Plant-Derived Molecules α-Boswellic Acid Acetate, Praeruptorin-A, and Salvianolic Acid-B Have Age-Related Differential Effects in Young and Senescent Human Fibroblasts In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Age-Related Differential Effects on Metabolic Activity and Cellular Growth
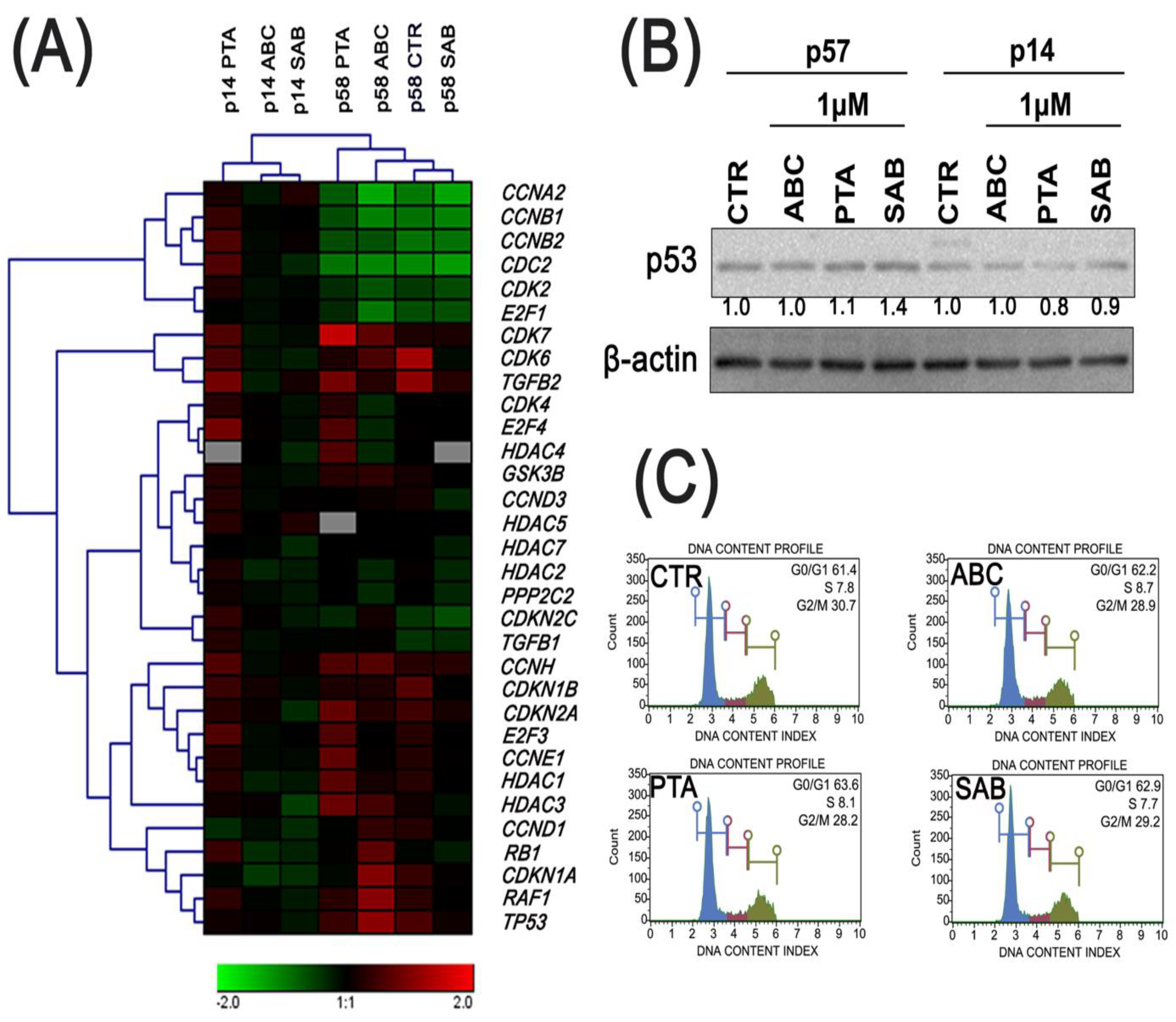
2.2. Cell cycle progression and the expression of proliferation-related genes
2.3. Oxidative Protein Damage, Protein Aggregation and Autophagy
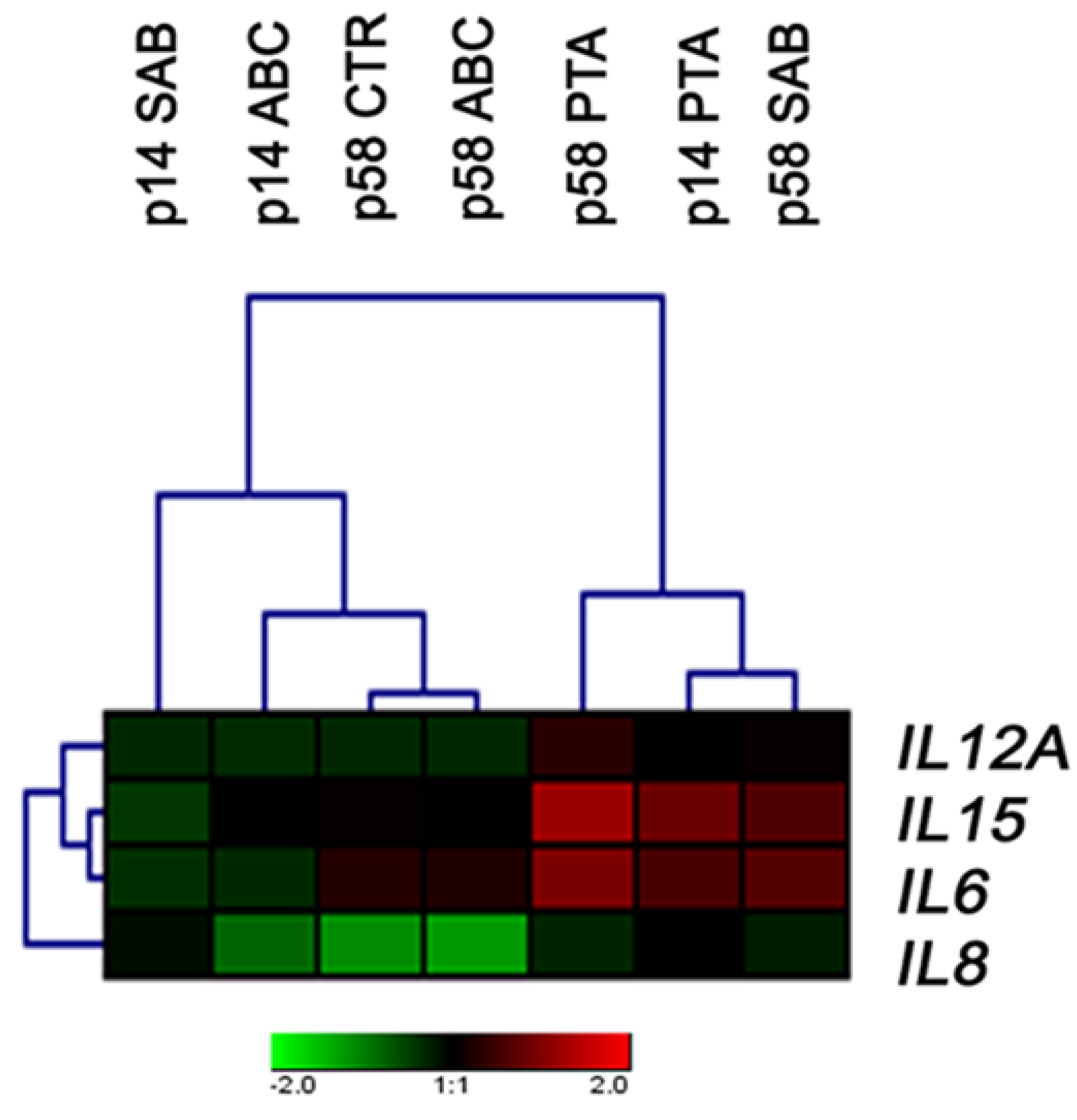
2.4. The Expression of Cytokine Genes
3. Materials and Methods
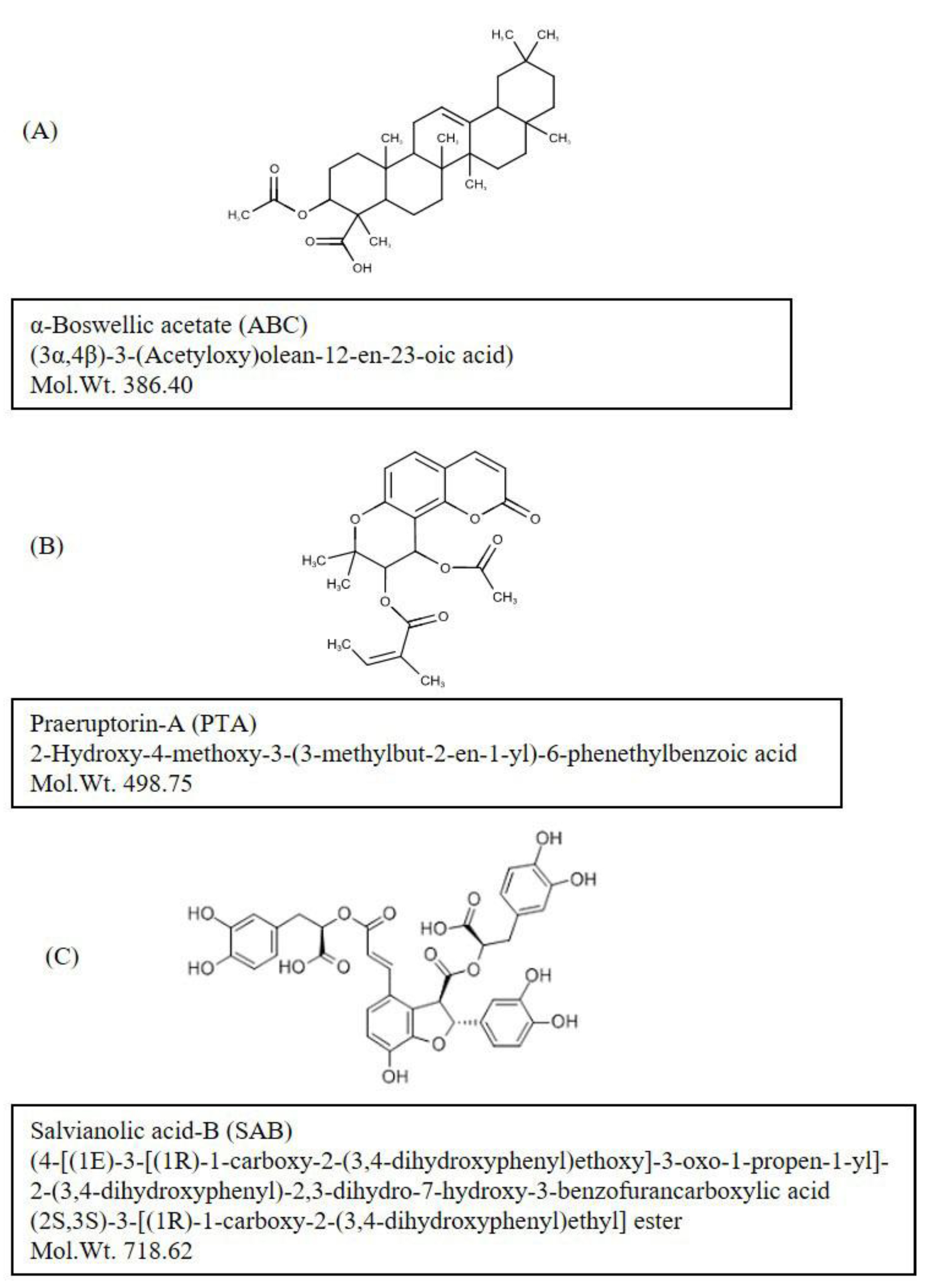
3.1. Phytochemicals
3.2. Cell Culture
3.3. Metabolic Activity, Short-Term Growth, Cell Cycle Analysis and Rejuvenation
3.4. Western Blotting
3.5. Protein Carbonylation and Aggregation
3.6. Real-Time PCR Using TaqMan® Arrays
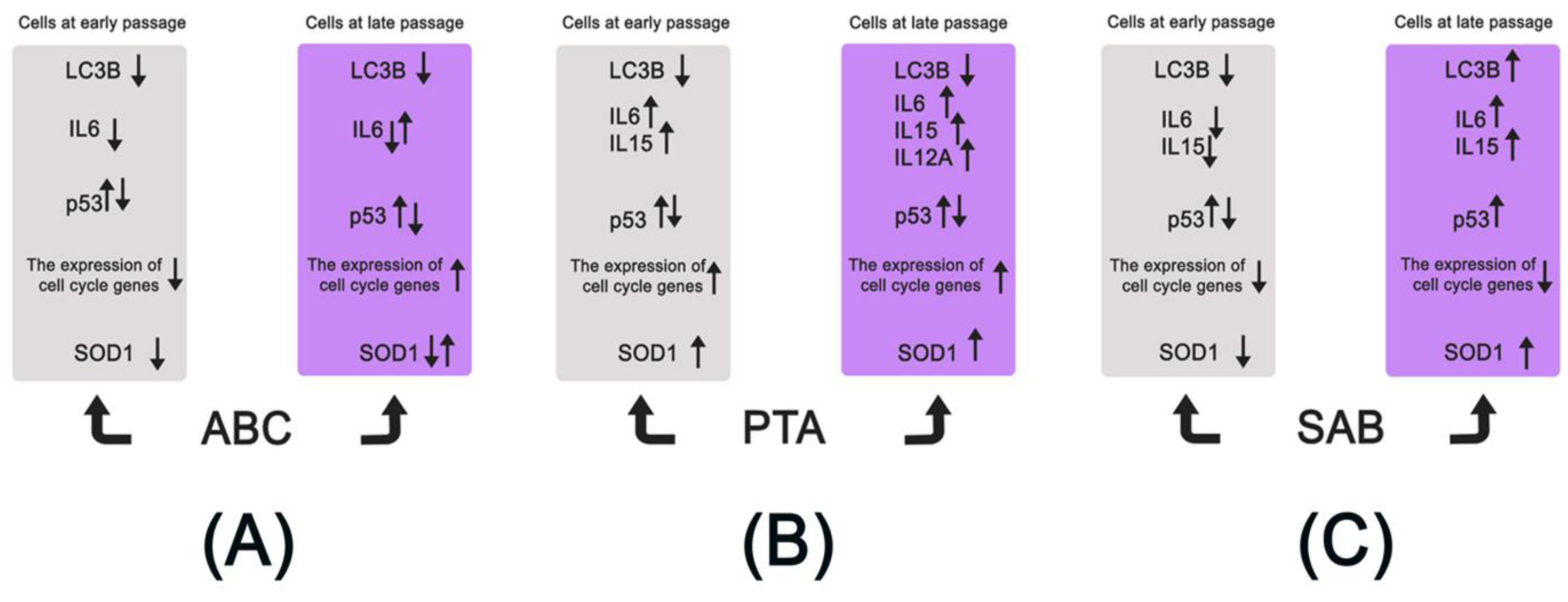
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obrenovich, M.E.; Nair, N.G.; Beyaz, A.; Aliev, G.; Reddy, V.P. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res. 2010, 13, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ahmad, W.; Bukhari, S.N. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant. Sci 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed]
- Maria, J.; Ingrid, Z. Effects of bioactive compounds on senescence and components of senescence associated secretory phenotypes in vitro. Food Funct. 2017, 8, 2394–2418. [Google Scholar] [CrossRef] [PubMed]
