Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review
Abstract
1. Introduction
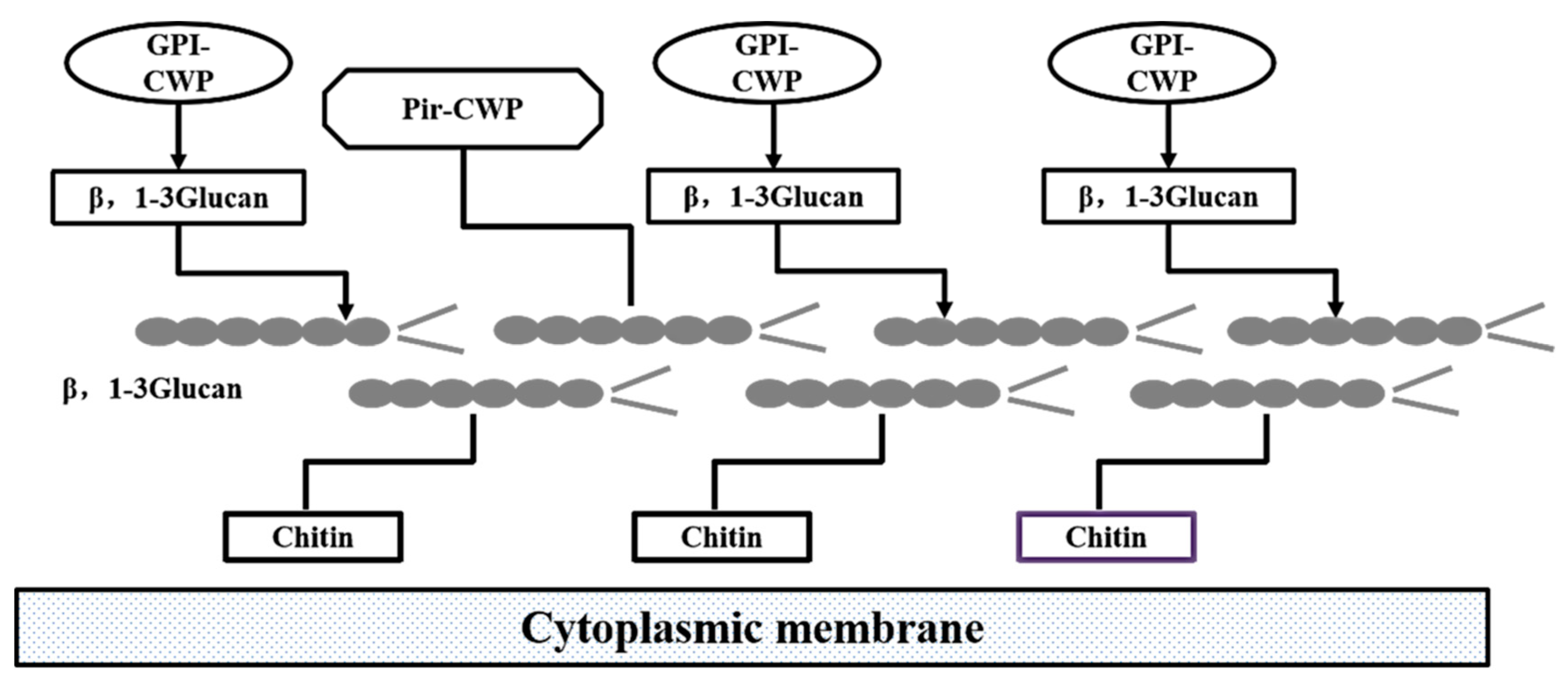
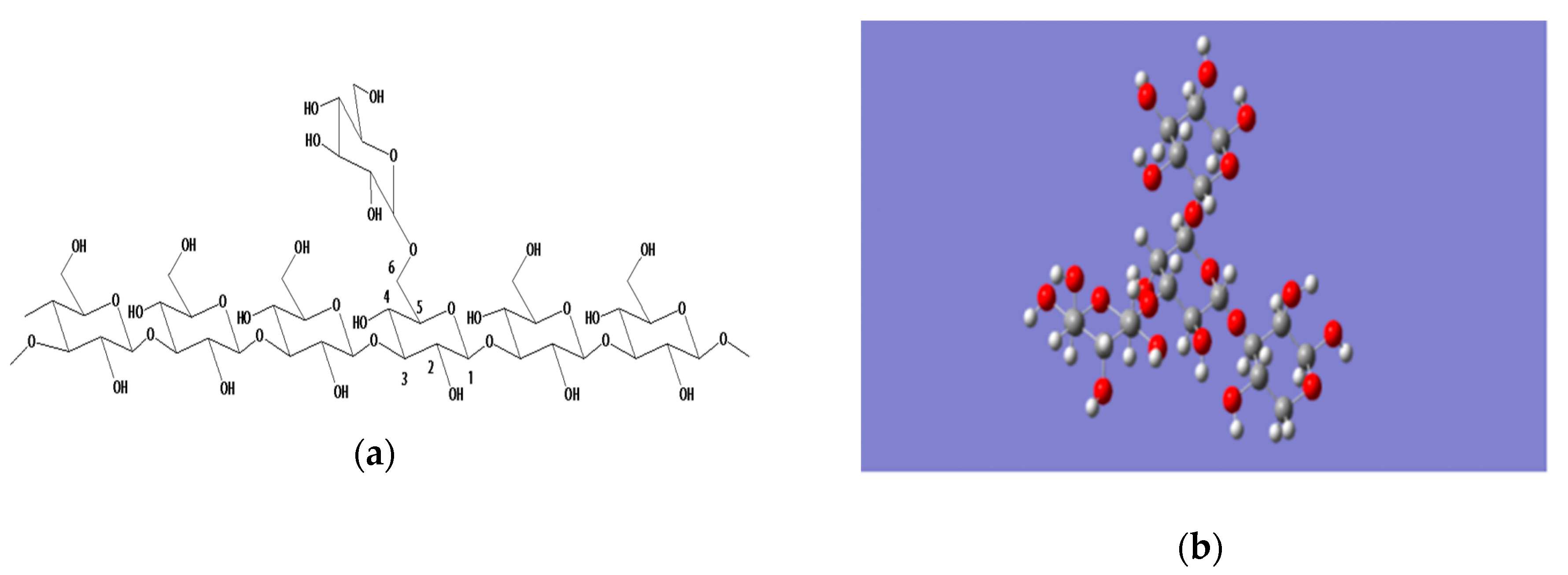
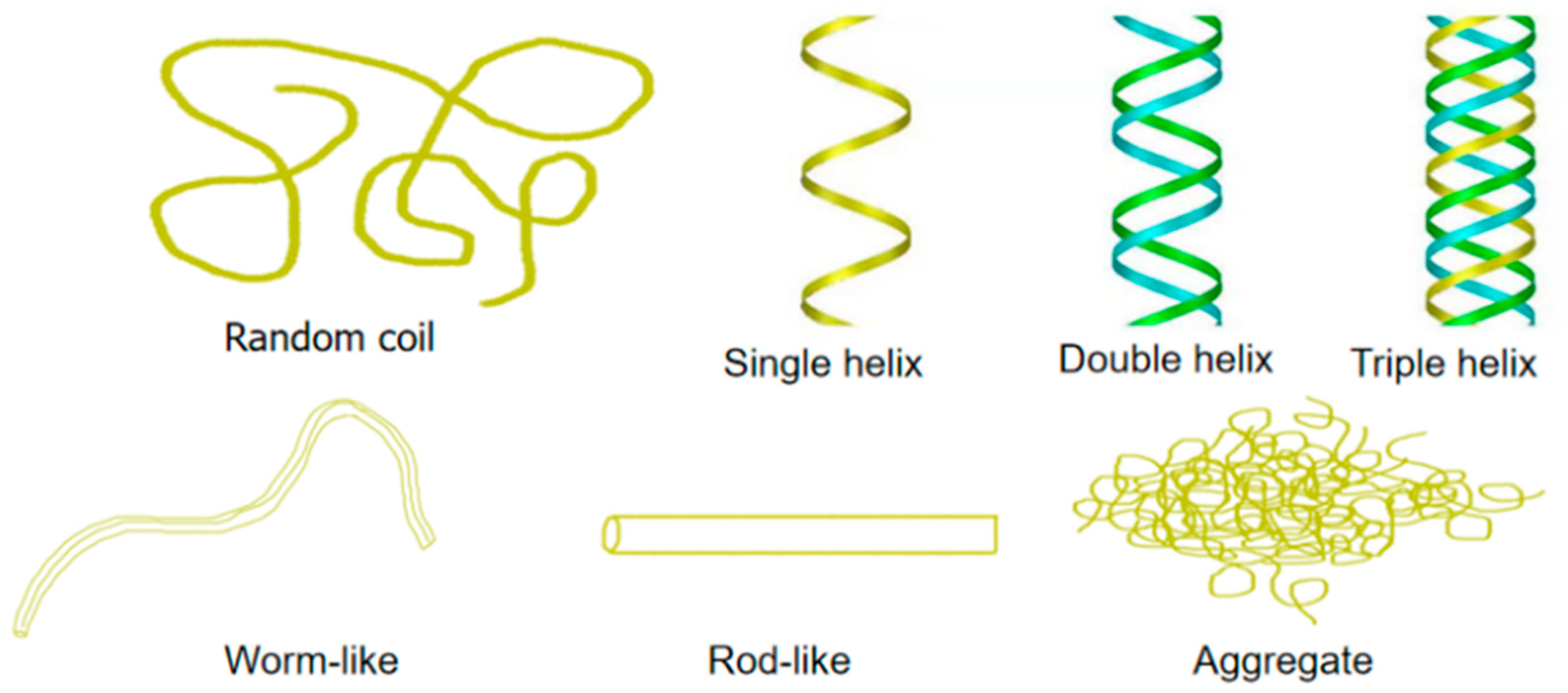
2. Advances in Research Yeast on β-Glucan Modification
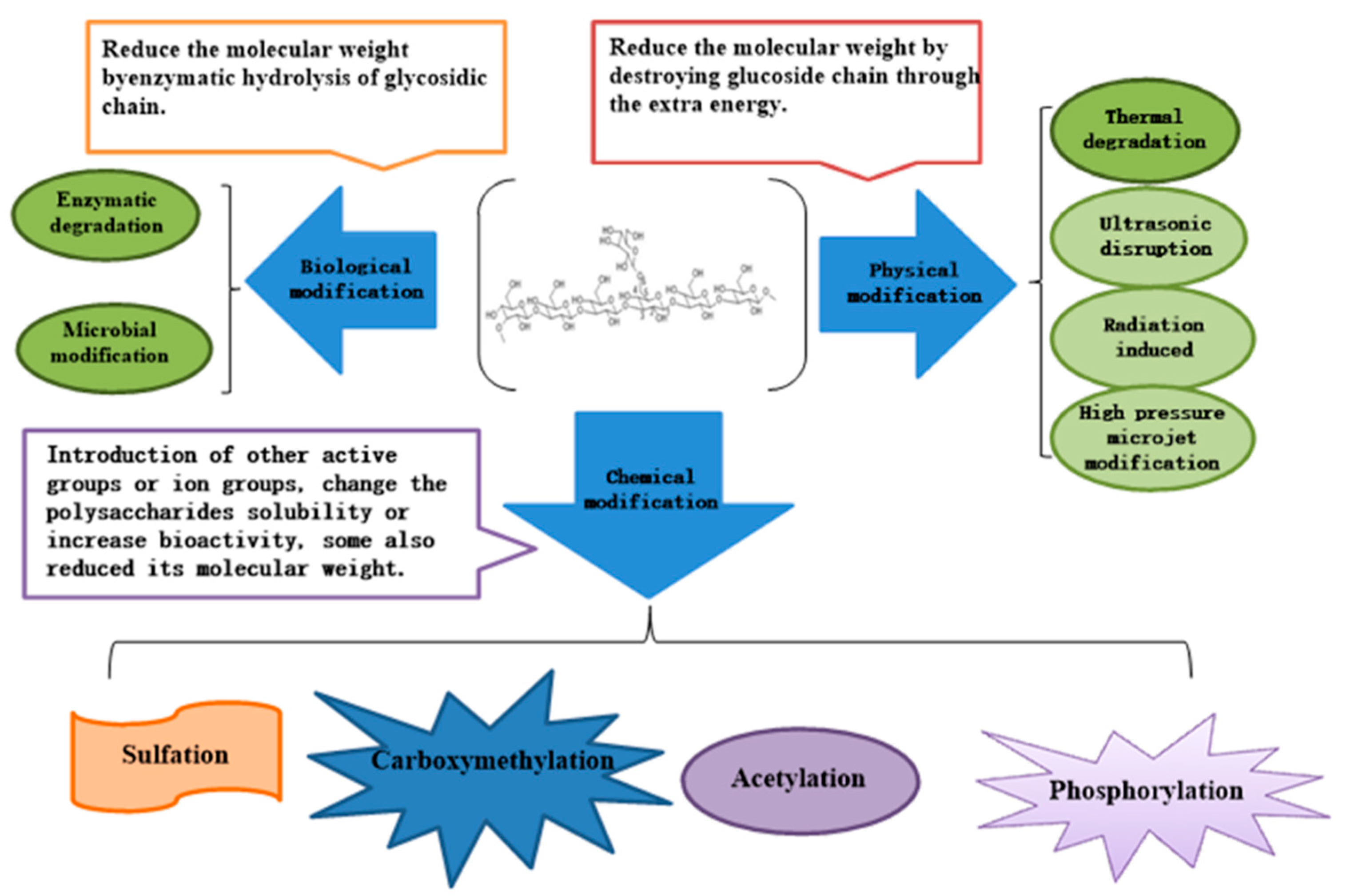
2.1. Physical Modification Method
2.1.1. Thermal Degradation
2.1.2. Irradiation
2.1.3. Ultrasonication
2.1.4. High-Pressure Micro-Jet Homogeneization
2.1.5. Supercritical Fluid Technology
2.2. Chemical Modification Method
2.2.1. Sulfation
2.2.2. Carboxymethylation Modification
2.2.3. Phosphorylation Modification
2.3. Biological Modification
2.3.1. Enzymatic Modification
2.3.2. Microbial Modification
3. Advances in Research on Biological Activities of Yeast β-Glucan
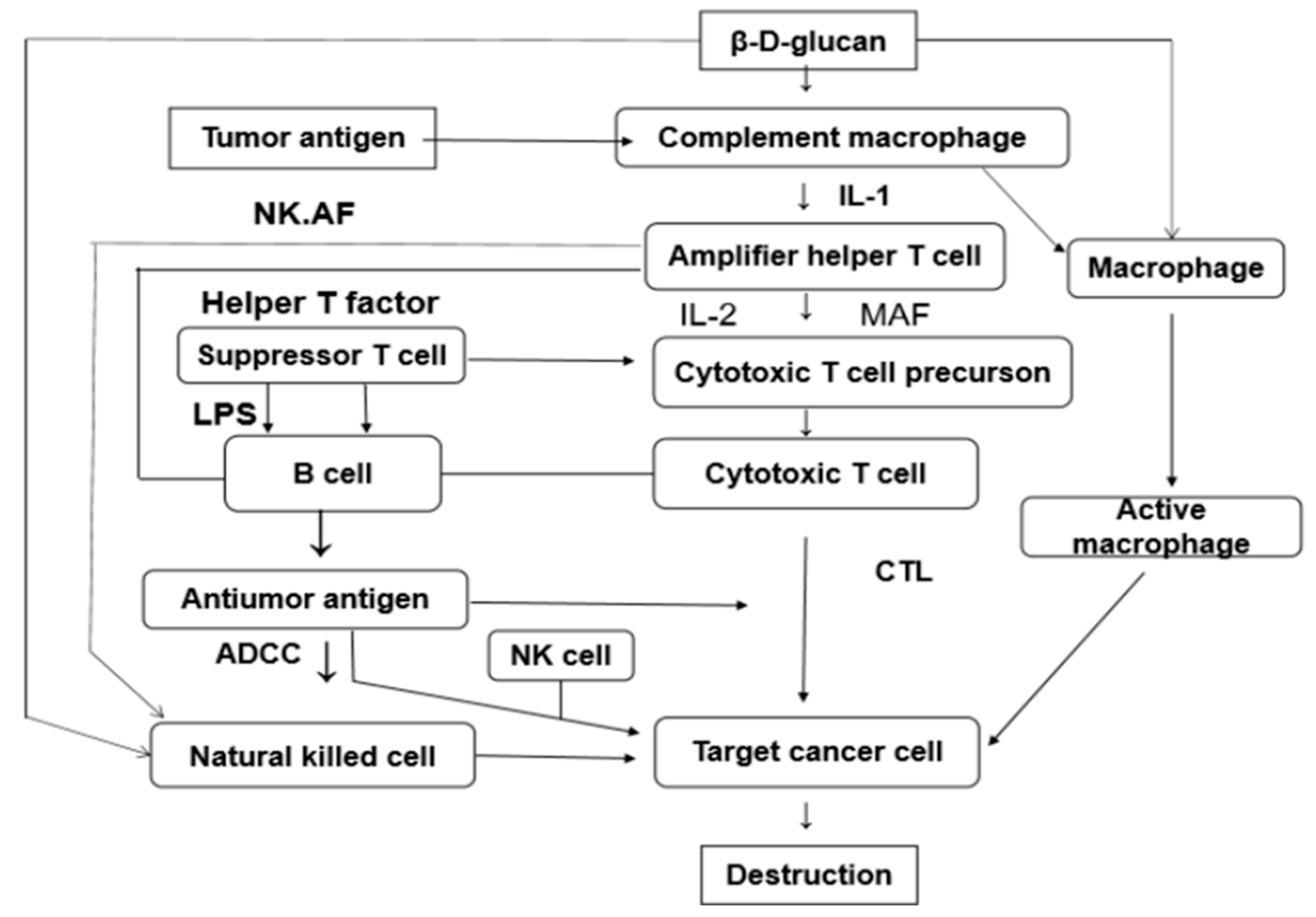
3.1. Immunomodulation and Anti-Tumor Activity
3.2. Antioxidant Promotes Wound Healing and Irradiation Resistance
3.3. Lowering Cholesterol and Blood Sugar
3.4. Other Biological Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hartland, R.P.; Vermeulen, C.A.; Klis, F.M.; Sietsma, J.H.; Wessels, J.G.H. The linkage of (1,3)-β-glucan to chitin during cell wall assembly in Saccharomyces cerevisiae. Yeast 1994, 10, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast, new structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [PubMed]
- Aguilar, U.B.; Francois, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Revi. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. protoc. 2012, 7, 1590. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Thielen, U.; Glenney, P.; Moranl, C. A study of Saccharomyces cerevisiae cell wall glucans. J. Inst. Brew. 2012, 115, 151–158. [Google Scholar] [CrossRef]
- Li, B.; Allendorf, D.J.; Hansen, R.; Marroquin, J.; Ding, C.; Cramer, D.E.; Yan, J. Yeast β-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J. Immunol. 2006, 177, 1661–1669. [Google Scholar] [CrossRef]
- Auclair, E. Yeast as an example of the mode of action of probiotics in monogastric and ruminant species. In Feed Manufacturing in the Mediterranean Region; CIHEAM-IAMZ: Reus, Spain, 2001; pp. 45–53. [Google Scholar]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms, a review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Wildenauer, F.X.; Lisdat, F.; Fleischer, L.G.; Kurz, T. Antioxidative activity of (1→ 3),(1→ 6)-β-d-glucan from Saccharomyces cerevisiae grown on different media. LWT-Food Sci. Technol. 2008, 41, 868–877. [Google Scholar] [CrossRef]
- Hakan, K.M.; Fatih, O.; Serdar, K.; Ahmet, A.; Engin, G.; Ahmet, S.; Zeki, O.; Erdener, T. The antioxidant effect of β-glucan on oxidative stress status in experimental spinal cord injury in rats. Neurosurg. Rev. 2005, 28, 298–302. [Google Scholar]
- Nicolosi, R.; Bell, S.J.; Bistrian, B.R.; Greenberg, I.; Forse, R.A.; Blackburn, G.L. Plasma lipid changes after supplementation with β-glucan fiber from yeast. Am. J. Clin. Nutr. 1999, 70, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Freimund, S.; Sauter, M.; Käppeli, O.; Dutlera, H. A new non-degrading isolation process for 1, 3-β-d-glucan of high purity from baker’s yeast Saccharomyces cerevisiae. Carbohydr. Polym. 2003, 54, 159–171. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan, a valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C.; Michaud, P. New developments and prospective applications for β (1, 3) glucans. Recent Pat. Biotechnol. 2007, 1, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G. (1→ 3, 1→ 6)-β-d-Glucans of yeasts and fungi and their biological activity. Stud. Nat. Prod. Chem. 2000, 23, 107–152. [Google Scholar]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Wang, Q.H.; Li, W.; Tang, S.S. Preparation of yeast dextran sulfate and anticoagulant activity. Food Res. Dev. 2011, 32, 32–35. (In Chinese) [Google Scholar]
