Design, Synthesis and Study of Nitrogen Monoxide Donors as Potent Hypolipidaemic and Anti-Inflammatory Agents
Abstract
1. Introduction
2. Results and Discussion
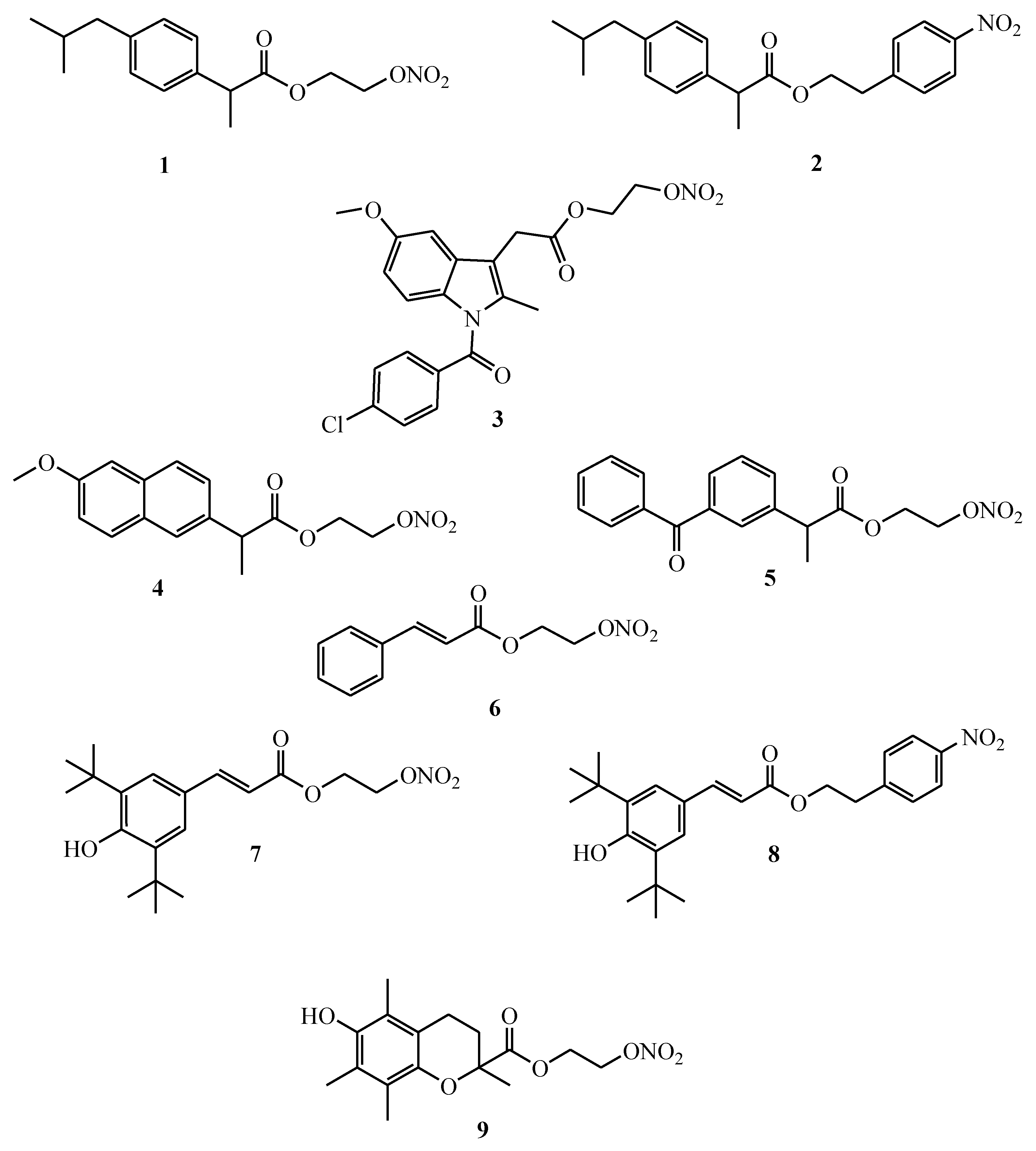
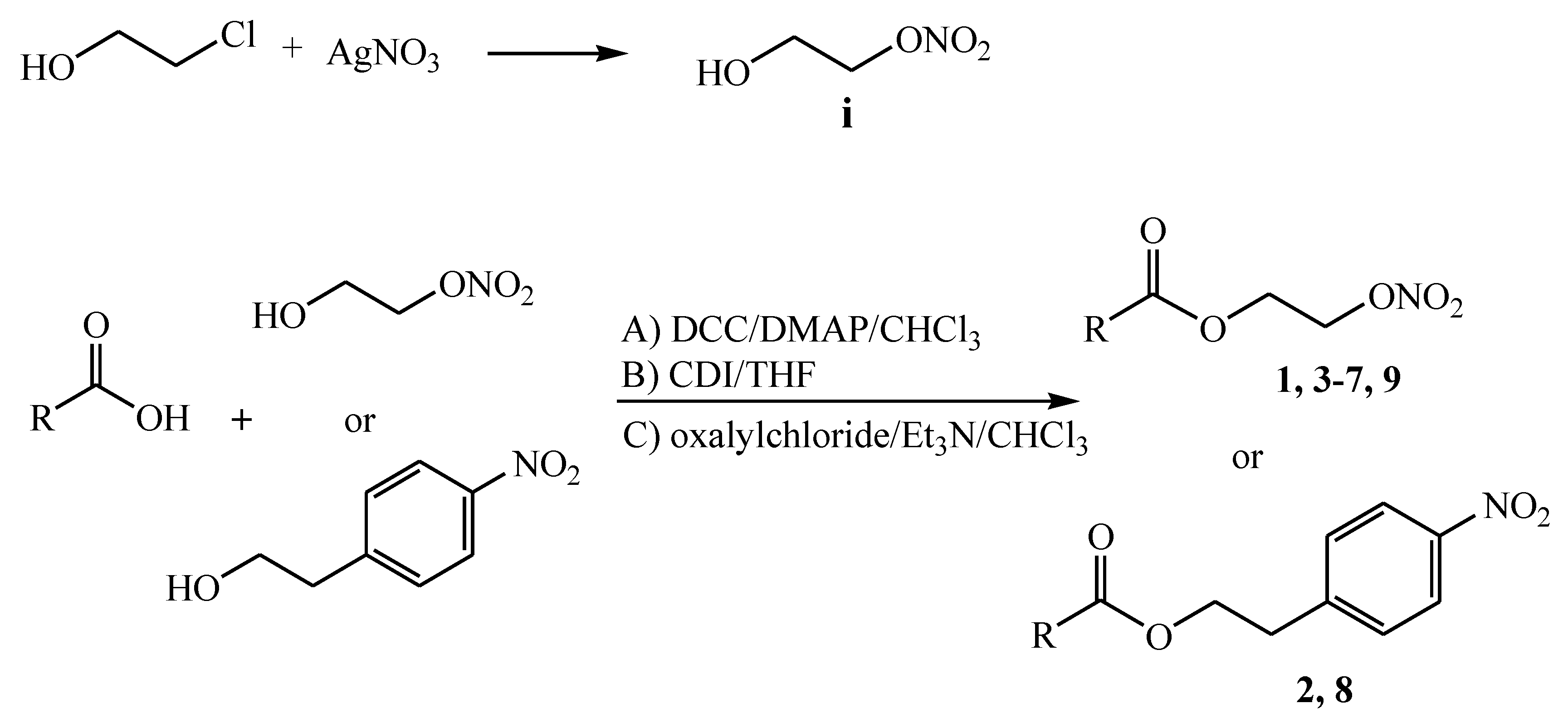
2.1. Synthesis
2.2. Hypolipidaemic Effect
2.3. Nitrogen Monoxide Release
2.4. Lipoxygenase Inhibitory Activity
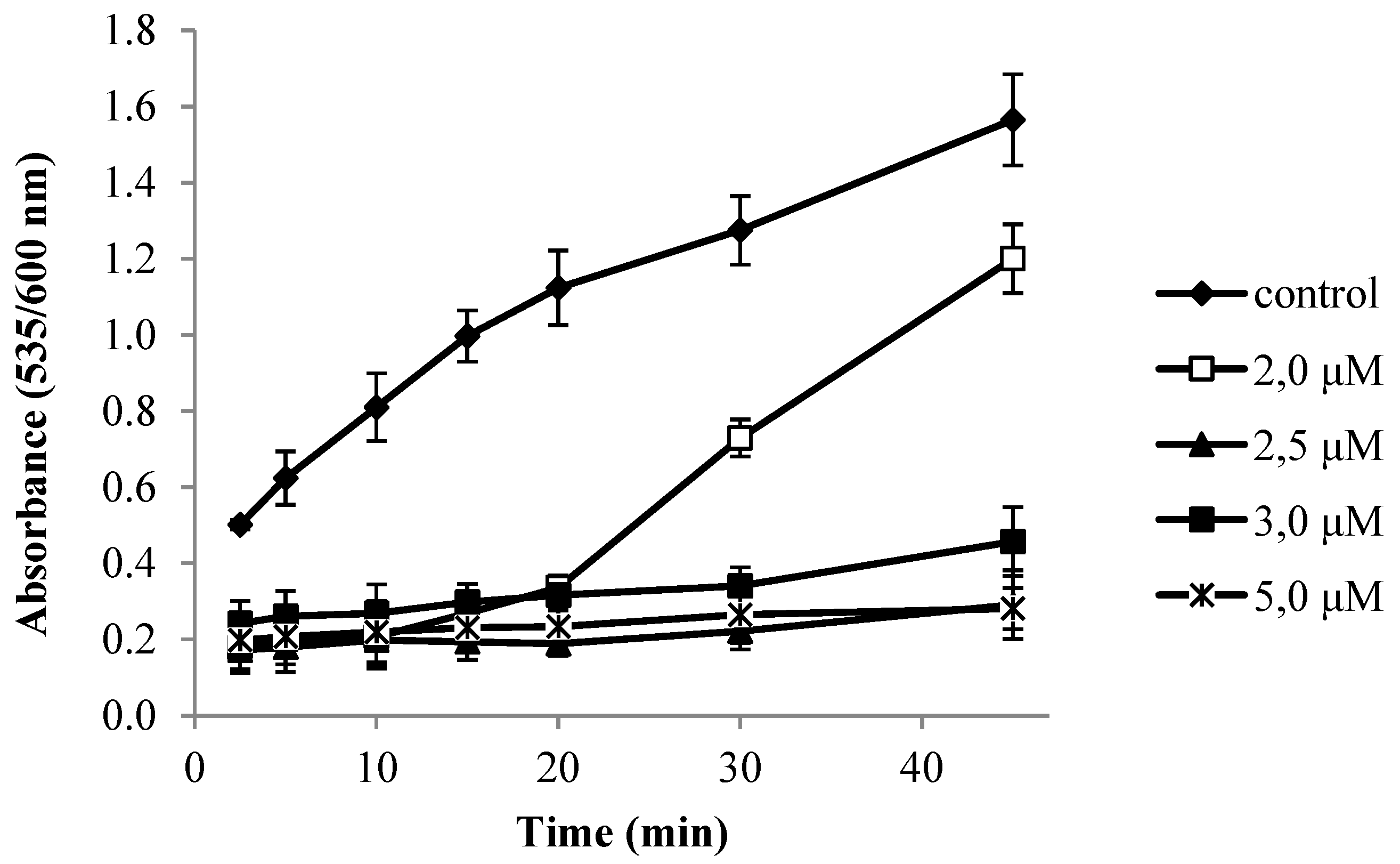
2.5. Antioxidant and Radical Scavenging Activity
2.6. In Vivo Anti-Inflammatory Activity
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. 2-Nitrooxy-Ethanol [9]
3.2.2. General Procedures for the Synthesis of Compounds 1–9
3.3. Biological Evaluation
3.3.1. Effect on Plasma Cholesterol, Triglyceride and LDL-Cholesterol Levels
3.3.2. In Vitro NO Release
3.3.3. In Vitro Evaluation of Lipoxygenase Activity
3.3.4. In Vitro Lipid Peroxidation
3.3.5. In Vitro Interaction with the Stable Radical 1,1-Diphenyl-2-Picrylhydrazyl (DPPH)
3.3.6. Carrageenan-Induced Paw Oedema
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez, A.; Calpena, A.C.; Clares, B. Evaluating the oxidative stress in inflammation: Role of melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Bäumer, A.T.; Temur, Y.; Kebben, D.; Jockenhovel, F.; Bohm, M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation 1999, 100, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Tziona, P.; Theodosis-Nobelos, P.; Rekka, E.A. Medicinal Chemistry approaches of controlling gastrointestinal side effects of non-steroidal anti-inflammatory drugs. Endogenous protective mechanisms and drug design. Med. Chem. 2017, 13, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Hofker, M. Lipoxygenases at the heart of atherosclerosis susceptibility. Eur. J. Hum. Genet. 