liou, G.-S. Novel aromatic polyamides bearing pendent diphenylamino or carbazolyl groups. J. Polym. Sci. A Polym. Chem. 2004, 42, 3302–3313. [Google Scholar] [CrossRef]
- Bolis, S.; Pasini, M.; Virgili, T. Ultrafast study of inter- and intrachain energy transfer in a core–polymer. J. Polym. Sci. B Polym. Phys. 2018, 56, 965–969. [Google Scholar] [CrossRef]
- Hu, L.; Liang, J.; Zhong, W.; Guo, T.; Zhong, Z.; Peng, F.; Fan, B.; Ying, L.; Cao, Y. Improving the electroluminescence performance of blue light-emitting poly(fluorene-co-dibenzothiophene-S,S-dioxide) by tuning the intra-molecular charge transfer effects and temperature-induced orientation of the emissive layer structure. J. Mater. Chem. C 2019, 7, 5630–5638. [Google Scholar] [CrossRef]
- Giovanella, U.; Pasini, M.; Destri, S.; Porzio, W.; Botta, C. Stabilized blue emission from polyfluorene-based light-emitting diodes: The role of triphenylamine. Synth. Met. 2008, 158, 113–119. [Google Scholar] [CrossRef]
- Bolognesi, A.; Betti, P.; Botta, C.; Destri, S.; Giovanella, U.; Moreau, J.; Pasini, M.; Porzio, W. From Block Copolymers to End-Capped Polymers: A Suitable Method To Control the Electro-Optical Properties of Polymeric Materials. Macromolecules 2009, 42, 1107–1113. [Google Scholar] [CrossRef]
- Vohra, V.; Calzaferri, G.; Destri, S.; Pasini, M.; Porzio, W.; Botta, C. Toward White Light Emission through Efficient Two-Step Energy Transfer in Hybrid Nanofibers. ACS Nano 2010, 4, 1409–1416. [Google Scholar] [CrossRef]
- Giovanella, U.; Betti, P.; Bolognesi, A.; Destri, S.; Melucci, M.; Pasini, M.; Porzio, W.; Botta, C. Core-type polyfluorene-based copolymers for low-cost light-emitting technologies. Org. Electron. 2010, 11, 2012–2018. [Google Scholar] [CrossRef]
- Giovanella, U.; Betti, P.; Botta, C.; Destri, S.; Moreau, J.; Pasini, M.; Porzio, W.; Vercelli, B.; Bolognesi, A. All-Conjugated Diblock Copolymer Approach To Improve Single Layer Green Electroluminescent Devices. Chem. Mater. 2011, 23, 810–816. [Google Scholar] [CrossRef]
- Ma, L.; Wu, Z.; Lei, T.; Yu, Y.; Yuan, F.; Ning, S.; Jiao, B.; Hou, X. Theoretical insight into the deep-blue amplified spontaneous emission of new organic semiconductor molecules. Org. Electron. 2014, 15, 3144–3153. [Google Scholar] [CrossRef]
- Svelto, O. Principles of Lasers, 5th ed.; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-1301-2. [Google Scholar]
- Xia, H.; Hu, C.; Chen, T.; Hu, D.; Zhang, M.; Xie, K. Advances in Conjugated Polymer Lasers. Polymers 2019, 11, 443. [Google Scholar] [CrossRef]
- Riedl, T.; Rabe, T.; Johannes, H.-H.; Kowalsky, W.; Wang, J.; Weimann, T.; Hinze, P.; Nehls, B.; Farrell, T.; Scherf, U. Tunable organic thin-film laser pumped by an inorganic violet diode laser. Appl. Phys. Lett. 2006, 88, 241116. [Google Scholar] [CrossRef]
- Yang, Y.; Turnbull, G.A.; Samuel, I.D.W. Hybrid optoelectronics: A polymer laser pumped by a nitride light-emitting diode. Appl. Phys. Lett. 2008, 92, 163306. [Google Scholar] [CrossRef]
- Lattante, S.; Cretí, A.; Lomascolo, M.; Anni, M. On the correlation between morphology and Amplified Spontaneous Emission properties of a polymer: Polymer blend. Org. Electron. 2016, 29, 44–49. [Google Scholar] [CrossRef]
- Sorek, Y.; Reisfeld, R.; Finkelstein, I.; Ruschin, S. Light amplification in a dye-doped glass planar waveguide. Appl. Phys. Lett. 1995, 66, 1169–1171. [Google Scholar] [CrossRef]
- Lahoz, F.; Oton, C.J.; Capuj, N.; Ferrer-González, M.; Cheylan, S.; Navarro-Urrios, D. Reduction of the amplified spontaneous emission threshold in semiconducting polymer waveguides on porous silica. Opt. Express 2009, 17, 16766–16775. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Tsiminis, G.; Richardson, S.; Turnbull, G.A.; Samuel, I.D.W.; Barcena, H.S.; Burn, P.L. Amplified spontaneous emission and lasing properties of bisfluorene-cored dendrimers. Appl. Phys. Lett. 2007, 91, 081108. [Google Scholar] [CrossRef]
- Tsiminis, G.; Montgomery, N.A.; Kanibolotsky, A.L.; Ruseckas, A.; Perepichka, I.F.; Skabara, P.J.; Turnbull, G.A.; Samuel, I.D.W. Laser characteristics of a family of benzene-cored star-shaped oligofluorenes. Semicond. Sci. Technol. 2012, 27, 094005. [Google Scholar] [CrossRef]
- McGehee, M.D.; Gupta, R.; Veenstra, S.; Miller, E.K.; Díaz-García, M.A.; Heeger, A.J. Amplified spontaneous emission from photopumped films of a conjugated polymer. Phys. Rev. B 1998, 58, 7035–7039. [Google Scholar] [CrossRef]
- Ego, C.; Grimsdale, A.C.; Uckert, F.; Yu, G.; Srdanov, G.; Müllen, K. Triphenylamine-Substituted Polyfluorene—A Stable Blue-Emitter with Improved Charge Injection for Light-Emitting Diodes. Adv. Mater. 2002, 14, 809–811. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds F4TPA are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgili, T.; Anni, M.; De Giorgi, M.L.; Borrego Varillas, R.; Squeo, B.M.; Pasini, M. Deep Blue Light Amplification from a Novel Triphenylamine Functionalized Fluorene Thin Film. Molecules 2020, 25, 79. https://doi.org/10.3390/molecules25010079
Virgili T, Anni M, De Giorgi ML, Borrego Varillas R, Squeo BM, Pasini M. Deep Blue Light Amplification from a Novel Triphenylamine Functionalized Fluorene Thin Film. Molecules. 2020; 25(1):79. https://doi.org/10.3390/molecules25010079
Chicago/Turabian StyleVirgili, Tersilla, Marco Anni, Maria Luisa De Giorgi, Rocio Borrego Varillas, Benedetta M. Squeo, and Mariacecilia Pasini. 2020. "Deep Blue Light Amplification from a Novel Triphenylamine Functionalized Fluorene Thin Film" Molecules 25, no. 1: 79. https://doi.org/10.3390/molecules25010079
APA StyleVirgili, T., Anni, M., De Giorgi, M. L., Borrego Varillas, R., Squeo, B. M., & Pasini, M. (2020). Deep Blue Light Amplification from a Novel Triphenylamine Functionalized Fluorene Thin Film. Molecules, 25(1), 79. https://doi.org/10.3390/molecules25010079










