Electrochemical Properties of Carbon Aerogel Electrodes: Dependence on Synthesis Temperature
Abstract
1. Introduction
2. Results and Discussion
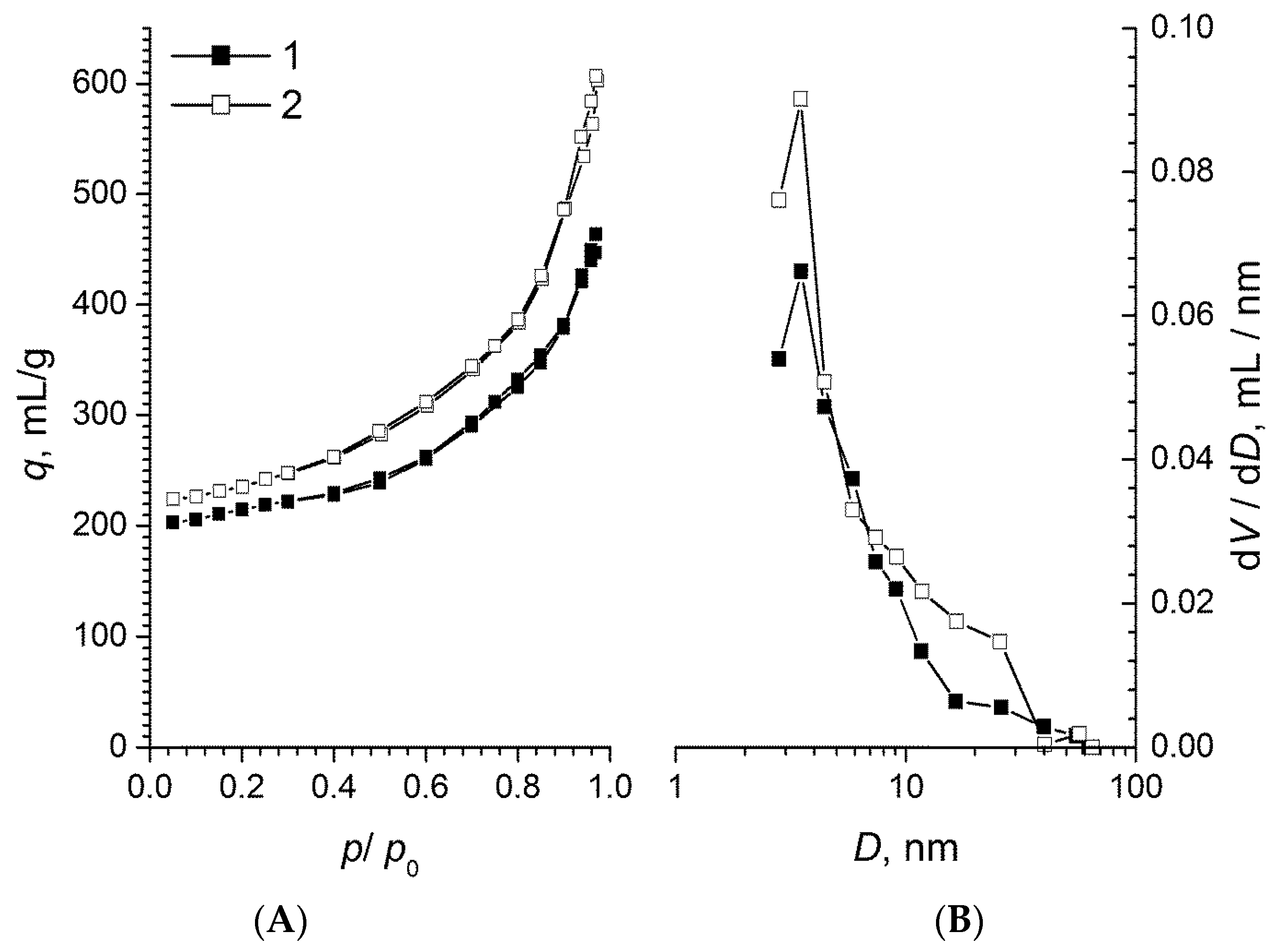
2.1. Texture Properties
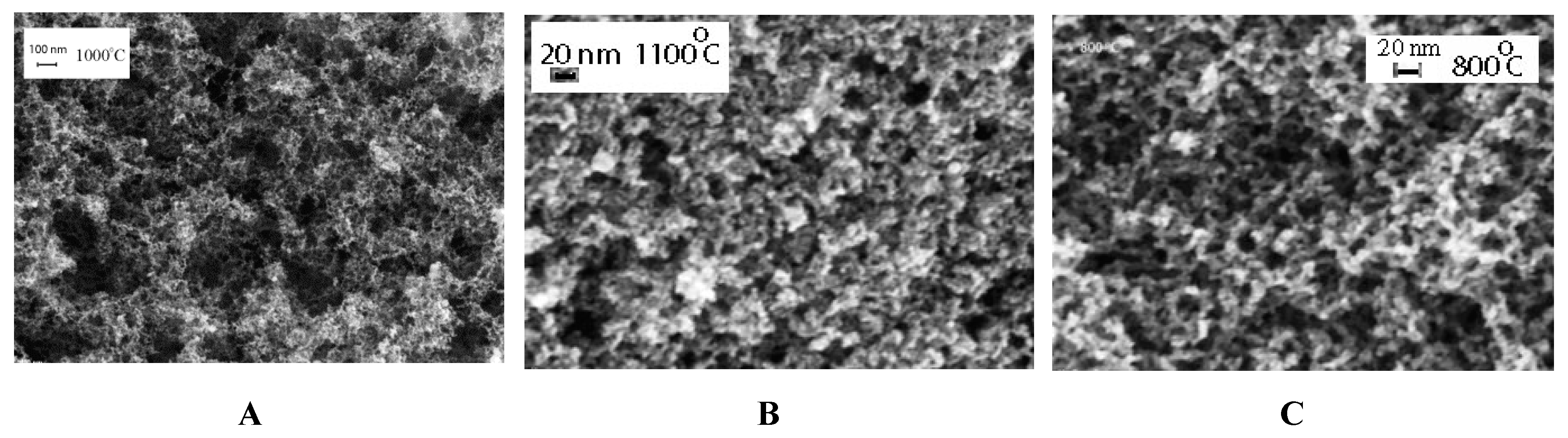
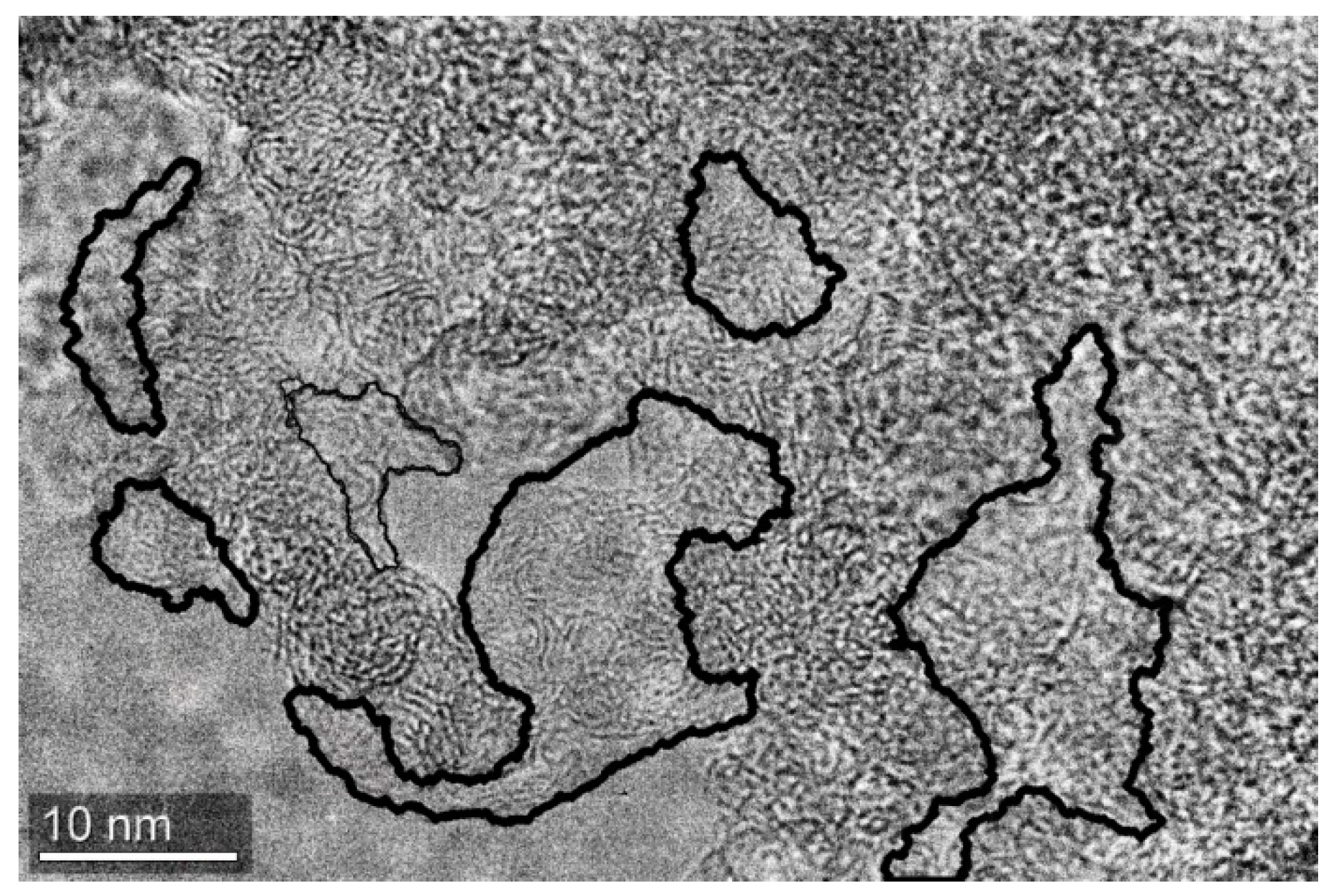
2.2. Scanning Electron Microscopy
2.3. Electric and Electrochemical Parameters
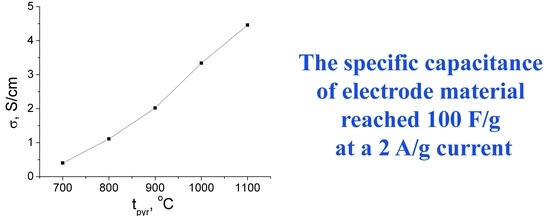
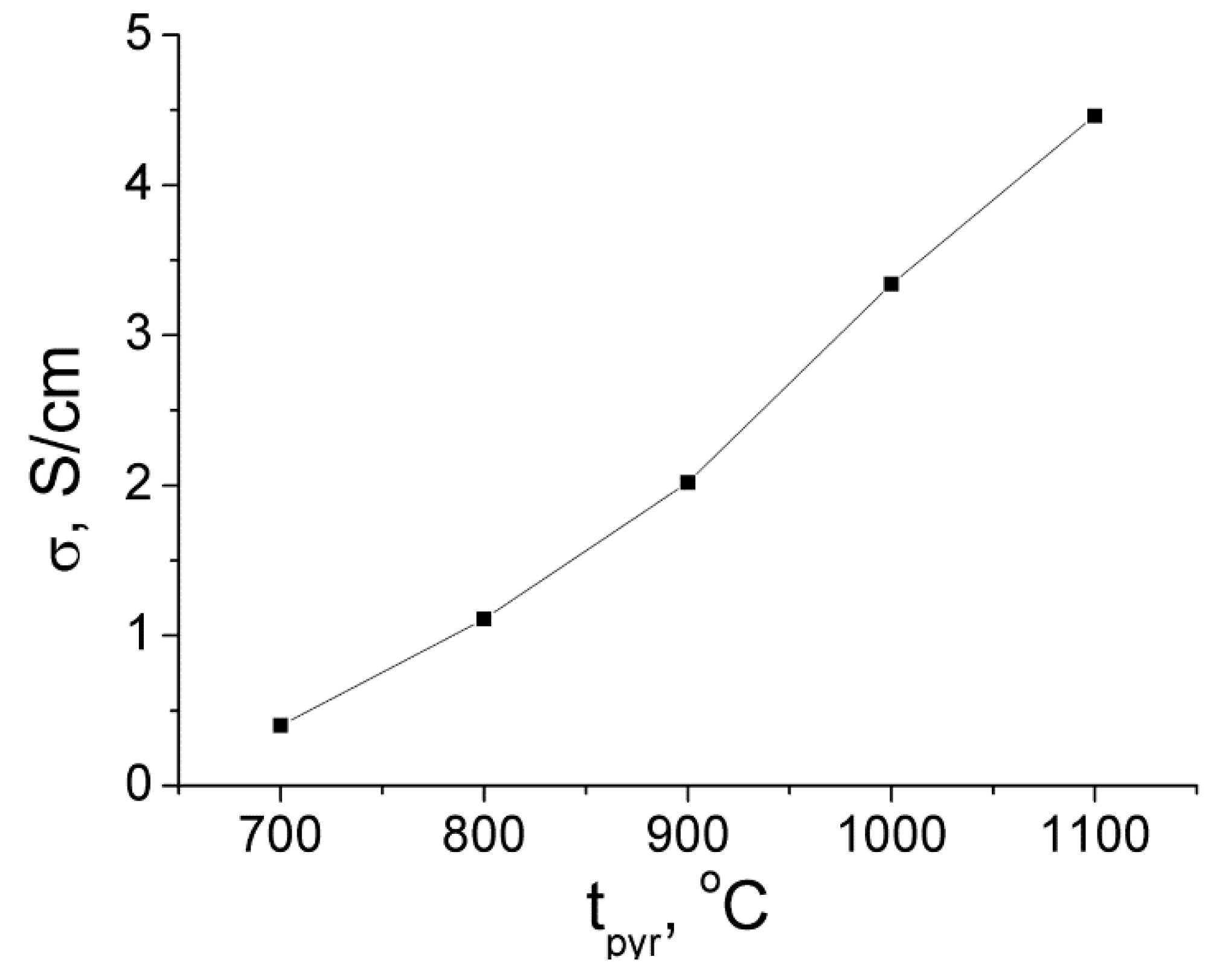
2.3.1. Electronic Conductivity
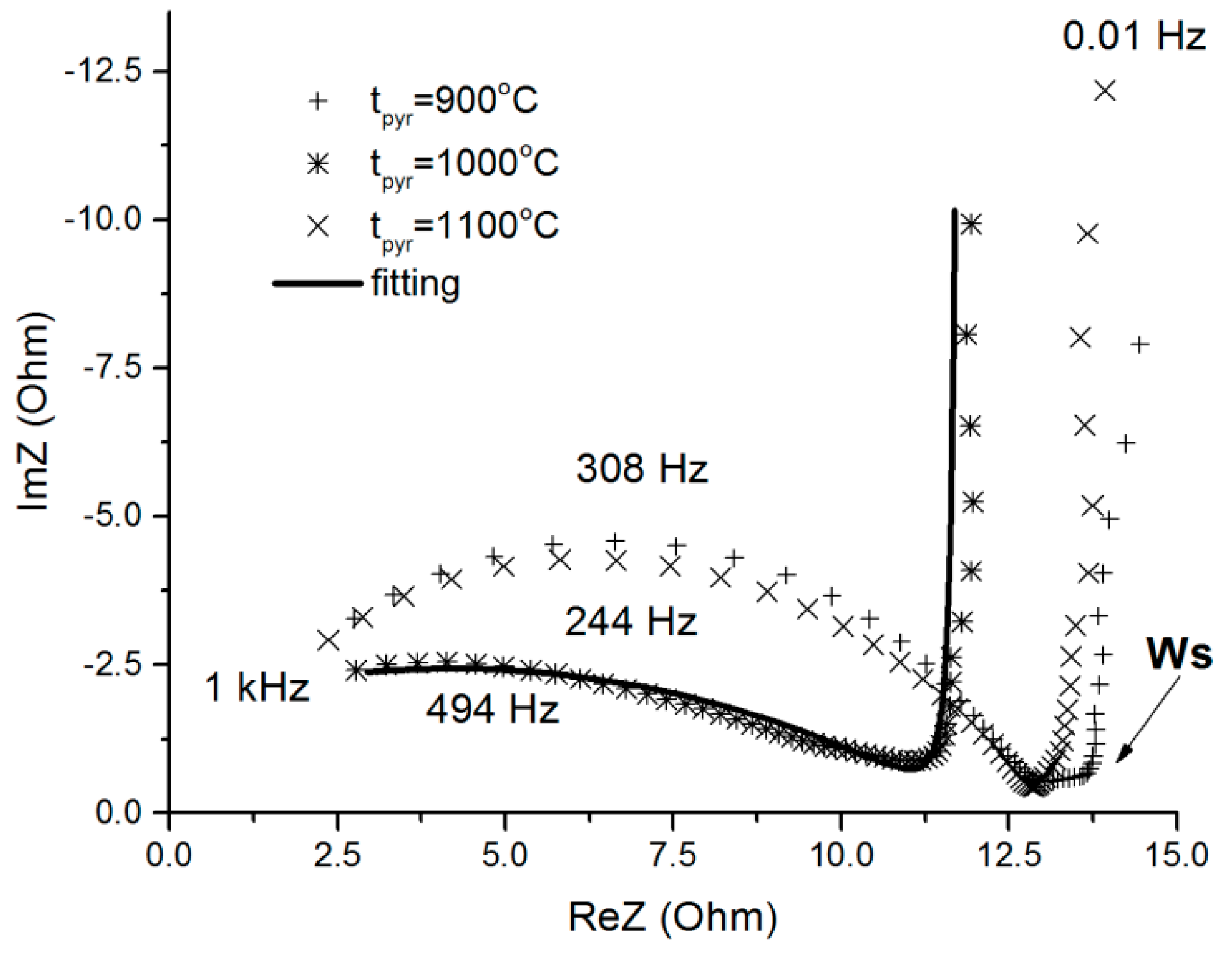
2.3.2. Characteristics of the Electrode Material from C-aerogels
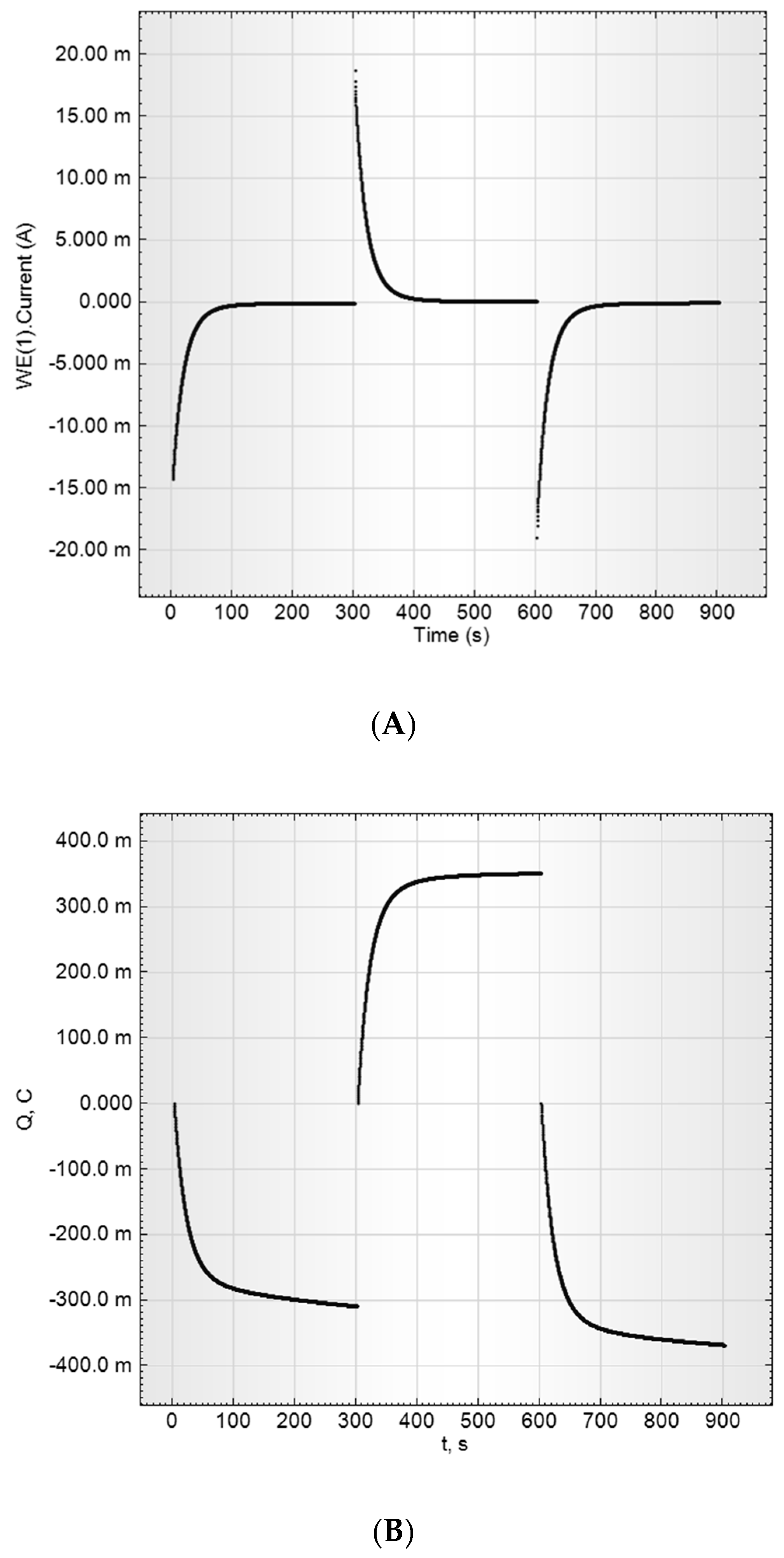
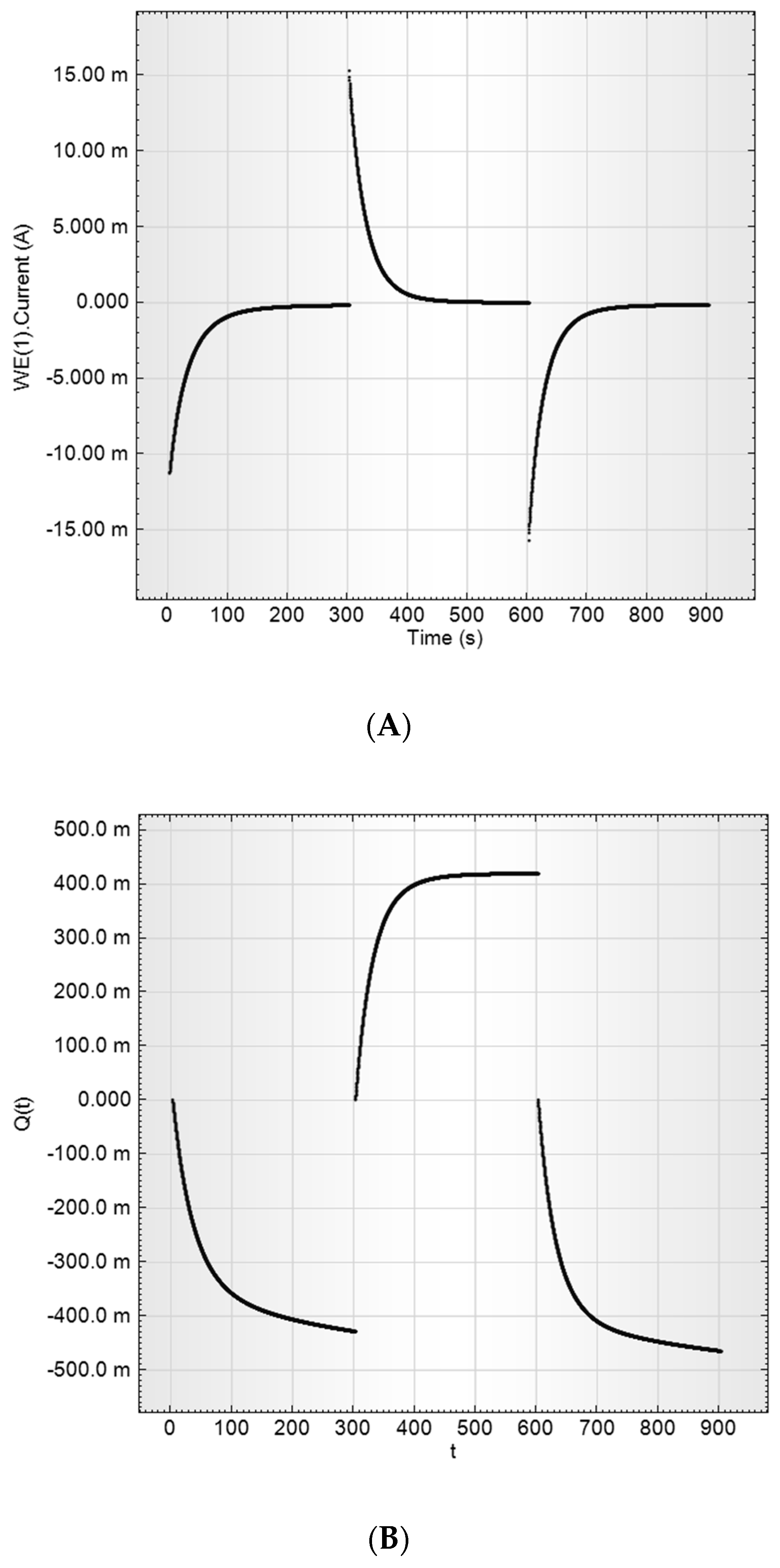
2.3.3. Direct Current Measurements
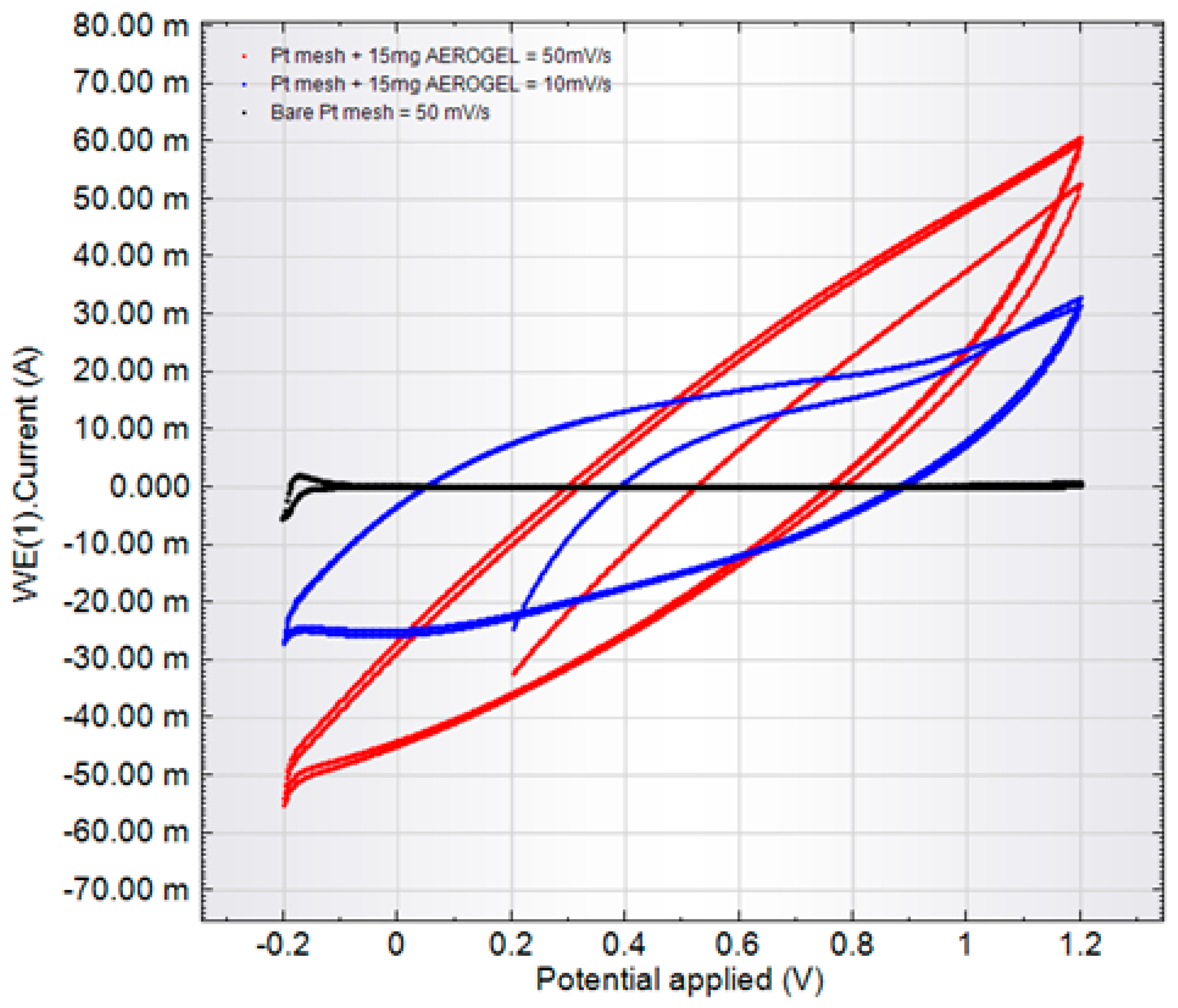
- CVA measurements
- Capacitance parameters determination by a current transient method (at constant potential)
3. Materials and Methods
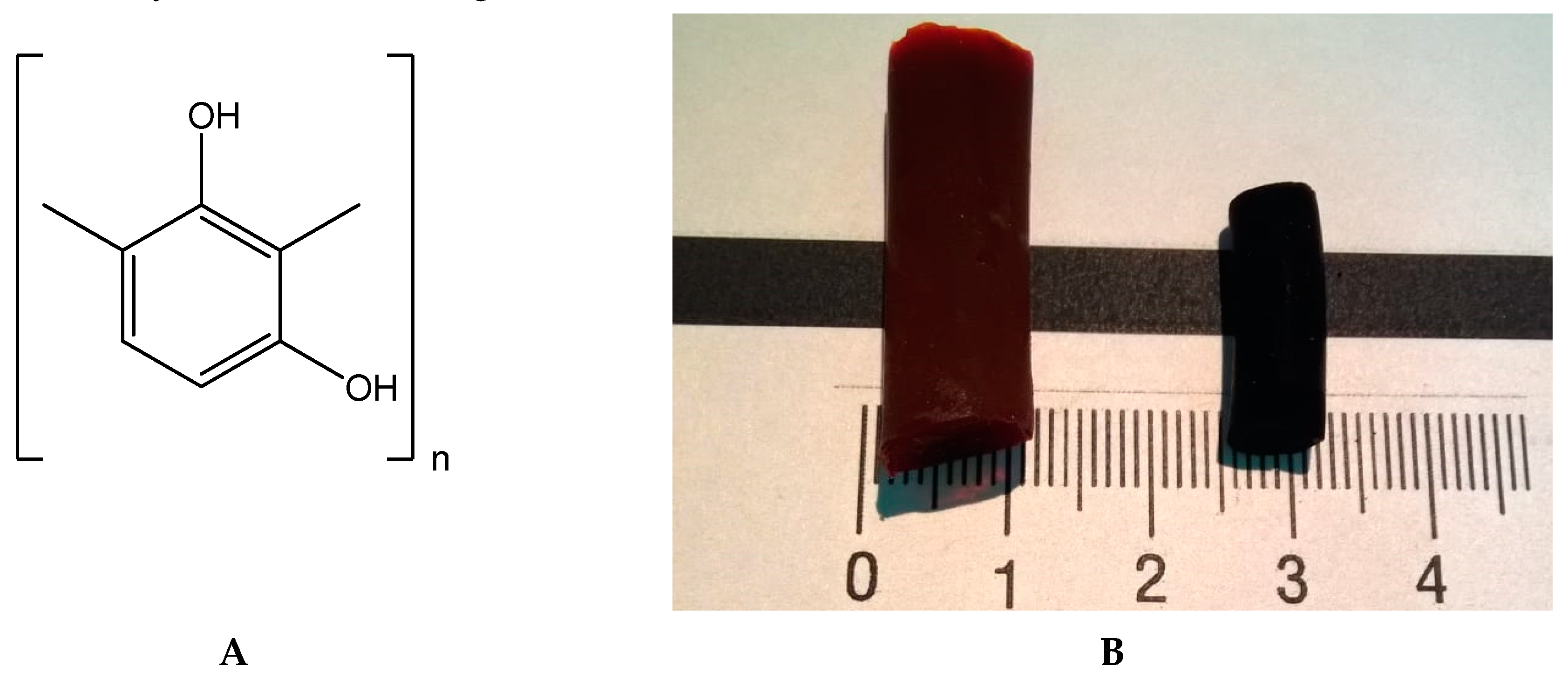
3.1. Materials
3.2. Preparation of Sols
3.3. Gelation of Sols