- BeMiller, J.N.; Bohn, J.N. β-d-Glucans as biological response modifiers, a review of structure-functional activity. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar]
- Villares, A.; Mateo-Vivaracho, L.; Guillamón, E. Structural features and healthy properties of polysaccharides occurring in mushrooms. Agriculture 2012, 2, 452–471. [Google Scholar] [CrossRef]
- Heinze, T.; Petzold-Welcke, K.; van Dam, J.E.G. Polysaccharides, Molecular and supramolecular structures. Terminology. EPNOE 2012, 23–64. [Google Scholar] [CrossRef]
- Belsito, M.D.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety Assessment of Microbial Polysaccharide Gums as Used in Cosmetics. Tentat. Rep. 2012, 35, 5S–49S. [Google Scholar]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Adachi, Y.; Ohno, N.; Yadomae, T.; Suzuki, Y.; Ohsawa, M.; Oikawa, S. Thermal denaturation of (1→ 3)-β-d-glucans in neutral aqueous solution above 130°, Effect on physicochemical properties. Carbohydr. Res. 1990, 198, 111–122. [Google Scholar] [CrossRef]
- Zhang, M. Heating-induced conformational change of a novel β-(1→3)-d-glucan from Pleurotus geestanus. Biopolymers 2009, 93, 121–131. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Ishibashi, K.; Yamanaka, D.; Adachi, Y.; Kanzaki, K. Modulation of an innate immune response by soluble yeast β-glucan prepared by a heat degradation method. Int. J. Biol. Macromol. 2017, 104, 367–376. [Google Scholar] [CrossRef]
- Li, Y.J.; Ha, Y.M.; Fan, B.; Wang, F.; Li, A. Application of Radiation Technology in Molecular Modification of Polysaccharides, A Review. Food Sci. 2009, 30, 403–408. (In Chinese) [Google Scholar]
- Byun, E.H.; Kim, J.H.; Sung, N.Y.; Choi, J.; Lim, S.T.; Kim, K.H.; Yook, H.S.; Byuna, M.W.; Lee, J.W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Liu, Y.Y. Study on the Preparation of Cell Wall Polysaccharides from Saccharomyces cerevisiae and Its Rheological Properties. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2009. (In Chinese). [Google Scholar]
- Methacanon, P.; Weerawatsophon, U.; Tanjak, P.; Rachtawee, P.; Prathumpai, W. Interleukin-8 stimulating activity of low molecular weight β-glucan depolymerized by γ-irradiation. Carbohydr. Polym. 2011, 86, 574–580. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Masoodi, F.A.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β-d-glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohydr. polym. 2016, 140, 442–450. [Google Scholar]
- Ogutu, F.O.; Mu, T.H.; Elahi, R.; Zhang, M.; Sun, H.N. Ultrasonic modification of selected polysaccharides-review. J. Food Process. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Huang, C.; Miao, M.; Jiang, B.; Cui, S.W.; Jia, X.; Zhang, T. Polysaccharides modification through green technology, Role of ultrasonication towards improving physicochemical properties of (1-3)(1-6)-α-d-glucans. Food hydrocoll. 2015, 50, 166–173. [Google Scholar] [CrossRef]
- Hunter, K.W., Jr.; Gault, R.A.; Berner, M.D. Preparation of microparticulate β-glucan from Saccharomyces cerevisiae for use in immune potentiation. Lett. Appl. microbial. 2002, 35, 267–271. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Hu, Y.; Liu, H. Ultrasonic Modification of the β-Glucans from Saccharomyces cerevisiae and It’s Effection on the Immunoactivity in Mice. J. Food Sci. Biotechnol. 2008, 4, 19. (In Chinese) [Google Scholar]
- Chen, J.; Chen, L.; Lin, S.; Liu, C.; Cheung, P.C.K. Preparation and structural characterization of a partially depolymerized beta-glucan obtained from Poria cocos sclerotium by ultrasonic treatment. Food Hydrocoll. 2015, 46, 1–9. [Google Scholar] [CrossRef]