- Gurau, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafe, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Rationale and methods of discovering hormetins as drugs for healthy ageing. Expert Opin. Drug Discov. 2012, 7, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Furst, R.; Zundorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm 2014, 2014, 146832. [Google Scholar] [CrossRef]
- Rattan, S.I.S.; Leonard, H. Cellular Ageing and Replicative Senescence. Healthy Ageing and Longevity; Springer: Dordrecht, The Netherlands, 2016; Volume 4. [Google Scholar]
- Rattan, S.I.; Clark, B.F. Kinetin delays the onset of ageing characteristics in human fibroblasts. Biochem. Biophys. Res. Commun. 1994, 201, 665–672. [Google Scholar] [CrossRef]
- Rattan, S.I.; Sodagam, L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res. 2005, 8, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Sodagam, L.; Lewinska, A.; Kwasniewicz, E.; Kokhanovska, S.; Wnuk, M.; Siems, K.; Rattan, S.I.S. Phytochemicals rosmarinic acid, ampelopsin, and amorfrutin-A can modulate age-related phenotype of serially passaged human skin fibroblasts in vitro. Front. Genet. 2019, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Luyten, W.; Antal, P.; Braeckman, B.P.; Bundy, J.; Cirulli, F.; Fang-Yen, C.; Fuellen, G.; Leroi, A.; Liu, Q.; Martorell, P.; et al. Ageing with elegans: A research proposal to map healthspan pathways. Biogerontology 2016, 17, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Banik, K.; Bordoloi, D.; Harsha, C.; Sailo, B.L.; Padmavathi, G.; Roy, N.K.; Gupta, S.C.; Aggarwal, B.B. Googling the guggul (commiphora and boswellia) for prevention of chronic diseases. Front. Pharmacol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wei, X.C.; Xu, X.R.; Zhang, H.Z.; Luo, C.H.; Feng, B.; Xu, R.C.; Zhao, S.Y.; Du, X.J.; Han, L.; et