3.4. Supercritical Drying
3.5. Pyrolysis Procedure
3.6. Characterization of Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: London, UK; New York, NY, USA, 2011. [Google Scholar]
- Pekala, R. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol–formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Schwan, M.; Ratke, L. Flexibilisation of resorcinol–formaldehyde aerogels. J. Mater. Sci. A 2013, 1, 13462–13468. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Du, A.; Zhu, X.; Shen, J.; Wu, G.; Zhang, Z.; Zhou, B. One-pot synthesis, characterization and properties of acid-catalyzed resorcinol/formaldehyde cross-linked silica aerogels and their conversion to hierarchical porous carbon monoliths. J. Sol-Gel Sci. Technol. 2012, 62, 294–303. [Google Scholar] [CrossRef]
- Berthon, S.; Barbieri, O.; Ehrburger-Dolle, F.; Geissler, E.; Achard, P.; Bley, F.; Hecht, A.-M.; Livet, F.; Pajonk, G.M.; Pinto, N.; et al. DLS and SAXS investigations of organic gels and aerogels. J. Non Cryst. Solids 2001, 285, 154–161. [Google Scholar] [CrossRef]
- Petricevic, R.; Glora, M.; Fricke, J. Planar fibre reinforced carbon aerogels for application in PEM fuel cells. Carbon 2001, 39, 857–867. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Malkova, A.N.; Sipyagina, N.A.; Straumal, E.A.; Baranchikov, A.E.; Yorov, K.E.; Ivanov, V.K. Facile synthesis of fluorinated resorcinol-formaldehyde aerogels. J. Fluorine Chem. 2017, 193, 1–7. [Google Scholar] [CrossRef]
- Oschatz, M.; Borchardt, L.; Pinkert, K.; Thieme, S.; Lohe, M.R.; Hoffmann, C.; Benusch, M.; Wisser, F.M.; Ziegler, C.; Giebeler, L.; et al. Hierarchical carbide-derived carbon foams with advanced mesostructure as a versatile electrochemical energy-storage material. Adv. Energy Mater. 2014, 4, 1300645. [Google Scholar] [CrossRef]
- Rose, M.; Korenblit, Y.; Kockrick, E.; Borchardt, L.; Oschatz, M.; Kaskel, S.; Yushin, G. Hierarchical micro- and mesoporous carbide-derived carbon as a high-performance