- Oboroceanu, D.; Wang, L.; Kroes-Nijboer, A.; Wang, L.; Brodkorb, A.; Venema, P.; Magner, E.; Auty, M.A.E. The effect of high pressure microfluidization on the structure and length distribution of whey protein fibrils. Int. Dairy J. 2011, 21, 823–830. [Google Scholar] [CrossRef]
- Grácia-Juliá, A.; René, M.; Cortés-Muñoz, M.; Picart, L.; López-Pedemonte, T.; Chevalier, D.; Dumay, E. Effect of dynamic high pressure on whey protein aggregation, A comparison with the effect of continuous short-time thermal treatments. Food Hydrocoll. 2008, 22, 1014–1032. [Google Scholar] [CrossRef]
- Zhang, W.Q. study on the effect of microfluidizer on the composition, structure and physiological functions of soluble soybean polysaccharides. Master’s Thesis, Nanchang University, Nan Chang, China, 2010. (In Chinese). [Google Scholar]
- Kan, M.; Zhang, Y.; Liu, C.M.; Liu, W.; Wang, J. Effect of Dynamic High Pressure Microfluidization on Molecular Weight Distribution and Functional Groups of Hemicellulose B Fractions Purified from Soybean Dregs Dietary Fibers. Food Sci. 2010, 31, 15–19. [Google Scholar]
- Duan, D.; Tu, Z.; Wang, H.; Sha, X.; Zhu, X. Physicochemical and rheological properties of modified rice amylose by dynamic high-pressure microfluidization. Int. J. Food Prop. 2017, 20, 734–744. [Google Scholar] [CrossRef][Green Version]
- Guo, L.; Wang, P.; Zhao, D.; Wang, G.; Zhou, F.; MA, X. Effect of High-pressure Homogenization on Structure and Functional Properties of Soybean β-Conglycinin. Food Sci. 2011, 19, 83–87. [Google Scholar]
- Huang, L.; Shen, M.; Zhang, X.; Jiang, L.; Song, Q.; Xie, J. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. Study on the Preparation, Modification and SolutionConformation of ß-glucans in Saccharomyces cerevisiae. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2013. (In Chinese). [Google Scholar]
- Kenar, J.A. Porous Structures from Bio-Based Polymers via Supercritical Drying. In Porous Lightweight Composites Reinforced with Fibrous Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 207–243. [Google Scholar]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2, Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Flax mucilage and barley beta-glucan aerogels obtained using supercritical carbon dioxide, Application as flax lignan carriers. Innov. Food Sci. Emerg. Technol. 2015, 28, 40–46. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Barley beta-glucan aerogels via supercritical CO2 drying. Food Res. Int. 2012, 48, 442–448. [Google Scholar] [CrossRef]
- Sibakov, J.; Myllymäki, O.; Holopainen, U.; Kaukovirta-Norja, A.; Hietaniemi, V.; Pihlava, J.M.; Poutanen, K.; Lehtinen, P. Lipid removal enhances separation of oat grain cell wall material from starch and protein. J. Cereal Sci. 2011, 54, 104–109. [Google Scholar] [CrossRef]
- Martinichen-Herrero, J.C.; Carbonero, E.R.; Gorin, P.A.J.; Lacominia, M. Anticoagulant and antithrombotic activity of a sulfate obtained from a glucan component of the lichen Parmotrema mantiqueirense Hale. Carbohydr. Polym. 2005, 60, 7–13. [Google Scholar] [CrossRef]
- Chang, Y.J.; Lee, S.; Yoo, M.A.; Lee, H.G. Structural and biological characterization of sulfated-derivatized oat β-glucan. J. Agric. Food Chem. 2006, 54, 3815–3818. [Google Scholar] [CrossRef]
- Han, M.D.; Han, J.S.; Hyun, S.H.; Shin, H.W. Solubilization of water-insoluble beta-glucan isolated from Ganoderma lucidum. J. Environ. Biol. 2008, 29, 237. [Google Scholar]
- Williams, D.L.; Pretus, H.A.; McNamee, R.B.; Jones, E.L.; Ensley, H.E.; Browder, I.L. Development of a water-soluble, sulfated (1→ 3)-β-d-glucan biological response modifier derived from Saccharomyces cerevisiae. Carbohydr. Res. 1992, 235, 247–257. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yao, S.J.; Guan, Y.X.; Wu, T.X.; Kennedy, J.F. A novel process for preparation of (1→3)-β-d-glucan sulphate by a heterogeneous reaction and its structural elucidation. Carbohydr. Polym. 2005, 59, 93–99. [Google Scholar] [CrossRef]
