Bio-Based Thermo-Reversible Aliphatic Polycarbonate Network
Abstract
1. Introduction
2. Results and Discussion
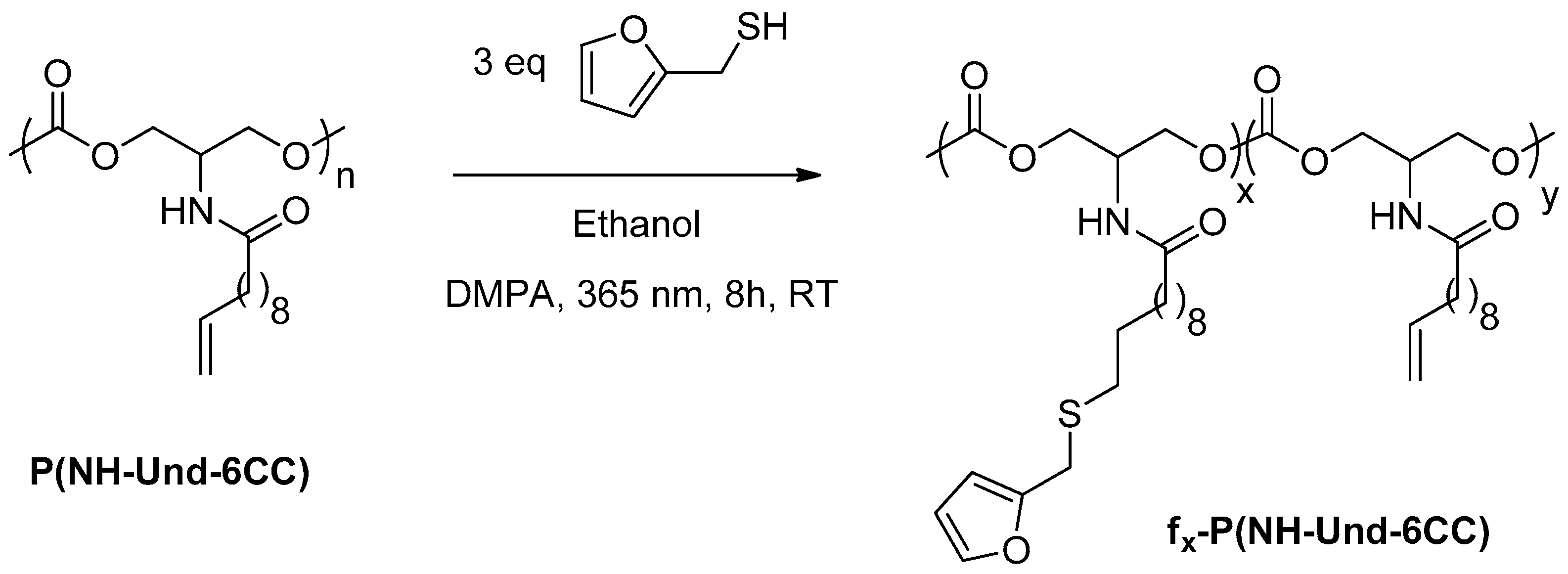
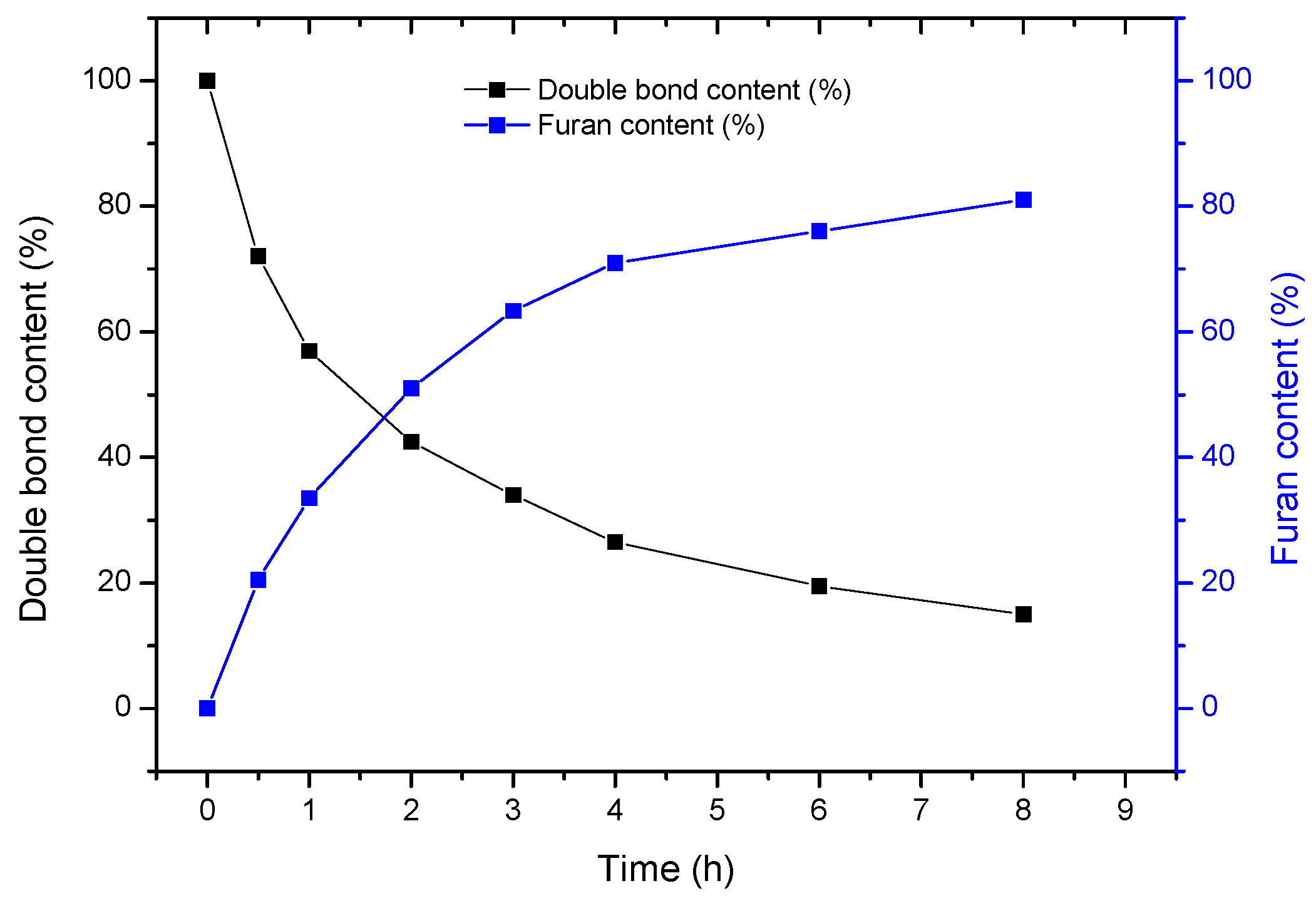
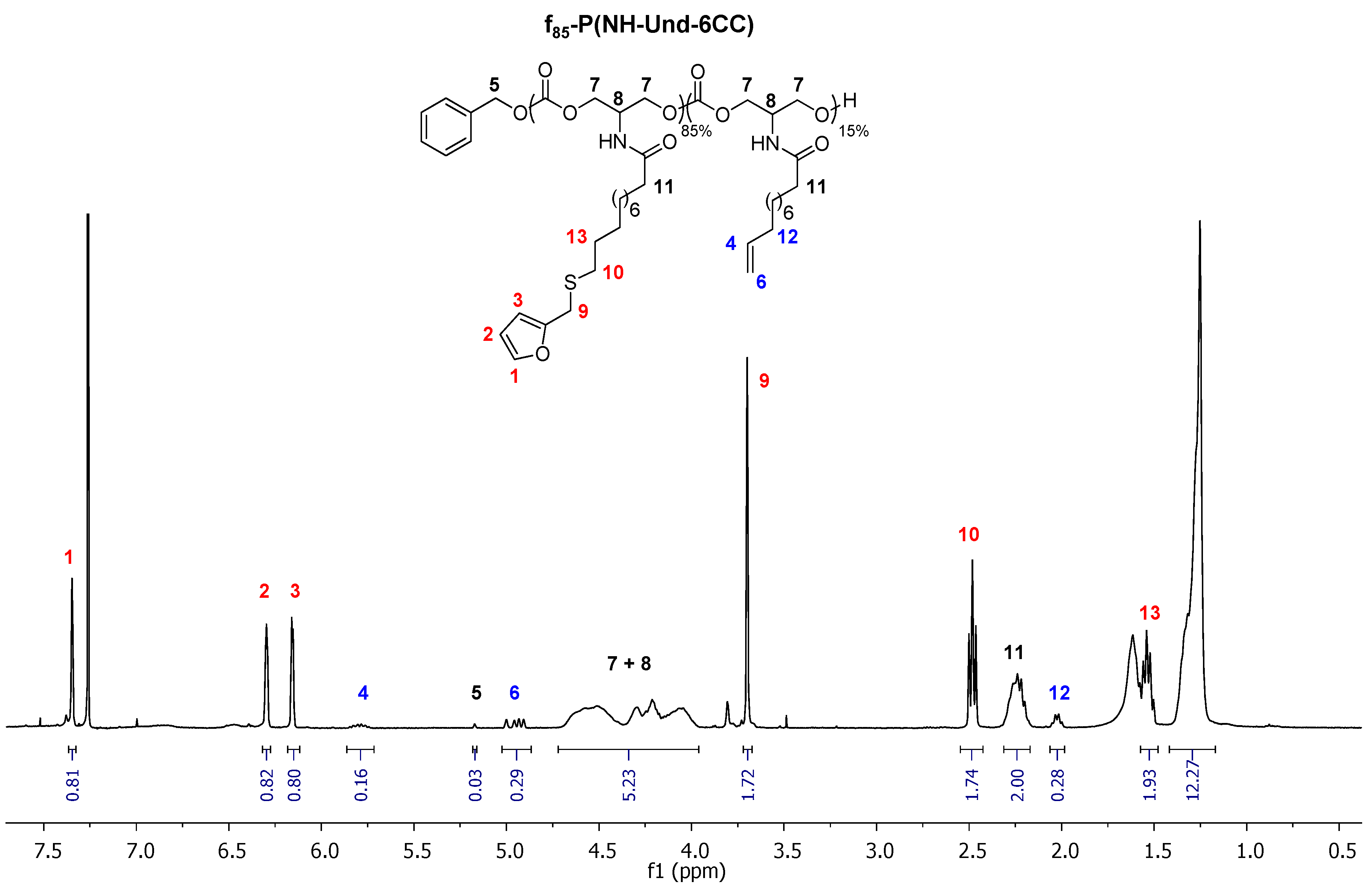
2.1. Grafting of Furan Moieties on the Polymer
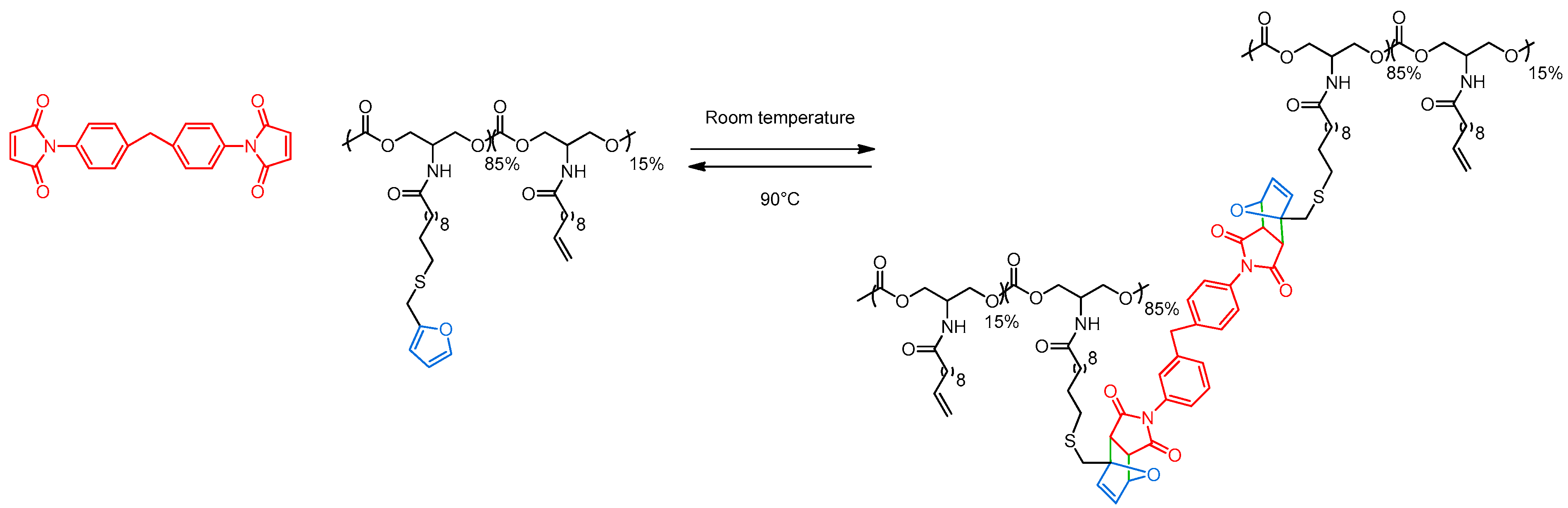
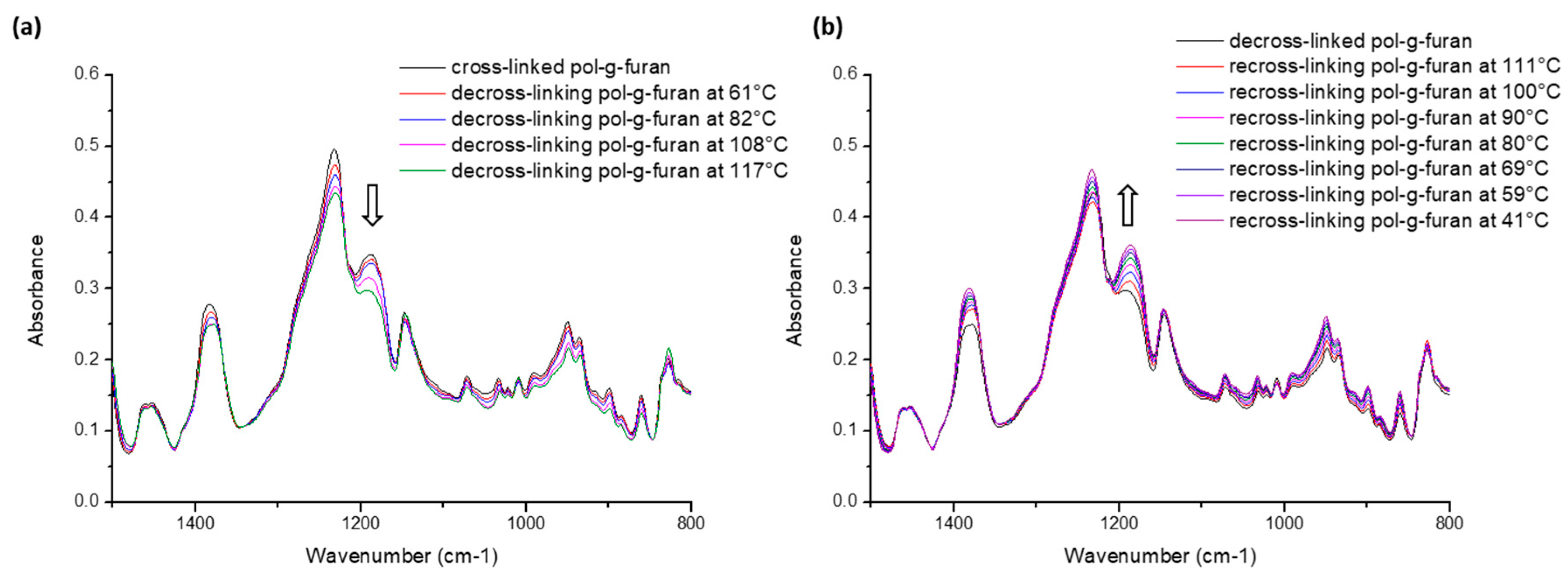
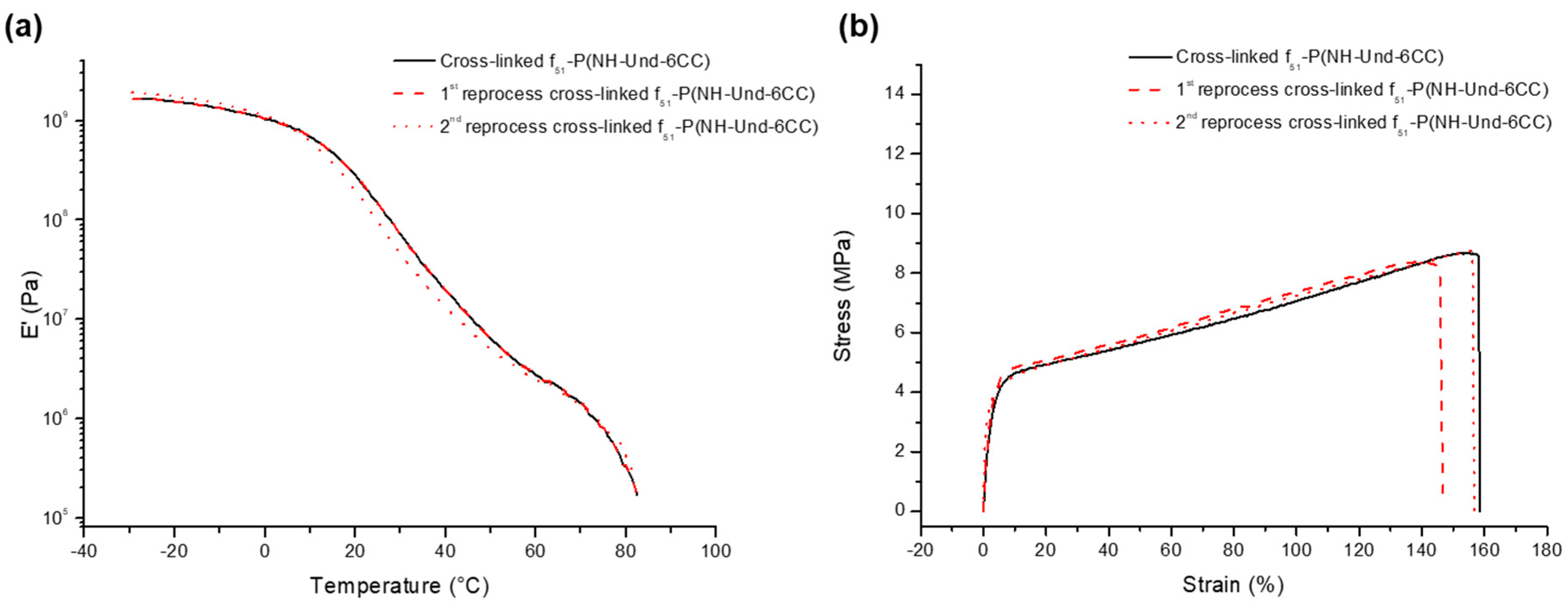
2.2. Reversible Cross-Linking and Reprocessability
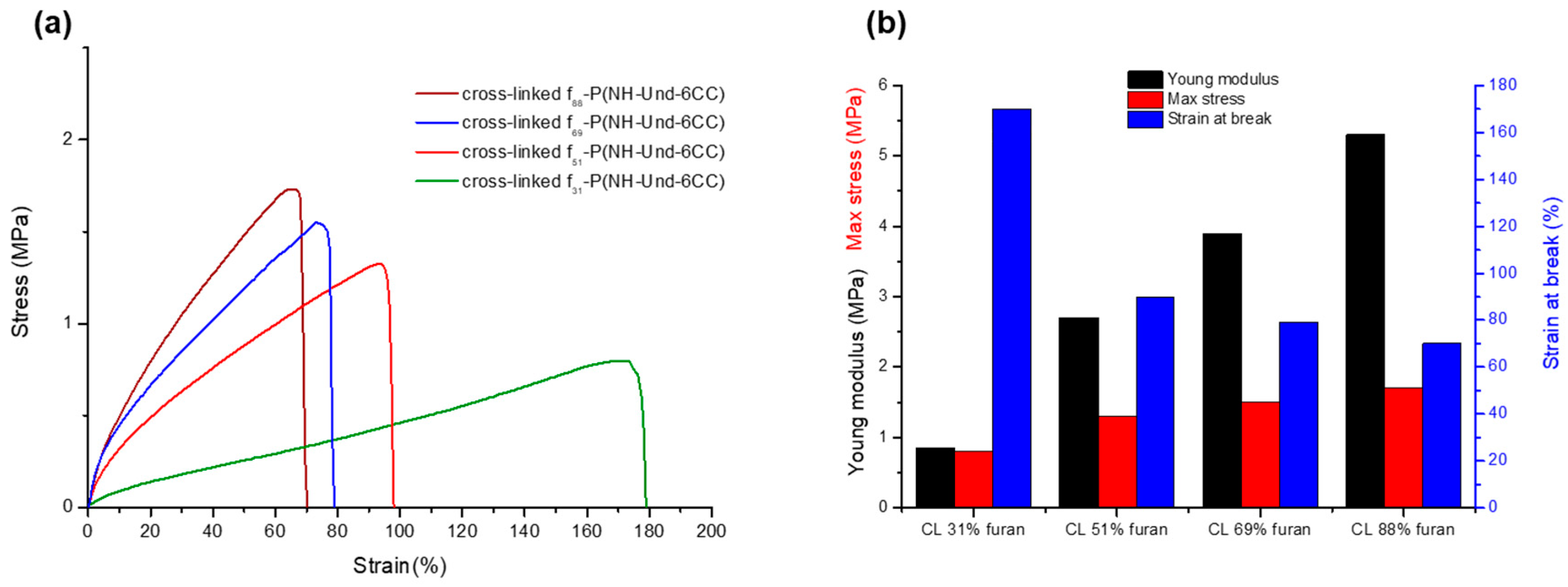
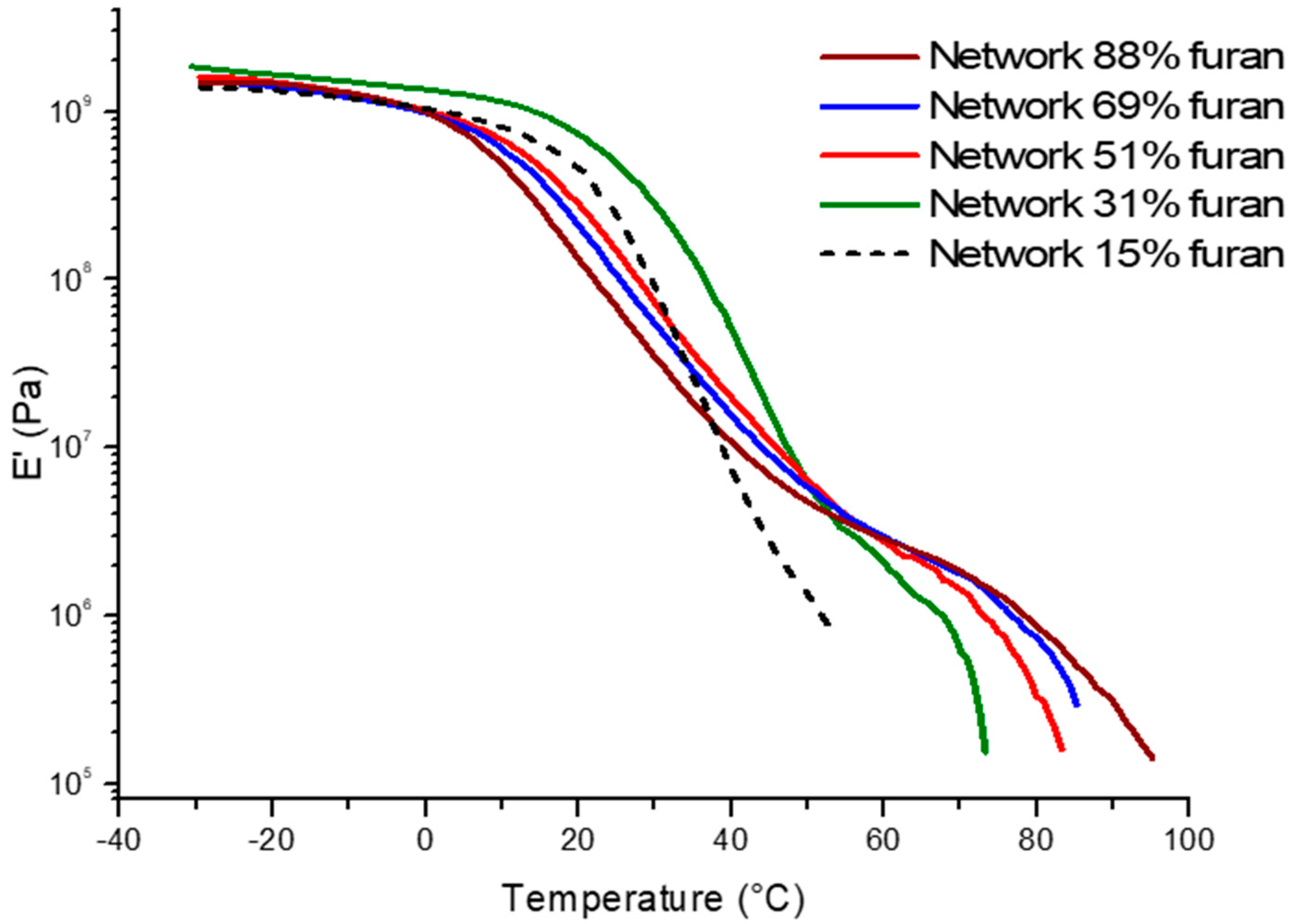
2.3. Tuneable Network Properties
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. General Procedure for the Synthesis of Furan-Functionalized fx-P(NH-Und-6CC)