2004, 12, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.K.; Lieb, D.C.; Dobrian, A.D.; Nadler, J.L. 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2013, 84, 104–105. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Wenzel, P.; Daiber, A.; Oelze, M.; Brandt, M.; Closs, E.; Xu, J.; Thum, T.; Bauersachs, J.; Ertl, G.; Zou, M.H.; et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis 2007, 198, 65–76. [Google Scholar] [CrossRef]
- Ziakas, G.N.; Rekka, E.A.; Gavalas, A.M.; Eleftheriou, P.T.; Tsiakitzis, K.C.; Kourounakis, P.N. Nitric oxide releasing derivatives of tolfenamic acid with anti-inflammatory activity and safe gastrointestinal profile. Bioorg. Med. Chem. 2005, 13, 6485–6492. [Google Scholar] [CrossRef]
- Kaushal, M.; Kutty, N.G.; Rao, C.M. Nitrooxyethylation reverses the healing-suppressant effect of ibuprofen. Mediat. Inflamm. 2006, 2006, 24396. [Google Scholar] [CrossRef]
- Bézière, N.; Goossens, L.; Pommery, J.; Vezin, H.; Touati, N.; Hénichart, J.P.; Pommery, N. New NSAIDs-NO hybrid molecules with antiproliferative properties on human prostatic cancer cell lines. Bioorg. Med. Chem. Lett. 2008, 18, 4655–4657. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Anter, E.; Keaney, F. Oxidative stress, antioxidants, and endothelial function. Curr. Med. Chem. 2004, 11, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Tooulia, K.K.; Theodosis-Nobelos, P.; Rekka, E.A. Thiomorpholine derivatives with hypolipidemic and antioxidant activity. Arch. Pharm. (Weinheim) 2015, 348, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Korolenko, T.A.; Tuzikov, F.V.; Vasil’eva, E.D.; Cherkanova, M.S.; Tuzikova, N.A. Fractional composition of blood serum lipoproteins in mice and rats with Triton WR 1339-induced lipemia. Bull. Exp. Biol. Med. 2010, 149, 567–570. [Google Scholar] [CrossRef]