- Alban, S.; Schauerte, A.; Franz, G. Anticoagulant sulfated polysaccharides, Part, I. Synthesis and structure–activity relationships of new pullulan sulfates. Carbohydr. Polym. 2002, 47, 267–276. [Google Scholar] [CrossRef]
- Guo, H.; Li, H.Y.; Liu, L.; Wu, C.Y.; Liu, H.; Zhao, L.; Zhang, Q.; Liu, Y.T.; Li, S.Q.; Qin, W.; et al. Effects of sulfated modification on the physicochemical properties and biological activities of β-glucans from Qingke (Tibetan hulless barley). Int. J. Biol. Macromol. 2019, 141, 41–50. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Wang, Y.; Cheung, C.K. Chain conformation of sulfated derivatives of β-glucan from sclerotia of Pleurotus tuber-regium. Carbohydr. Res. 2003, 338, 2863–2870. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.M.; Dekker, R.F.H.; Barbosa, A.M.; Teixeira, S.D.; Salomé, K. Carboxymethylation of (1→6)-β-glucan (lasiodiplodan), Preparation, characterization and antioxidant evaluation. Carbohydr. Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Xue, C.; Dong, M.; Pan, Y. Study on the Carboxymethylation of Glucan from Yeast. Food Ferment. Ind. 2004, 11, 28–30. (In Chinese) [Google Scholar]
- Wang, M.; Tao, G. Studies on the strycture characteristic of modified yeast glucans—CMG. Nat. Product Res. Dev. 1997, 3, 43–48. (In Chinese) [Google Scholar]
- Wang, Y.; Zhang, L. Chain conformation of carboxymethylated derivatives of (1→ 3)-β-d-glucan from Poria cocos sclerotium. Carbohydr. Polym. 2006, 65, 504–509. [Google Scholar] [CrossRef]
- Wang, M.; Ding, X. The Physiochemical Properties and the Conformation in the Solution of Modified Yeast Glucan—CMG. Chinese J. Biochem. Mol. Biol. 1998, 14, 636–640. (In Chinese) [Google Scholar]
- Wang, Y.; Yu, Y.; Mao, J. Carboxymethylated β-glucan derived from Poria cocos with biological activities. J. Agric. Food Chem. 2009, 57, 10913–10915. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šandula, J.; Korolenko, T.A.; Falameeva, O.V.; Poteryaeva, O.N.; Zhanaeva, S.Y.; Levina, O.A.; Filatova, T.G.; Kaledin, V.I. Increased efficiency of Lewis lung carcinoma chemotherapy with a macrophage stimulator—yeast carboxymethyl glucan. Int. Immunopharmacol. 2002, 2, 775–781. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Huang, S.; Li, H. The antioxidant activities of six (1→ 3)-β-d-glucan derivatives prepared from yeast cell wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Li, C.; Ha, T.; Ozment-Skelton, T.; Kalbfleisch, J.H.; Preiszner, J.; Brooks, L.; Breuel, K.; Schweitzer, J.B. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J. Imm. 2004, 172, 449–456. [Google Scholar] [CrossRef]
- Müller, A.; Rice, P.J.; Ensley, H.E.; Coogan, P.S.; Kalbfleish, J.H.; Kelley, J.L.; Love, E.J.; Portera, C.A.; Ha, T.; Browder, I.W.; et al. Receptor binding and internalization of a water-soluble (1--> 3)-beta-D-glucan biologic response modifier in two monocyte/macrophage cell lines. J. Imm. 1996, 156, 3418–3425. [Google Scholar]
- Williams, D.L.; McNamee, R.B.; Jones, E.L.; Pretus, H.A.; Ensley, H.E.; Browder, I.W.; Di Luzio, N.R. A method for the solubilization of a (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991, 219, 203–213. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Zhang, L.; Zeng, F. Chain conformation and anti-tumor activities of phosphorylated (1→ 3)-β-d-glucan from Poria cocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L. Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1→ 3)-α-d-glucan from Poria cocos mycelia. Carbohydr. Polym. 2011, 83, 1363–1369. [Google Scholar] [CrossRef]
