The Effect of Calcium on the Cohesive Strength and Flexural Properties of Low-Methoxyl Pectin Biopolymers
Abstract
1. Introduction
2. Results
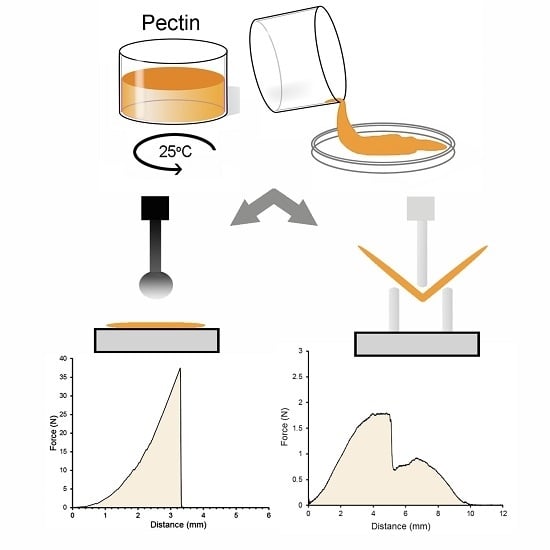
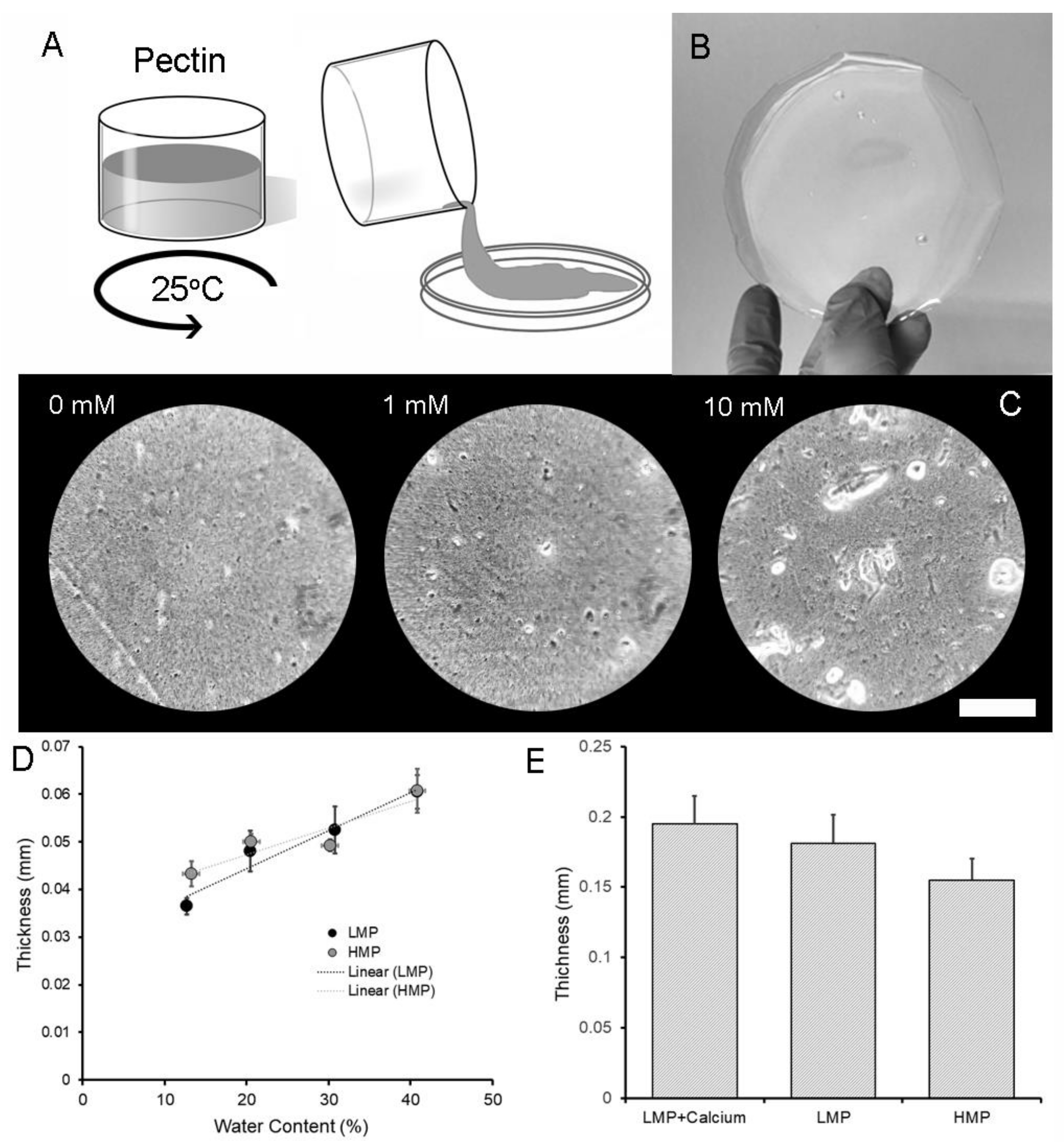
2.1. LMP and HMP Polymer Films
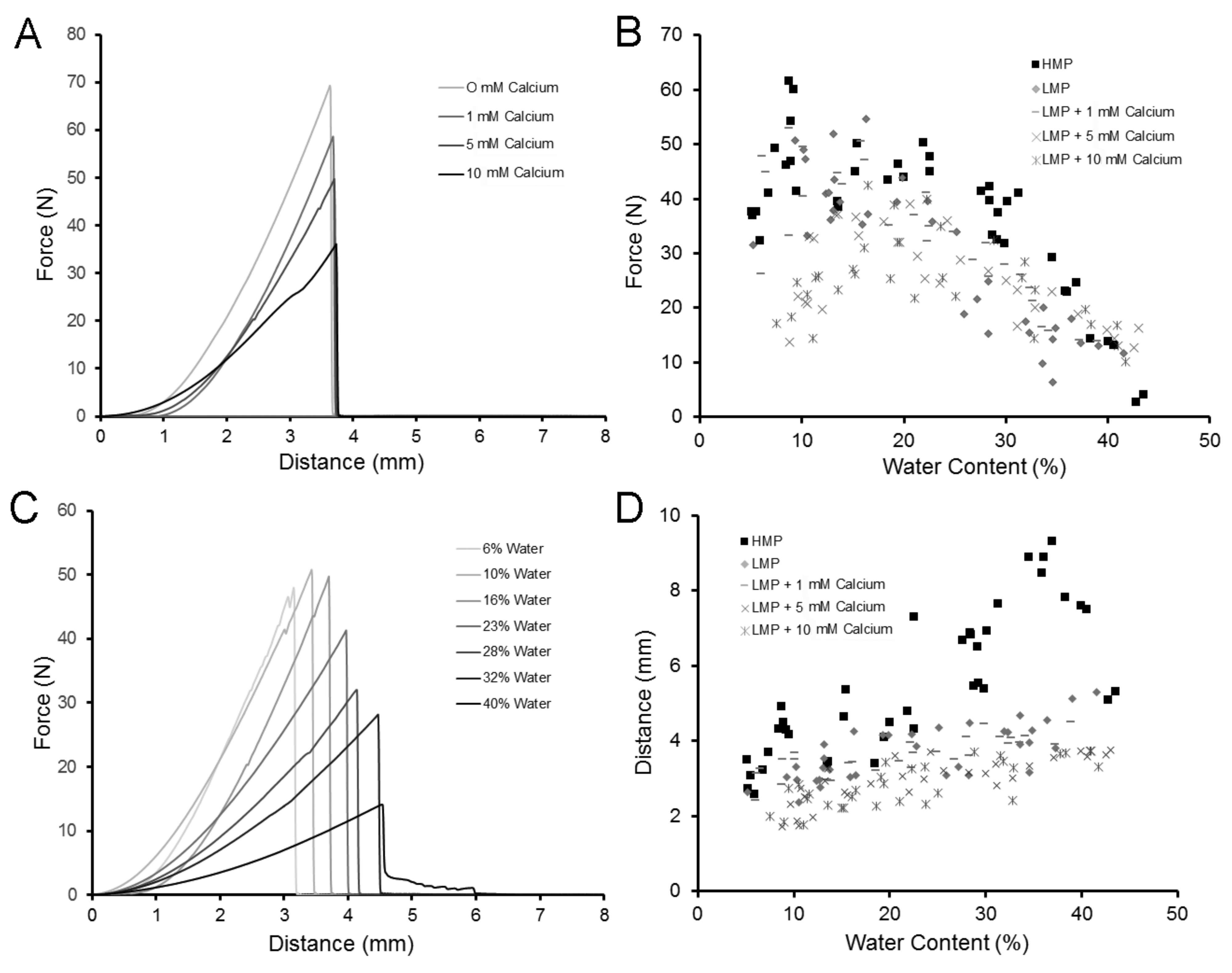
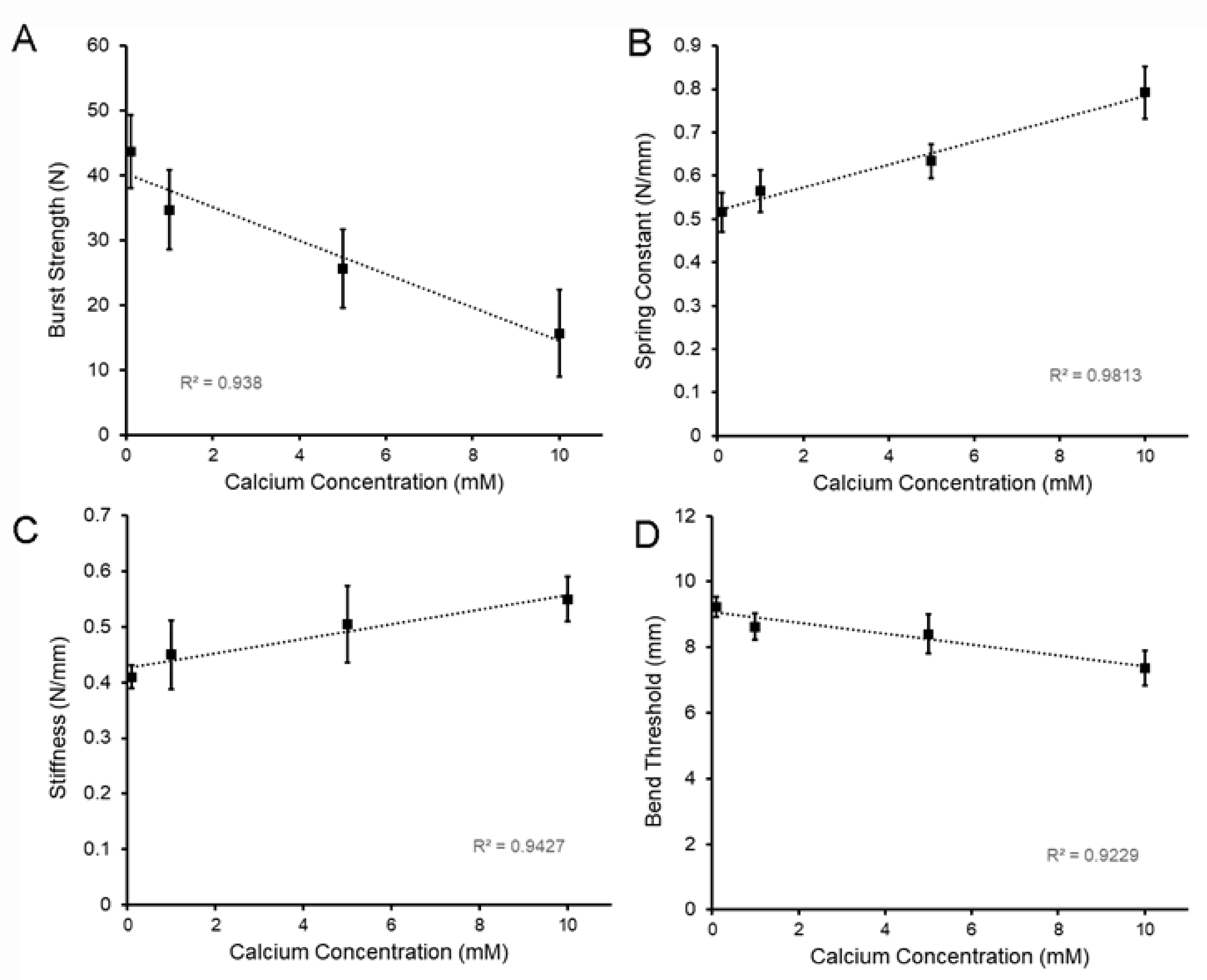
2.2. Strength and Extensibility of LMP Films
2.3. Flexural Properties of LMP Films
2.4. Physical Properties of LMP Films
3. Discussion
4. Methods
4.1. Pectin
4.2. Pectin Dissolution in Water
4.3. Humidification Chamber
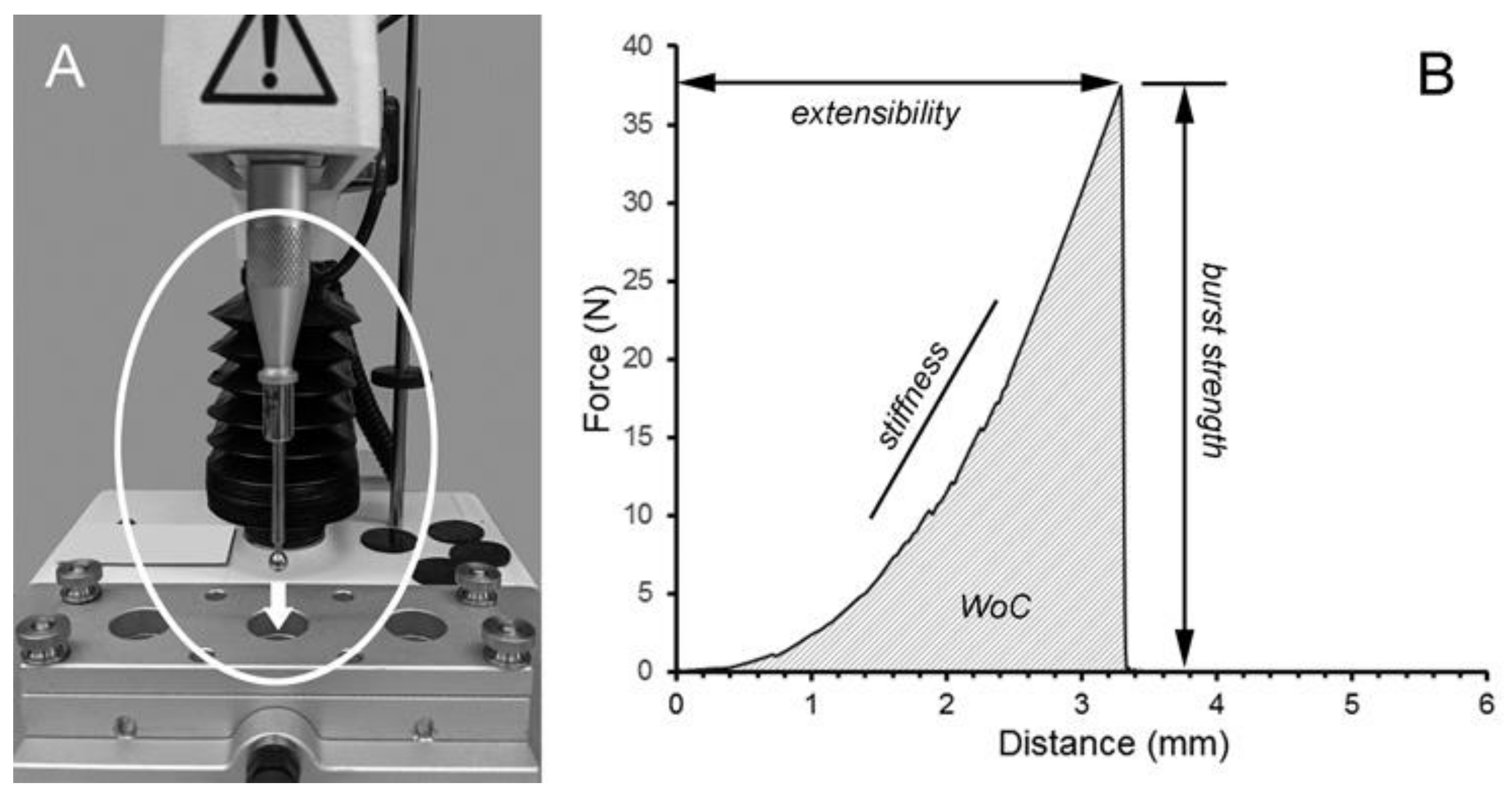
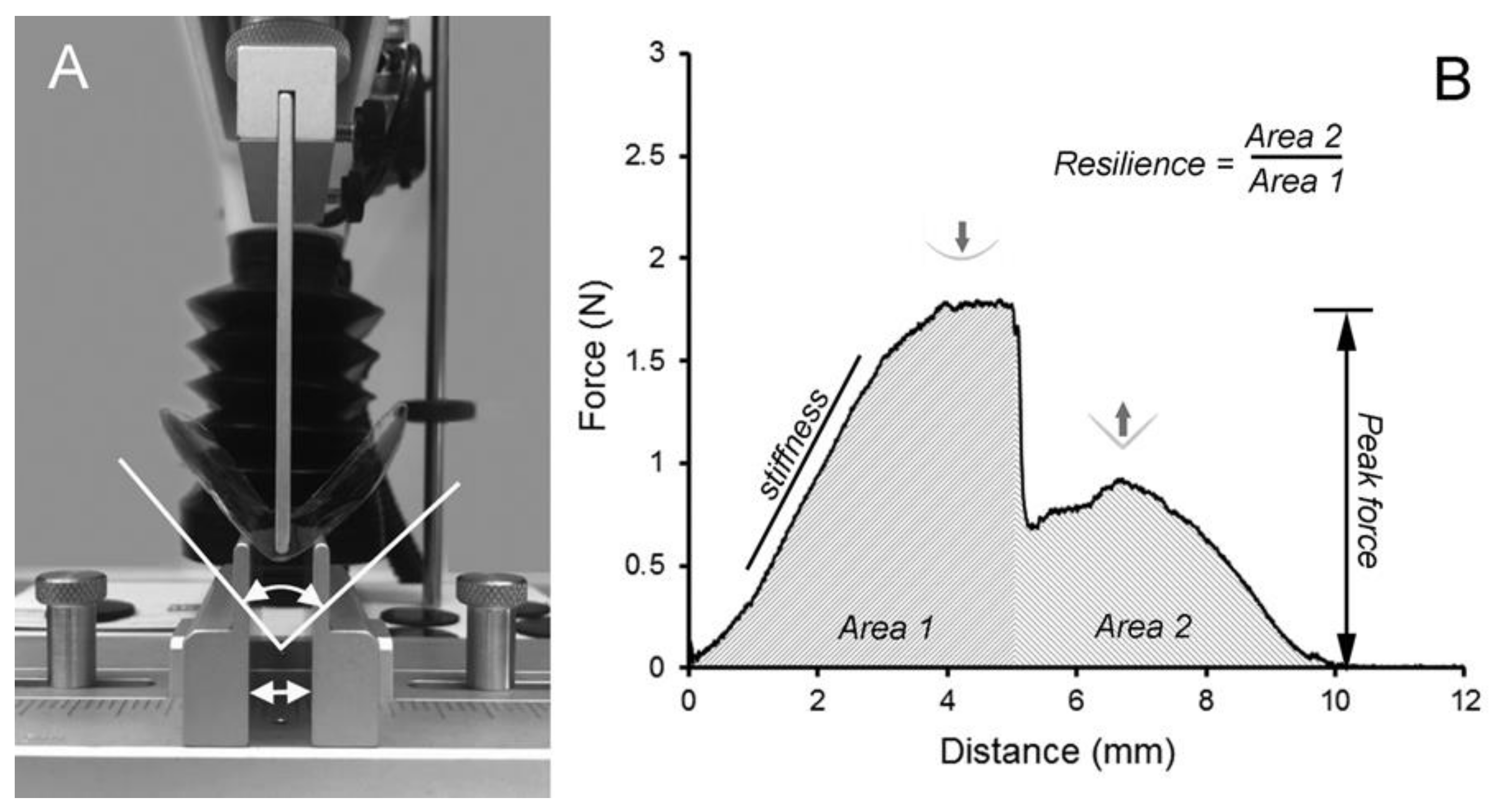
4.4. Adhesion Testing
4.5. Film Thickness
4.6. Fracture Mechanics
4.7. Bend Properties of Pectin Films.
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HMP | high-methoxyl pectin |
| LMP | low-methoxyl pectin |
| Wc | water content |
| WoC | work of cohesion |
| SD | standard deviation |
References
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011, 7, 152–162. [Google Scholar] [CrossRef]