electrode material in supercapacitors. Small 2011, 7, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Nickel, W.; Thommes, M.; Cychosz, K.A.; Leistner, M.; Adam, M.; Mondin, G.; Strubel, P.; Borchardt, L.; Kaskel, S. Evolution of porosity in carbide-derived carbon aerogels. J. Mater. Chem. A 2014, 2, 18472–18479. [Google Scholar] [CrossRef]
- Yang, I.; Kwon, D.; Kim, M.-S.; Jung, J.C. A comparative study of activated carbon aerogel and commercial activated carbons as electrode materials for organic electric double-layer capacitors. Carbon 2018, 132, 503–511. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Chen, M.; Shi, S.; Cao, Y. Preparing hierarchical porous carbon aerogels based on enzymatic hydrolysis lignin through ambient drying for supercapacitor electrodes. Microporous Mesoporous Mat. 2018, 265, 258–265. [Google Scholar] [CrossRef]
- Lu, C.; Huang, Y.H.; Hong, J.S.; Wu, Y.J.; Li, J.; Cheng, J.P. The effects of melamine on the formation of carbon xerogel derived from resorcinol and formaldehyde and its performance for supercapacitor. J. Colloid Interface Sci. 2018, 524, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Laheäär, A.; Peikolainen, A.-L.; Koel, M.; Jänes, A.; Lust, E. Comparison of carbon aerogel and carbide-derived carbon as electrode materials for non-aqueous supercapacitors with high performance. J. Solid State Electrochem. 2012, 16, 2717–2722. [Google Scholar] [CrossRef]
- Mayer, S.T.; Pekala, R.W.; Kaschmitter, J.L. The aerocapacitor: An electrochemical double-layer energy-storage device. J. Electrochem. Soc. 1993, 140, 446–451. [Google Scholar] [CrossRef]
- Kim, S.J.; Hwang, S.W.; Hyun, S.H. Preparation of carbon aerogel electrodes for supercapacitor and their electrochemical characteristics. J. Mater. Sci. 2005, 40, 725–731. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Zanto, E.J.; Al-Muhtaseb, S.A.; Ritter, J.A. Sol-gel-derived carbon aerogels and xerogels: Design of experiments approach to materials synthesis. Ind. Eng. Chem. Res. 2002, 41, 3151–3162. [Google Scholar] [CrossRef]