3.4. General Procedure for Polycarbonates Cross-Linking by Diels-Alder Reaction
3.5. General Procedure for Polycarbonates Decross-Linking by Retro-Diels–Alder Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Artham, T.; Doble, M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G. ROP of Cyclic Carbonates and ROP of Macrocycles. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar]
- Tempelaar, S.; Mespouille, L.; Coulembier, O.; Dubois, P.; Dove, A.P. Synthesis and post-polymerisation modifications of aliphatic poly(carbonate)s prepared by ring-opening polymerisation. Chem. Soc. Rev. 2013, 42, 1312–1336. [Google Scholar] [CrossRef] [PubMed]
- Montero De Espinosa, L.; Meier, M.A.R.; De Espinosa, L.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science? Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Martina, M.; Hutmacher, D.W. Biodegradable polymers applied in tissue engineering research: A review. Polym. Int. 2007, 56, 145–157. [Google Scholar] [CrossRef]
- Amsden, B. Curable, biodegradable elastomers: Emerging biomaterials for drug delivery and tissue engineering. Soft Matter 2007, 3, 1335. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Biodegradable elastomeric scaffolds for soft tissue engineering. J. Control. Release 2003, 87, 69–79. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater. Sci. 2016, 4, 9–24. [Google Scholar] [CrossRef]