- Roubroeks, J.P.; Andersson, R.; Mastromauro, D.I.; Christensen, B.E.; Åman, P. Molecular weight, structure and shape of oat (1→ 3),(1→ 4)-β-d-glucan fractions obtained by enzymatic degradation with (1→ 4)-β-d-glucan 4-glucanohydrolase from Trichodermareesei. Carbohydr. Polym. 2001, 46, 275–285. [Google Scholar] [CrossRef]
- Leathers, T.D.; Sutivisedsak, N. Enzymatic modification of schizophyllan. Biotechnol. Lett. 2014, 37, 673–678. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Sibakov, J.; Nappa, M.; Hytönen, E.; Koksel, H.; Koksel, K. Extraction of soluble β-glucan from oat and barley fractions, Process efficiency and dispersion stability. J. Cereal Sci. 2018, 81, 60–68. [Google Scholar] [CrossRef]
- Kery, V.; Kogan, G.; Zjacova, K. Hydrolysis of yeast cell wall glucan by extracellular β-(1,3)–glucanase from Aspergillus Niger. Enzyme Microb. Technol. 1991, 13, 87–90. [Google Scholar]
- Duan, H.; Xiong, S.; Liu, H. Study on Solubility and Properties of Enzymolysates of Yeast β-1,3-Glucan. Food Sci. 2008, 29, 185–190. (In Chinese) [Google Scholar]
- Yu, Y.; Bai, X.; Du, Y. Study on preparation and activity of soluble yeast glucan. Chinese J. Biochem. Pharm. 2010, 31, 254–256. (In Chinese) [Google Scholar]
- Liang, Y.; Zhu, L.; Gao, M.; Wu, J.; Zhan, X. Effective production of biologically active water-soluble β-1, 3-glucan by a coupled system of Agrobacterium sp. and Trichoderma harzianum. Prep. Biochem. Biotechnol. 2018, 48, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Petravić-Tominac, V.; Zechner-Krpan, V.; Grba, S.; Srečec, S.; Panjkota-Krbavčić, I.; Vidović, L. Biological effects of yeast β-glucans. Agric. Conspec. Sci. 2010, 75, 149–158. [Google Scholar]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The production of yeast cell wall using an agroindustrial waste influences the wall thickness and is implicated on the aflatoxin B1 adsorption process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Zou, S.; Duan, B.; Sun, M.; Xu, X. Yeast β-glucan suppresses the chronic inflammation and improves the microenvironment in adipose tissues of ob/ob mice. J. Agric. Food Chem. 2018, 66, 621–629. [Google Scholar] [CrossRef]
- Fusté, N.P.; Guasch, M.; Guillen, P.; Anerillas, C.; Cemeli, T.; Pedraza, T.; Ferrezuelo, F.; Encinas, M.; Moralejo, M.; Garí, E. Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydr. Polym. 2019, 210, 389–398. [Google Scholar] [CrossRef]
- Bacha, U.; Nasir, M.; Iqbal, S.; Anjum, A.A. Nutraceutical, anti-inflammatory, and immune modulatory effects of β-glucan isolated from yeast. BioMed. Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Xia, C.H.; Zhang, Y.; Zhang, X. Study on the Effect of Yeast β-dextran on Immunity in Mice. Sichuan Food Ferment. 2015, 52, 19–21. (In Chinese) [Google Scholar]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.Y. Regulation of yeast β-glucan in animal immunity. Pigs Poultry 2014, 33, 77–78. (In Chinese) [Google Scholar]
- Chan, G.C.F.; Chan, W.K.; Sze, D.M.Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Wei, G. Effect of Yeast Polysaccharide on Performance and Immune Function of Broilers. China Feed 2018, 48–52. (In Chinese) [Google Scholar]
- Xu, H.; Zou, S.; Xu, X.; Zhang, L. Anti-tumor effect of β-glucan from Lentinus edodes and the underlying mechanism. Sci. Rep. 2016, 6, 28802. [Google Scholar] [CrossRef]
- Chung, T.K.H.; Cheung, T.H.; Lo, W.K.; Yim, S.F.; Yu, M.Y.; Krajewski, S.; Reed, J.C.; Wong, Y.F. Expression of apoptotic regulators and their significance in cervical cancer. Cancer Lett. 2002, 180, 63–68. [Google Scholar] [CrossRef]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R.; et al. Effects of low molecular weight yeast β-glucan on antioxidant and immunological activities in mice. Int. J. Mol. Sci. 2015, 16, 21575–21590. [Google Scholar] [CrossRef]