- Branchi, A.; Fiorenza, A.M.; Rovellini, A.; Torri, A.; Muzio, F.; Macor, S.; Sommariva, D. Lowering effects of four different statins on serum triglyceride level. Eur. J. Clin. Pharmacol. 1999, 55, 499–502. [Google Scholar] [CrossRef]
- Aji, W.; Ravalli, S.; Szabolcs, M.; Jiang, X.C.; Sciacca, R.R.; Michler, R.E.; Cannon, P.J. L-arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation 1997, 95, 430–437. [Google Scholar] [CrossRef]
- Folcik, V.A.; Nivar-Aristy, R.A.; Krajewski, L.P.; Cathcart, M.K. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J. Clin. Investig. 1995, 96, 504–510. [Google Scholar] [CrossRef]
- Bushnell, E.A.C.; Berryman, V.E.J.; Gauld, J.W.; Boyd, R.J. The importance of the MM environment and the selection of the QM method in QM/MM calculations: Applications to enzymatic reactions. In Advances in Protein Chemistry and Structural Biology; Karabencheva-Christova, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 100, Chapter 6. [Google Scholar]
- Jacob, P.J.; Manju, S.L.; Ethiraj, K.R.; Elia, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Theodosis-Nobelos, P.; Kourounakis, P.N.; Rekka, E.A. Anti-inflammatory and hypolipidemic effect of novel conjugates with trolox and other antioxidant acids. Med. Chem. 2017, 13, 214–225. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef]