- Feng, J.; Zhuo, R.X.; Zhang, X.Z. Construction of functional aliphatic polycarbonates for biomedical applications. Prog. Polym. Sci. 2012, 37, 211–236. [Google Scholar] [CrossRef]
- Pascual, A.; Tan, J.P.K.; Yuen, A.; Chan, J.M.W.; Coady, D.J.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y.; Sardon, H. Broad-Spectrum Antimicrobial Polycarbonate Hydrogels with Fast Degradability Biomacromolecules. Biomacromolecules 2015, 16, 1169–1178. [Google Scholar] [CrossRef]
- Yuen, A.Y.; Lopez-Martinez, E.; Gomez-Bengoa, E.; Cortajarena, A.L.; Aguirresarobe, R.H.; Bossion, A.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y.; Sardon, H. Preparation of Biodegradable Cationic Polycarbonates and Hydrogels through the Direct Polymerization of Quaternized Cyclic Carbonates. ACS Biomater. Sci. Eng. 2017, 3, 1567–1575. [Google Scholar] [CrossRef]
- Martín, C.; Kleij, A.W. Terpolymers Derived from Limonene Oxide and Carbon Dioxide: Access to Cross-Linked Polycarbonates with Improved Thermal Properties. Macromolecules 2016, 49, 6285–6295. [Google Scholar] [CrossRef]
- Stevens, D.M.; Tempelaar, S.; Dove, A.P.; Harth, E. Nanosponge Formation from Organocatalytically Synthesized Poly(carbonate) Copolymers. ACS Macro Lett. 2012, 1, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Schüller-Ravoo, S.; Feijen, J.; Grijpma, D.W. Preparation of Flexible and Elastic Poly(trimethylene carbonate) Structures by Stereolithography. Macromol. Biosci. 