- Pooyan, P.; Tannenbaum, R.; Garmestani, H. Mechanical behavior of a cellulose-reinforced scaffold in vascular tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 7, 50–59. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Srinivasan, S.; Lakshmanan, V.-K.; Tamura, H.; Nair, S.V.; Jayakumar, R. Beta-Chitin hydrogel/nano hydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 85, 584–591. [Google Scholar] [CrossRef]
- Khanarian, N.T.; Haney, N.M.; Burga, R.A.; Lu, H.H. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 2012, 33, 5247–5258. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Coimbra, P.; Ferreira, P.; de Sousa, H.C.; Batista, P.; Rodrigues, M.A.; Corriea, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Dreaden, T.M.; Theobald, L.K.; Tran, N.M.; Beal, T.L.; Eid, M.; Gao, M.Y.; Shirley, R.B.; Stoffel, M.T.; Kumar, M.V.; et al. Pectin induces apoptosis in human prostate cancer cells: Correlation of apoptotic function with pectin structure. Glycobiology 2007, 17, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.Y.; Mohnen, D. Evolving Views of Pectin Biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.H.; Hao, Z.Y.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant. Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Villanova, J.C.O.; Ayres, E.; Orefice, R.L. Design, characterization and preliminary in vitro evaluation of a mucoadhesive polymer based on modified pectin and acrylic monomers with potential use as a pharmaceutical excipient. Carbohydr. Polym. 2015, 121, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.B.; Kienzle, A.; Ysasi, A.B.; Valenzuela, C.D.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharides as air-tight sealants of the human pleura. J. Biol. Mater. Res. 2018, 107, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rolin, C.; Nielsen, B.U.; Glahn, P.-E. Pectin. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Oakenfull, D.G. The Chemistry of High-Methoxyl Pectins. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 87–108. [Google Scholar]
- Axelos, M.A.V.; Thibault, J.F. The Chemistry of Low-Methoxyl Pectin Gelation. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 109–118. [Google Scholar]