- Di Rosa, M.; Giroud, J.P.; Willoughby, D.A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 1971, 104, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Tziona, P.; Poptsis, A.; Athanasekou, C.; Kourounakis, P.N.; Rekka, E.A. Novel polyfunctional esters of ibuprofen and ketoprofen with hypolipidemic, lipoxygenase inhibitory and enhanced anti-inflammatory activity. Med. Chem. Res. 2017, 26, 461–472. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Gavalas, A.; Rekka, E.A. Amides of non-steroidal anti-inflammatory drugs with thiomorpholine can yield hypolipidemic agents with improved anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2016, 26, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Tsiakitzis, K.C.; Papagiouvannis, G.; Theodosis-Nobelos, P.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Synthesis, antioxidant and anti-inflammatory effects of antioxidant acid amides with GABA and n-acyl-pyrrolidin-2-ones. Curr. Chem. Biol. 2017, 11, 127–139. [Google Scholar] [CrossRef]
- Gund, M.; Gaikwad, P.; Borhade, N.; Burhan, A.; Desai, D.C.; Sharma, A.; Dhiman, M.; Patil, M.; Sheikh, J.; Thakre, G.; et al. Gastric-sparing nitric oxide-releasable ‘true’ prodrugs of aspirin and naproxen. Bioorg. Med. Chem. Lett. 2014, 24, 5587–5592. [Google Scholar] [CrossRef]
- Cena, C.; Boschi, D.; Tron, G.C.; Chegaev, K.; Lazzarato, L.; Di Stilo, A.; Aragno, M.; Fruttero, R.; Gasco, A. Development of a new class of potential antiatherosclerosis agents: NO-donor antioxidants. Bioorg. Med. Chem. Lett. 2004, 14, 5971–5974. [Google Scholar] [CrossRef]
- López, G.V.; Batthyány, C.; Blanco, F.; Botti, H.; Trostchansky, A.; Migliaro, E.; Radi, R.; González, M.; Cerecetto, H.; Rubbo, H. Design, synthesis, and biological characterization of potential antiatherogenic nitric oxide releasing tocopherol analogs. Bioorg. Med. Chem. 2005, 13, 5787–5796. [Google Scholar] [CrossRef]