- Vol’fkovich, Y.M.; Mikhalin, A.A.; Bograchev, D.A.; Sosenkin, V.E. Carbon electrodes with high pseudocapacitance for supercapacitors. Rus. J. Electrochem. 2012, 48, 424–433. [Google Scholar] [CrossRef]
Sample Availability: Samples of all aerogels listed in the paper are available from the authors. |
| Series | Ssp, m2/g | Density, g/cm3 |
|---|---|---|
| RF | 430 ± 40 | 0.14 ± 0.02 |
| RF-700 | 700 ± 70 | 0.16 ± 0.01 |
| RF-800 | 730 ± 80 | 0.18 ± 0.02 |
| RF-900 | 760 ± 80 | 0.15 ± 0.01 |
| RF-1000 | 760 ± 80 | 0.14 ± 0.01 |
| RF-1100 | 740 ± 80 | 0.15 ± 0.01 |
| RF-900 | RF-1000 | RF-1100 | |
|---|---|---|---|
| R1, Ohm | 11.3 ± 0.2 | 15.29 ± 0,04 | 13.47 ± 0.04 |
| T | 0.000144 ± 0.000004 | 0.00287 ± 0.00008 | 0.00038 ± 0.00001 |
| W | 6900 ± 200 | 350 ±10 | 2631 ± 70 |
| p | 0.845 ± 0.006 | 0.393 ± 0.003 | 0.716 ± 0.0004 |
| C1, F | 2.07 ± 0.01 | 1.59 ± 0.02 | 1.25 ± 0.0015 |