al. Seeing the unseen of the combination of two natural resins, frankincense and myrrh: Changes in chemical constituents and pharmacological activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Entschladen, F.; Liu, H.; Niggemann, B.; Fang, Q.; Zaenker, K.S.; Han, R. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cells. Cancer Detect. Prev. 2003, 27, 67–75. [Google Scholar] [CrossRef]
- Xia, L.; Chen, D.; Han, R.; Fang, Q.; Waxman, S.; Jing, Y. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol. Cancer Ther. 2005, 4, 381–388. [Google Scholar]
- Tipton, D.A.; Lyle, B.; Babich, H.; Dabbous, M. In vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. Toxicol. In Vitro 2003, 17, 301–310. [Google Scholar] [CrossRef]
- Sarkhail, P.; Shafiee, A.; Sarkheil, P. Biological activities and pharmacokinetics of praeruptorins from Peucedanum species: A systematic review. Biomed. Res. Int. 2013, 2013, 343808. [Google Scholar] [CrossRef]
- Hung, C.Y.; Lee, C.H.; Chiou, H.L.; Lin, C.L.; Chen, P.N.; Lin, M.T.; Hsieh, Y.H.; Chou, M.C. Praeruptorin-b inhibits 12-o-tetradecanoylphorbol-13-acetate-induced cell invasion by targeting akt/nf-kappab via matrix metalloproteinase-2/-9 expression in human cervical cancer cells. Cell Physiol. Biochem. 2019, 52, 1255–1266. [Google Scholar] [PubMed]