- Basu, S.; Shivhare, U.S.; Muley, S. Moisture adsorption isotherms and glass transition temperature of pectin. J. Food Sci. Technol. Mysore 2013, 50, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, C.; Hermansson, A.M. Synergistic rheological behaviour of mixed HM/LM pectin gels. Food Hydrocoll. 2007, 21, 480–486. [Google Scholar] [CrossRef]
- Capel, F.; Nicolai, T.; Durand, D. Influence of chain length and polymer concentration on the gelation of (amidated) low-methoxyl pectin induced by calcium. Biomacromolecules 2005, 6, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Powell, D.A.; Gidley, M.J.; Rees, D.A. Conformations and interactions of pectins: 1. Polymorphism between gel and solid states of calcium polygalacturonate. J. Mol. Biol. 1982, 155, 507–516. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations—Egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Sriamornsak, P. Application of pectin in oral drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef]
- Sousa, A.G.; Nielsen, H.L.; Armagan, I.; Larsen, J.; Sorensen, S.O. The impact of rhamnogalacturonan-I side chain monosaccharides on the rheological properties of citrus pectin. Food Hydrocoll. 2015, 47, 130–139. [Google Scholar] [CrossRef]
- Braccini, I.; Perez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Plazinski, W. Molecular Basis of Calcium Binding by Polyguluronate Chains. Revising the Egg-Box Model. J. Comput. Chem. 2011, 32, 2988–2995. [Google Scholar] [CrossRef]
- Daas, P.J.H.; Boxma, B.; Hopman, A.; Voragen, A.G.J.; Schols, H.A. Nonesterified galacturonic acid sequence homology of pectins. Biopolymers 2001, 58, 1–8. [Google Scholar] [CrossRef]
- Sperber, B.; Schols, H.A.; Stuart, M.A.C.; Norde, W.; Voragen, A.G.J. Influence of the overall charge and local charge density of pectin on the complex formation between pectin and beta-lactoglobulin. Food Hydrocoll. 2009, 23, 765–772. [Google Scholar] [CrossRef]
- Vithanage, C.R.; Grimson, M.J.; Wills, P.R.; Harrison, P.; Smith, B.G. Rheological and structural properties of high-methoxyl esterified, low-methoxyl esterified and low-methoxyl amidated pectin gels. J. Texture Stud. 2010, 41, 899–927. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Van de Walle, D.; Van Loey, A.M.; Dewettinck, K.; Hendrickx, M.E. Evaluation of cation-facilitated pectin-gel properties: Cryo-SEM visualisation and rheological properties. Food Hydrocoll. 