- Thompson, I.J.; Oyston, P.C.F.; Williamson, D.E. Potential of the β-glucans to enhance innate resistance to biological agents. Expert Rev. Anti-Infect. Ther. 2010, 8, 339–352. [Google Scholar] [CrossRef]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Guizard, C.; Chanzy, H.; Sarko, A. Molecular and crystal structure of dextrans, a combined electron and X-ray diffraction study. 1. The anhydrous, high-temperature polymorph. Macromol. 1984, 17, 100–107. [Google Scholar] [CrossRef]
- Zhang, M.; Cheung, P.C.K.; Zhang, L.; Chiu, C.M.; Ooi, V.E.C. Carboxymethylated β-glucans from mushroom sclerotium of Pleurotus tuber-regium as novel water-soluble anti-tumor agent. Carbohydr. Polym. 2004, 57, 319–325. [Google Scholar] [CrossRef]
- Gu, Y.; Takagi, Y.; Nakamura, T.; Hasegawa, T.; Suzuki, I.; Oshima, M.; Tawaraya, H.; Niwano, Y. Enhancement of radioprotection and anti-tumor immunity by yeast-derived β-glucan in mice. J. Med. Food 2005, 8, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Shiomi, Y.; Minato, K.; Kawakami, S.; Ashida, H.; Tsuchida, H. Fucogalactan isolated from Sarcodon aspratus elicits release of tumor necrosis factor-α and nitric oxide from murine macrophages. Immunopharmacology 2000, 46, 113–121. [Google Scholar] [CrossRef]
- Shi, J.; LI, Y.; Shi, F. Improve Water-Solubility of β-d-Glucan from Saccharomyces cerevisiae by Mechanical Activation. J. Food Sci. Biotechnol. 2015, 34, 536–541. (In Chinese) [Google Scholar]
- Mo, L.; Chen, Y.; Li, W.; Guo, S.; Wang, X.; An, H.; Zhan, Y. Anti-tumor effects of (1→ 3)-β-d-glucan from Saccharomyces cerevisiae in S180 tumor-bearing mice. Int. J. Biol. Macromol. 2017, 95, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Cai, Y.; Gunn, L.; Ding, C.; Li, B.; Kloecker, G.; Qian, K.; Vasilakos, J.; Saijo, S.; Iwakura, Y.; et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood 2011, 117, 6825–6836. [Google Scholar] [CrossRef]
- Gu, K.; Zhao, L.; Wang, L.; Bu, D.; Liu, N.; Wang, J.P. Effects of Dietary Supplementation of Yeast β-Glucan on Performance, Serum Biochemical Indices and Antioxidant Capacity of Transition Dairy Cows. Chin. J. Anim. Nutr. 2018, 30, 2164–2171. (In Chinese) [Google Scholar]
- Ahmad, H.; Tian, J.; Wang, J.; Khan, M.A.; Wang, Y.; Zhang, L.; Wang, T. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J. Agric. Food Chem. 2012, 6, 7111–7120. [Google Scholar] [CrossRef]
- Reverberi, M.; Fabbri, A.A.; Zjalic, S.; Ricelli, A.; Punelli, F.; Fanelli, C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 2005, 69, 207–215. [Google Scholar] [CrossRef]
- Shan-bai, D.H.X.; Xiao-bo, H.U. Study on Antitumor and Antioxidant Activities of Yeast β-1, 3-glucan Hydrolysates in vivo. J. Huazhong Agric. Univ. 2007, 6. Available online: http://www.cqvip.com/Main/Detail.aspx?id=26364942 (accessed on 21 November 2019).
- Tang, Q.; Huang, G. Progress in polysaccharide derivatization and properties. Mini Rev. Med. Chem. 2016, 16, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Huang, K.; Zhao, Y.; Chen, F.; Qin, S. Effect of selenium sources in diets on selenium content in blood and the antioxidant capacity of hen. J. Nanjing Agric. Univ. 2008, 2. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-NJNY200802020.htm (accessed on 21 November 2019).
- Şener, G.; Toklu, H.; Ercan, F.; Erkanlı, G. Protective effect of β-glucan against oxidative organ injury in a rat model of sepsis. Int. Immunopharmacol. 2005, 5, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Ying, S.Y.; Choi, P.C.L. Evaluation of Hyphecan (1-4, 2-acetamide-deoxy-B-D-glucan polymer) on wound healing in a rodent model. Ann. Colleg. Surgeons Hong Kong 2002, 6, 113–117. [Google Scholar] [CrossRef]