- Wu, M.H.; Lin, C.L.; Chiou, H.L.; Yang, S.F.; Lin, C.Y.; Liu, C.J.; Hsieh, Y.H. Praeruptorin A inhibits human cervical cancer cell growth and invasion by suppressing MMP-2 expression and ERK1/2 signaling. Int. J. Mol. Sci. 2017, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.J.; Ci, W.; Wang, G.F.; Zhang, J.Y.; Wu, S.Y.; Xu, W.; Jin, H.; Zhu, Z.G.; Zhang, J.J.; Pang, J.X.; et al. Praeruptorin A inhibits lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-kappaB pathway activation. Phytother Res. 2011, 25, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bi, H.; Jin, J.; Niu, L.; Cai, D.; Deng, R.; Li, Y.; Wang, Y.; Huang, M. Effects of praeruptorin A and praeruptorin C, a racemate isolated from Peucedanum praeruptorum, on MRP2 through the CAR pathway. Planta Med. 2013, 79, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, J.; Wu, F.; Li, J.; Zhou, L.; Kong, L. Effects of (+/-)-praeruptorin A on airway inflammation, airway hyperresponsiveness and NF-kappaB signaling pathway in a mouse model of allergic airway disease. Eur. J. Pharmacol. 2012, 683, 316–324. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, Y.C.; Hu, W.L.; Hung, Y.C. Oxidative stress and salvia miltiorrhiza in aging-associated cardiovascular diseases. Oxid Med. Cell Longev. 2016, 2016, 4797102. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Gu, X. Salvianolic acid B, a potential chemopreventive agent, for head and neck squamous cell cancer. J. Oncol. 2011, 2011, 534548. [Google Scholar] [CrossRef]
- Lee, H.P.; Liu, Y.C.; Chen, P.C.; Tai, H.C.; Li, T.M.; Fong, Y.C.; Chang, C.S.; Wu, M.H.; Chiu, L.P.; Wang, C.J.; et al. Tanshinone IIA inhibits angiogenesis in human endothelial progenitor cells in vitro and in vivo. Oncotarget 2017, 8, 109217–109227. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Lin, C.W.; Wang, P.H.; Hsin, M.C.; Yang, S.F. The potential of Chinese herbal medicines in the treatment of cervical cancer. Integr. Cancer Ther. 2019, 18, 1534735419861693. [Google Scholar] [CrossRef]