- López, G.V.; Blanco, F.; Hernández, P.; Ferreira, A.; Piro, O.E.; Batthyány, C.; González, M.; Rubbo, H.; Cerecetto, H. Second generation of alpha-tocopherol analogs-nitric oxide donors: Synthesis, physicochemical, and biological characterization. Bioorg. Med. Chem. 2007, 15, 6262–6272. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | Dose (i.p.) (μmol/kg) | % Reduction | ||
|---|---|---|---|---|
| TC a | TG b | LDL-C c | ||
| 1 | 150 | 62.2 ** | 30.1 ** | 80.0 * |
| 3 | 150 | 71.7 ** | 31.8 ** | 62.3 * |
| 4 | 150 | 78.2 *** | 38.9 *** | 84.6 *** |
| 5 | 150 | 46.6 ** | 40.2 *** | 57.8 *** |
| 6 | 150 | 70.9 *** | 54.4 *** | 74.8 *** |
| 7 | 150 | 65.5 ** | 45.7 *** | 60.6 * |
| 9 | 150 | 55.4 *** | 30.0 *** | 65.0 *** |
| Simvastatin | 150 | 73.0 *** | - | 70.0 *** |
| Ibuprofen | 300 | 41.0 *** | 38.0 *** | 41.6 *** |
| Naproxen | 500 | 53.0 *** | 43.5 *** | 25.5 *** |
| Compound | ΝΟ Release (μΜ)/Compound | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5 | 6 | 7 | 9 | |
| 500 μM | 116 | 102 | 73 | 100 | 98 | 77 | 93 |
| 250 μM | 82 | 58 | 37 | 57 | 51 | 38 | 49 |
| 125 μM | 40 | 28 | 17 | 26 | 22 | 17 | 23 |
| 62.5 μM | 17 | 13 | 7 | 11 | 11 | 6.5 | 10 |
| 31.25 μM | 7 | 5 | 2.5 | 4 | 5 | 2 | 3 |
| Compound | IC50 (μM) | clogP |
|---|---|---|
| 1 | 46 | 4.65 |
| 2 | 24 | 5.92 |
| 3 | 107 | 5.15 |
| 4 | >>300 | 3.78 |
| 5 | 86 | 3.73 |
| 6 | 220 | 3.18 |
| 7 | 44 | 6.16 |
| 8 | 10.5 | 7.44 |
| 9 | 120 | 4.16 |
| Ibuprofen | 200 | |
| Ketoprofen | 220 | |
| Trolox | >>300 | 3.10 |
| NDGA | 1.3 |
| Compound | Percent Interaction with DPPH | Inhibition of Lipid Peroxidation IC50 (μΜ) | ||