- Medeiros, S.D.V.; Cordeiro, S.L.; Cavalcanti, J.E.C.; Melchuna, K.M.; Lima, A.; Filho, I.A.; Medeiros, A.C.; Rocha, K.B.F.; Oliveira, E.M.; Faria, E.D.B.; et al. Effects of purified Saccharomyces cerevisiae (1→ 3)-β-glucan on venous ulcer healing. Int. J. Mol. Sci. 2012, 13, 8142–8158. [Google Scholar] [CrossRef]
- Cherng, J.H. The strategies of natural polysaccharide in wound healing. Wound Healing-Current Perspectives. IntechOpen 2018. [Google Scholar] [CrossRef]
- Berdal, M.; Appelbom, H.I.; Eikrem, J.H.; Lund, Å.; Busund, L.T.; Hanes, R.; Sejelid, R.; Jnssen, T. Aminated β-1, 3-D-glucan has a dose-dependent effect on wound healing in diabetic db/db mice. Wound Repair Regen. 2011, 19, 579–587. [Google Scholar] [CrossRef]
- Harangozó, M. Penetration of radionuclides across the skin, Glucans as possible inhibitors of metals permeation. J. Radioan. Nucl. Chem. 2001, 250, 189–191. [Google Scholar] [CrossRef]
- Zulli, F.; Suter, F.; Biltz, H.; Nissen, H.P. Improving skin function with CM-glucan, a biological response modifier from yeast. Int. J. Cosmet. Sci. 1998, 20, 79–86. [Google Scholar] [CrossRef]
- Pathchen, M.L.; Petruczenko, A. Glucan effect of the suevival of diction exposure, mice after radiction exposure. Acta Physiol. Pol. 1984, 35, 23–26. [Google Scholar]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→ 3, 1→ 4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Wang, L.; Newman, R.K.; Newman, C.W.; Hofer, P.J. Barley β-glucans alter intestinal viscosity and reduce plasma cholesterol concentrations in chicks. J. Nutr. 1992, 122, 2292–2297. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans, a randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I. Production of microbial polysaccharides for use in food. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Woodhead Publishing: Technological Educational Institute of Larissa, Larissa, Greece, 2013; pp. 413–468. [Google Scholar]
- Kim, H.J.; White, P.J. In vitro bile-acid binding and fermentation of high, medium, and low molecular weight β-glucan. J. Agric. Food Chem. 2009, 58, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, R.; Bell, S.J.; Bistrian, B.R. Changes in plasma lipids from a yeast-derived-glucan fiber in hypercholesterolemic patients. Submitted to Am. J. Clin. Nutr. 1998, 34, 189–203. [Google Scholar]
- Kalra, S.; Jood, S. Effect of dietary barley β-glucan on cholesterol and lipoprotein fractions in rat. J. Cereal Sci. 2000, 31, 141–145. [Google Scholar] [CrossRef]
- Peng, C.; Qu, X.; Yang, X.; Peng, Y.D. The Function of β-glucans and Application in Live stock Production. Feed Rev. 2016, 6, 32–35. (In Chinese) [Google Scholar]
- Wang, Z.; Li, L.; Li, B.; Guo, S. Anticoagulant property of a semi-synthesized sodium β-1,4-glucan sulfate. Acta Pharm. Sinica 2006, 41, 323–327. [Google Scholar]
- Fruhauf, S.; Kunz-Vekiru, E.; Schatzmayr, G.; Moll, W.D. Detection of hydrolyzed zearalenone (HZEN) as intermediate in Zearalenone catabolism of Clonostachys rosea. New Biotechnol. 2012, 29, S167. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, J.P.; Zhou, X.H. The function of yeast cell wall polysaccharide and its detection methods. China Feed 2015, 11, 40–42. (In Chinese) [Google Scholar]
- Zhang, L.; Xu, X. Adsorptive Properties of β-d-Glucans Extracted from Spent Brewer Yeast Cell Wall against Zearalenone. Food Sci. 2006, 27, 75–78. (In Chinese) [Google Scholar]