| Method * | RF-900 | RF-1000 | RF-1100 | |
|---|---|---|---|---|
| impedance | R, Ohm | 11.3 ± 0.2 | 15.29 ± 0.04 | 13.47 ± 0.04 |
| transient | ESR, Ohm | 26 | 35 | 32 |
| impedance | W(diffusion) Ohm/Hz-p | 6900 ± 200 | 350 ± 10 | 2631 ± 70 |
| impedance | p | 0.845 ± 0.006 | 0.393 ± 0.003 | 0.716 ± 0.0004 |
| impedance | C, F | 2.07 ± 0.01 | 1.59 ± 0.02 | 1.25 ± 0.0015 |
| CVA | C, F | 1.39 | 1.07 | 1.14 |
| transient | C, F | 2.1 | 1.75 | 1.35 |
| impedance | C(sp), F/g | 152 | 106 | 107 |
| CVA | C(sp), F/g | 102 | 71 | 97 |
| transient | C(sp), F/g | 154 | 117 | 115 |
| transient | tdischarge95%, sec | >100 | 80 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkova, A.N.; Sipyagina, N.A.; Gozhikova, I.O.; Dobrovolsky, Y.A.; Konev, D.V.; Baranchikov, A.E.; Ivanova, O.S.; Ukshe, A.E.; Lermontov, S.A. Electrochemical Properties of Carbon Aerogel Electrodes: Dependence on Synthesis Temperature. Molecules 2019, 24, 3847. https://doi.org/10.3390/molecules24213847