|---|---|---|---|---|
| 200 μΜ | 100 μΜ | 50 μΜ | ||
| 7 | 87 | 49 | 21 | 41 |
| 8 | 88 | 57 | 23 | 150 |
| 9 | 92 | 90 | 55 | 2.3 |
| Trolox | 92 | 90 | 38 | 25 |
| Compound | % Oedema Reduction |
|---|---|
| 1 | 76 ** |
| 2 | 53 * |
| 3 | 29 ** |
| 4 | 70 ** |
| 5 | 75 ** |
| 6 | 61 ** |
| 7 | 55 ** |
| 8 | 51 ** |
| 9 | 57 ** |
| Ibuprofen | 36 * |
| Indomethacin | 42 * |
| Naproxen | 11 * |
| Ketoprofen | 47 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosis-Nobelos, P.; Papagiouvanis, G.; Pantelidou, M.; Kourounakis, P.N.; Athanasekou, C.; Rekka, E.A. Design, Synthesis and Study of Nitrogen Monoxide Donors as Potent Hypolipidaemic and Anti-Inflammatory Agents. Molecules 2020, 25, 19. https://doi.org/10.3390/molecules25010019
Theodosis-Nobelos P, Papagiouvanis G, Pantelidou M, Kourounakis PN, Athanasekou C, Rekka EA. Design, Synthesis and Study of Nitrogen Monoxide Donors as Potent Hypolipidaemic and Anti-Inflammatory Agents. Molecules. 2020; 25(1):19. https://doi.org/10.3390/molecules25010019
Chicago/Turabian StyleTheodosis-Nobelos, Panagiotis, Georgios Papagiouvanis, Maria Pantelidou, Panos N. Kourounakis, Chrysoula Athanasekou, and Eleni A. Rekka. 2020. "Design, Synthesis and Study of Nitrogen Monoxide Donors as Potent Hypolipidaemic and Anti-Inflammatory Agents" Molecules 25, no. 1: 19. https://doi.org/10.3390/molecules25010019
APA StyleTheodosis-Nobelos, P., Papagiouvanis, G., Pantelidou, M., Kourounakis, P. N., Athanasekou, C., & Rekka, E. A. (2020). Design, Synthesis and Study of Nitrogen Monoxide Donors as Potent Hypolipidaemic and Anti-Inflammatory Agents. Molecules, 25(1), 19. https://doi.org/10.3390/molecules25010019