2016, 61, 172–182. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Munyensanga, C.; Celus, M.; Van de Walle, D.; Dewettinck, K.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Molar mass influence on pectin-Ca2+ adsorption capacity, interaction energy and associated functionality: Gel microstructure and stiffness. Food Hydrocoll. 2018, 85, 331–342. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of Pectin Structural Properties on Interactions with Divalent Cations and Its Associated Functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J. Biol. Mat. Res. Part A 2019. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A. The general mechanism of water sorption on foodstuffs—Importance of the multitemperature fitting of data and the hierarchy of models. J. Food Eng. 2007, 82, 528–535. [Google Scholar] [CrossRef]
- Zheng, Y.; Pierce, A.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Analysis of pectin biopolymer phase states using acoustic emissions. Carbohydr. Polym. 2019, 227, 115282. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, C.; Zheng, Y.; Pierce, A.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Ackermann, M.; Mentzer, S.J. The Effect of Calcium on the Cohesive Strength and Flexural Properties of Low-Methoxyl Pectin Biopolymers. Molecules 2020, 25, 75. https://doi.org/10.3390/molecules25010075
Byun C, Zheng Y, Pierce A, Wagner WL, Scheller HV, Mohnen D, Ackermann M, Mentzer SJ. The Effect of Calcium on the Cohesive Strength and Flexural Properties of Low-Methoxyl Pectin Biopolymers. Molecules. 2020; 25(1):75. https://doi.org/10.3390/molecules25010075
Chicago/Turabian StyleByun, Christine, Yifan Zheng, Aidan Pierce, Willi L. Wagner, Henrik V. Scheller, Debra Mohnen, Maximilian Ackermann, and Steven J. Mentzer. 2020. "The Effect of Calcium on the Cohesive Strength and Flexural Properties of Low-Methoxyl Pectin Biopolymers" Molecules 25, no. 1: 75. https://doi.org/10.3390/molecules25010075
APA StyleByun, C., Zheng, Y., Pierce, A., Wagner, W. L., Scheller, H. V., Mohnen, D., Ackermann, M., & Mentzer, S. J. (2020). The Effect of Calcium on the Cohesive Strength and Flexural Properties of Low-Methoxyl Pectin Biopolymers. Molecules, 25(1), 75. https://doi.org/10.3390/molecules25010075