- Wang, E.; Tomaszewski, G. Granular presence of terminin is the marker to distinguish between the senescent and quiescent states. J. Cell Physiol. 1991, 147, 514–522. [Google Scholar] [CrossRef]
- Wang, E. Are all nonproliferating cells similar? Exp. Gerontol. 1992, 27, 419–423. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, Z.; Lu, Y.; Yao, Y.; Zhang, Y.; Ma, Z.; Kuai, M.; Sun, X.; Sun, S.; Jing, Y.; et al. Cardioprotective effect of salvianolic acid B on acute myocardial infarction by promoting autophagy and neovascularization and inhibiting apoptosis. J. Pharm. Pharmacol. 2016, 68, 941–952. [Google Scholar] [CrossRef]
- Jing, Z.; Fei, W.; Zhou, J.; Zhang, L.; Chen, L.; Zhang, X.; Liang, X.; Xie, J.; Fang, Y.; Sui, X.; et al. Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 2016, 7, 61509–61519. [Google Scholar] [CrossRef]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. Akt/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Li, Q.; Zhang, F.; Sun, M.; Wan, Z.; Wang, S. Protective effects of salvianolic acid B against hydrogen peroxide-induced apoptosis of human umbilical vein endothelial cells and underlying mechanisms. Int. J. Mol. Med. 2019, 44, 457–468. [Google Scholar] [CrossRef]
- Wu, J.Z.; Ardah, M.; Haikal, C.; Svanbergsson, A.; Diepenbroek, M.; Vaikath, N.N.; Li, W.; Wang, Z.Y.; Outeiro, T.F.; El-Agnaf, O.M.; et al. Dihydromyricetin and salvianolic acid B inhibit alpha-synuclein aggregation and enhance chaperone-mediated autophagy. Transl. Neurodegener. 2019, 8, 18. [Google Scholar] [CrossRef]