- Murphy, P.; Dal Bello, F.; O’Doherty, J.; Arendt, E.K.; Sweeney, T.; Coffey, A. Analysis of bacterial community shifts in the gastrointestinal tract of pigs fed diets supplemented with β-glucan from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae. Animal 2013, 7, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Shao, Y.; Wang, Z.; Guo, Y. Effects of dietary yeast β-glucans supplementation on growth performance, gut morphology, intestinal Clostridium perfringens population and immune response of broiler chickens challenged with necrotic enteritis. Anim. Feed Sci. Technol. 2016, 215, 144–155. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Ma, T.; Tu, Y.; Zhang, N.; Guo, J.; Deng, K.; Zhou, Y.; Yun, Q.; Diao, Q. Effects of dietary yeast β-glucan on nutrient digestibility and serum profiles in pre-ruminant Holstein calves. J. Integr. Agric. 2015, 14, 749–757. [Google Scholar] [CrossRef]
- Jaskari, J.; Kontula, P.; Siitonen, A.; Jousimies-Somer, H.; Mattila-Sandholm, T.; Poutanen, K. Oat β-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 1998, 49, 175–181. [Google Scholar] [CrossRef]
- Zhou, Y.; Diao, Q.; Tu, Y. Effects of Yeast β-glucan on Gastrointestinal Development in Early-weaning Calves. Chinese J. Anim. Nutr. 2009, 21, 846–852. (In Chinese) [Google Scholar]
- Wang, Z.; Qiang, W.; Guo, Y.; Wang, Y.; Wang, Y. Effect of Dietary β-1,3-glucan on Immune Functions and Intestinal Microflora Levels of Broilers Challenged with Salmonella pullorum. China Poultry 2010, 32, 14–18. (In Chinese) [Google Scholar]
- Refstie, S.; Baeverfjord, G.; Seim, R.R.; Elvebø, O. Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture 2010, 305, 109–116. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, H.; She, W. Effect of yeast polysaccharide on growth performance, immune function andintestinal flora of weaned piglets. China Feed 2018, 16, 46–50. (In Chinese) [Google Scholar]
- Cui, Y.; Ma, X. Biological Function of Yeast Polysaccharide and Its Effect on Piglet’s Gut Health. Chin. J. Anim. Nutr. 2018, 30, 857–864. (In Chinese) [Google Scholar]
| Water Soluble β-Glucan | Modification Methods | Isolation and Purification | Yield | MW | Ref. |
|---|---|---|---|---|---|
| 1,3-/1,6-glucan | Heat degradation | Centrifuged at 3,000 rpm for 20 min; filtered through a 0.45 µm disposable syringe filter | 89.8% | 70.8 × 104 −0.13 × 104 | [27] |
| Black yeast β-Glucan | Irradiation 10, 30 and 50 kGy | _ | 55.76%, 75.81%, 81.72% | 6.2 × 104, 3.2 × 104, 2.5 × 104 | [29] |
| Poria cocos β-glucan | Ultrasonic treatment | Ultrafiltration using a membrane with a MW cut-off of 10 kDa | PCS90 6.30 mg/mL (Solubility) | 4.3 × 104 | [39] |
| Yeast β-glucan | High pressure micro-jet | Ethanol precipitationcentrifugation | 79.3% | [47] | |
| G. lucidum β-glucan | Sulfation | Ultrafiltration system (Sartorius. Co. SM 17521), using a 10,000 MW cut-off filter | 85% | 9.3 × 103 | [55] |
| Saccharomyces cerevisiae (1→3)-β-d-glucan | Phosphorylation | 1 µm pre-filter Pellicon tangential flow dialyzer (Millipore, Bedford, MA) | 70% | 1.28 × 106 −0.25 × 105, 3.57 × 106 −1.10 × 105, 12.23 × 106 −3.04 × 105 | [71] |
| Yeast β-1,3-glucan | Enzymic | Sephadex G-100 | 52% | 6.380 × 108, 4.785 × 107, 1.206 × 106 | [78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Lan, P.; He, Y.; Li, C.; Ma, X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules 2020, 25, 57. https://doi.org/10.3390/molecules25010057
Yuan H, Lan P, He Y, Li C, Ma X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules. 2020; 25(1):57. https://doi.org/10.3390/molecules25010057
Chicago/Turabian StyleYuan, Hongjie, Ping Lan, Yan He, Chengliang Li, and Xia Ma. 2020. "Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review" Molecules 25, no. 1: 57. https://doi.org/10.3390/molecules25010057
APA StyleYuan, H., Lan, P., He, Y., Li, C., & Ma, X. (2020). Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules, 25(1), 57. https://doi.org/10.3390/molecules25010057