Malkova AN, Sipyagina NA, Gozhikova IO, Dobrovolsky YA, Konev DV, Baranchikov AE, Ivanova OS, Ukshe AE, Lermontov SA. Electrochemical Properties of Carbon Aerogel Electrodes: Dependence on Synthesis Temperature. Molecules. 2019; 24(21):3847. https://doi.org/10.3390/molecules24213847
Chicago/Turabian StyleMalkova, Alena N., Nataliya A. Sipyagina, Inna O. Gozhikova, Yury A. Dobrovolsky, Dmitry V. Konev, Alexander E. Baranchikov, Olga S. Ivanova, Alexander E. Ukshe, and Sergey A. Lermontov. 2019. "Electrochemical Properties of Carbon Aerogel Electrodes: Dependence on Synthesis Temperature" Molecules 24, no. 21: 3847. https://doi.org/10.3390/molecules24213847
APA StyleMalkova, A. N., Sipyagina, N. A., Gozhikova, I. O., Dobrovolsky, Y. A., Konev, D. V., Baranchikov, A. E., Ivanova, O. S., Ukshe, A. E., & Lermontov, S. A. (2019). Electrochemical Properties of Carbon Aerogel Electrodes: Dependence on Synthesis Temperature. Molecules, 24(21), 3847. https://doi.org/10.3390/molecules24213847