- Zhao, D.H.; Wu, Y.J.; Liu, S.T.; Liu, R.Y. Salvianolic acid B attenuates lipopolysaccharide-induced acute lung injury in rats through inhibition of apoptosis, oxidative stress and inflammation. Exp. Ther. Med. 2017, 14, 759–764. [Google Scholar] [CrossRef]
- Syrovets, T.; Buchele, B.; Krauss, C.; Laumonnier, Y.; Simmet, T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J. Immunol. 2005, 174, 498–506. [Google Scholar] [CrossRef]
- Sodagam, L.; Lewinska, A.; Wnuk, M.; Rattan, S.I.S. Chronic exposure to rapamycin and episodic serum starvation modulate ageing of human fibroblasts in vitro. Biogerontology 2017, 18, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2019, 28, 101337. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Klukowska-Rotzler, J.; Deregowska, A.; Adamczyk-Grochala, J.; Wnuk, M. C-myc activation promotes cofilin-mediated f-actin cytoskeleton remodeling and telomere homeostasis as a response to oxidant-based DNA damage in medulloblastoma cells. Redox Biol. 2019, 24, 101163. [Google Scholar] [CrossRef] [PubMed]
- Moros, M.; Lewinska, A.; Onorato, G.; Antognazza, M.R.; Di Maria, F.; Blasio, M.; Lanzani, G.; Tino, A.; Wnuk, M.; Tortiglione, C. Light-triggered modulation of cell antioxidant defense by polymer semiconducting nanoparticles in a model organism. MRS Commun. 2018, 8, 918–925. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Semik, E.; Zabek, T.; Wnuk, M. Reduced levels of methyltransferase DNMT2 sensitize human fibroblasts to oxidative stress and DNA damage that is accompanied by changes in proliferation-related miRNA expression. Redox Biol. 2018, 14, 20–34. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds acetyl-α-boswellic acid, praeruptorin-A and salvianolic acid-B are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewinska, A.; Sodagam, L.; Bloniarz, D.; Siems, K.; Wnuk, M.; Rattan, S.I.S. Plant-Derived Molecules α-Boswellic Acid Acetate, Praeruptorin-A, and Salvianolic Acid-B Have Age-Related Differential Effects in Young and Senescent Human Fibroblasts In Vitro. Molecules 2020, 25, 141. https://doi.org/10.3390/molecules25010141
Lewinska A, Sodagam L, Bloniarz D, Siems K, Wnuk M, Rattan SIS. Plant-Derived Molecules α-Boswellic Acid Acetate, Praeruptorin-A, and Salvianolic Acid-B Have Age-Related Differential Effects in Young and Senescent Human Fibroblasts In Vitro. Molecules. 2020; 25(1):141. https://doi.org/10.3390/molecules25010141
Chicago/Turabian StyleLewinska, Anna, Lakshman Sodagam, Dominika Bloniarz, Karsten Siems, Maciej Wnuk, and Suresh I. S. Rattan. 2020. "Plant-Derived Molecules α-Boswellic Acid Acetate, Praeruptorin-A, and Salvianolic Acid-B Have Age-Related Differential Effects in Young and Senescent Human Fibroblasts In Vitro" Molecules 25, no. 1: 141. https://doi.org/10.3390/molecules25010141
APA StyleLewinska, A., Sodagam, L., Bloniarz, D., Siems, K., Wnuk, M., & Rattan, S. I. S. (2020). Plant-Derived Molecules α-Boswellic Acid Acetate, Praeruptorin-A, and Salvianolic Acid-B Have Age-Related Differential Effects in Young and Senescent Human Fibroblasts In Vitro. Molecules, 25(1), 141. https://doi.org/10.3390/molecules25010141