2011, 11, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Durand, P.-L.; Chollet, G.; Grau, E.; Cramail, H. Versatile cross-linked fatty acid-based polycarbonate networks obtained by thiol–ene coupling reaction. RSC Adv. 2019, 9, 145–150. [Google Scholar] [CrossRef]
- Guimard, N.K.; Oehlenschlaeger, K.K.; Zhou, J.; Hilf, S.; Schmidt, F.G.; Barner-Kowollik, C. Current Trends in the Field of Self-Healing Materials. Macromol. Chem. Phys. 2012, 213, 131–143. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Billiet, S.; Hillewaere, X.K.D.; Teixeira, R.F.A.; Du Prez, F.E. Chemistry of crosslinking processes for self-healing polymers. Macromol. Rapid Commun. 2013, 34, 290–309. [Google Scholar] [CrossRef]
- Bekas, D.G.; Tsirka, K.; Baltzis, D.; Paipetis, A.S. Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring techniques. Compos. Part B Eng. 2016, 87, 92–119. [Google Scholar] [CrossRef]
- Durand, P.-L.; Chollet, G.; Grau, E.; Cramail, H. Simple and Efficient Approach toward Photosensitive Biobased Aliphatic Polycarbonate Materials. ACS Macro. Lett. 2018, 7, 250–254. [Google Scholar] [CrossRef]
- The Top 10 Emerging Technologies for 2013. Available online: https://www.weforum.org/agenda/2013/02/top-10-emerging-technologies-for-2013/ (accessed on 19 November 2019).
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Bai, N.; Saito, K.; Simon, G.P. Synthesis of a diamine cross-linker containing Diels–Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym. Chem. 2013, 4, 724–730. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Gandini, A. Thermoreversible nonlinear Diels-Alder polymerization of furan/plant oil monomers. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2260–2270. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, Y.; Huang, C.; Xiao, L.; Chen, Z.; Yang, K.; Wang, Y. Self-healable and recyclable triple-shape PPDO–PTMEG co-network constructed through thermoreversible Diels–Alder reaction. Polym. Chem. 2012, 3, 1390–1393. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels–Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Gheneim, R.; Perez-Berumen, C.; Gandini, A. Diels–Alder Reactions with Novel Polymeric Dienes and Dienophiles: Synthesis of Reversibly Cross-Linked Elastomers. Macromolecules 2002, 35, 7246–7253. [Google Scholar] [CrossRef]
- Diaz, M.M.; Van Assche, G.; Maurer, F.H.J.; Van Mele, B. Thermophysical characterization of a reversible dynamic polymer network based on kinetics and equilibrium of an amorphous furan-maleimide Diels-Alder cycloaddition. Polymer 2017, 120, 176–188. [Google Scholar] [CrossRef]
- Polgar, L.M.; Van Duin, M.; Broekhuis, A.A.; Picchioni, F. Use of Diels–Alder Chemistry for Thermoreversible Cross-Linking of Rubbers: The Next Step toward Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Defize, T.; Riva, R.; Thomassin, J.-M.; Jérôme, C.; Alexandre, M. Thermo-Reversible Reactions for the Preparation of Smart Materials: Recyclable Covalently-Crosslinked Shape Memory Polymers. Macromol. Symp. 2011, 309–310, 154–161. [Google Scholar] [CrossRef]
- Roper, T.M.; Guymon, C.A.; Jönsson, E.S.; Hoyle, C.E. Influence of the alkene structure on the mechanism and kinetics of thiol–alkene photopolymerizations with real-time infrared spectroscopy. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6283–6298. [Google Scholar] [CrossRef]


| Polymer | Reaction Time (h) | Furan Content a (mol%) | Mn b (g mol−1) | [Ɖ] b | Tg (°C) c |
|---|---|---|---|---|---|
| P(NH-Und-6CC) | 0 | 0 | 5700 | 1.07 | 23.1 |
| f31-P(NH-Und-6CC) | 1 | 31 | 6200 | 1.15 | −6.6 |
| f51-P(NH-Und-6CC) | 2 | 51 | 6650 | 1.12 | −13.5 |
| f69-P(NH-Und-6CC) | 4 | 69 | 7300 | 1.19 | −49.0 |
| f88-P(NH-Und-6CC) | 12 | 88 | 7850 | 1.22 | −59.6 |
| Performed at RT | Performed at 65 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| Furan Content a | Td(5%) fx-Network (°C) b | Tg fx-Network (°C) c | Young Modulus (MPa) d | Young Modulus (MPa) e | Max Stress (MPa) e | Elongation at Break (%) e | Gel Content (%) | Swelling Ratio (%) |
| 88% | 108 | 4.6 | 31 ± 2 | 5.3 | 1.7 | 70 | 96.5 | 238 |
| 69% | 107 | 6.8 | 43 ± 2 | 3.9 | 1.5 | 79 | 93.7 | 296 |
| 51% | 110 | 13.4 | 90 ± 15 | 2.7 | 1.3 | 98 | 96 | 318 |
| 31% | 112 | 10.2 | 230 ± 70 | 0.85 | 0.8 | 170 | 71 | 765 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durand, P.-L.; Grau, E.; Cramail, H. Bio-Based Thermo-Reversible Aliphatic Polycarbonate Network. Molecules 2020, 25, 74. https://doi.org/10.3390/molecules25010074
Durand P-L, Grau E, Cramail H. Bio-Based Thermo-Reversible Aliphatic Polycarbonate Network. Molecules. 2020; 25(1):74. https://doi.org/10.3390/molecules25010074
Chicago/Turabian StyleDurand, Pierre-Luc, Etienne Grau, and Henri Cramail. 2020. "Bio-Based Thermo-Reversible Aliphatic Polycarbonate Network" Molecules 25, no. 1: 74. https://doi.org/10.3390/molecules25010074
APA StyleDurand, P.-L., Grau, E., & Cramail, H. (2020). Bio-Based Thermo-Reversible Aliphatic Polycarbonate Network. Molecules, 25(1), 74. https://doi.org/10.3390/molecules25010074





