Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck)
Abstract
:1. Introduction
2. Results
2.1. Identification of Flavonoids and Limonoids
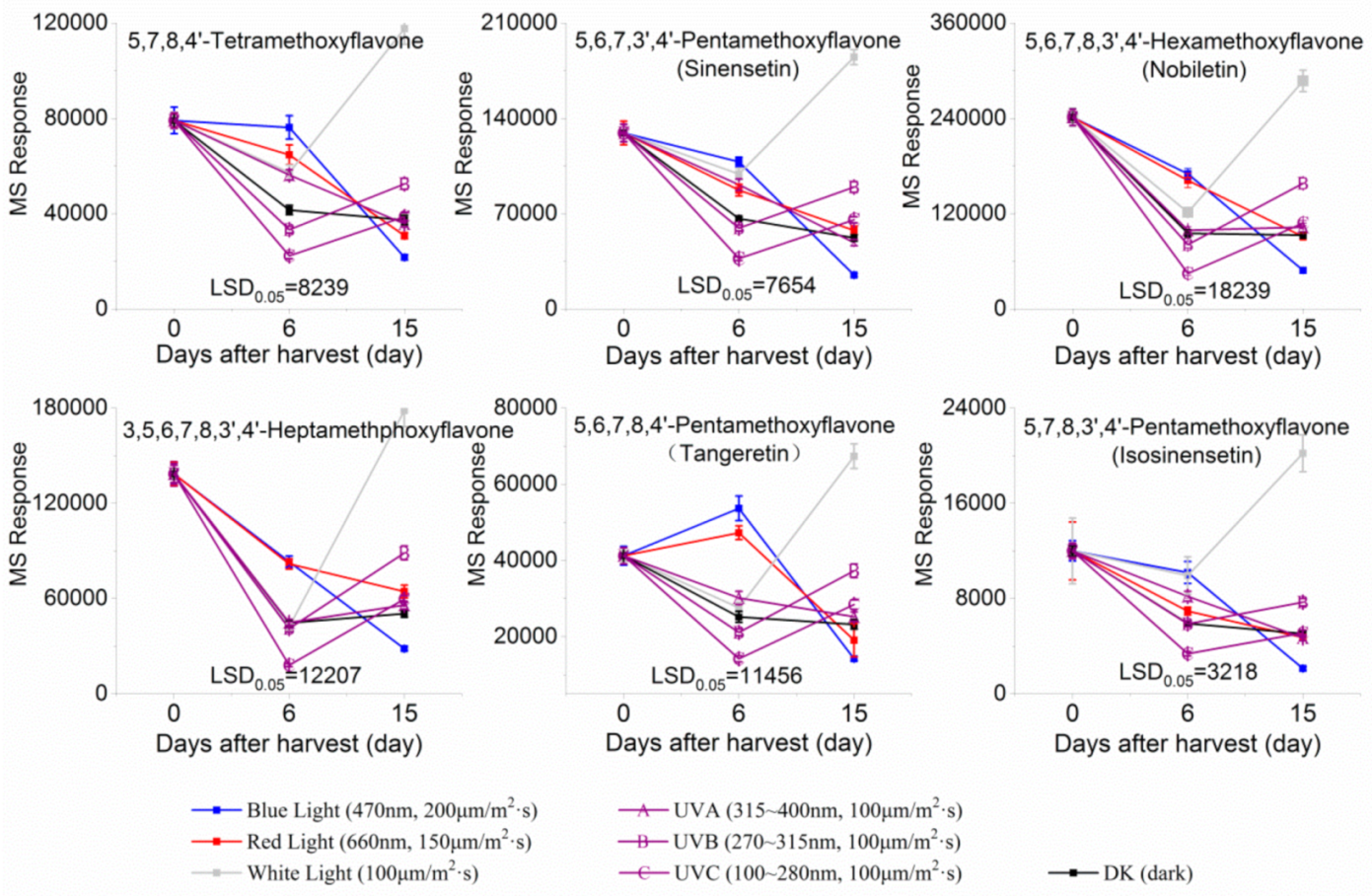
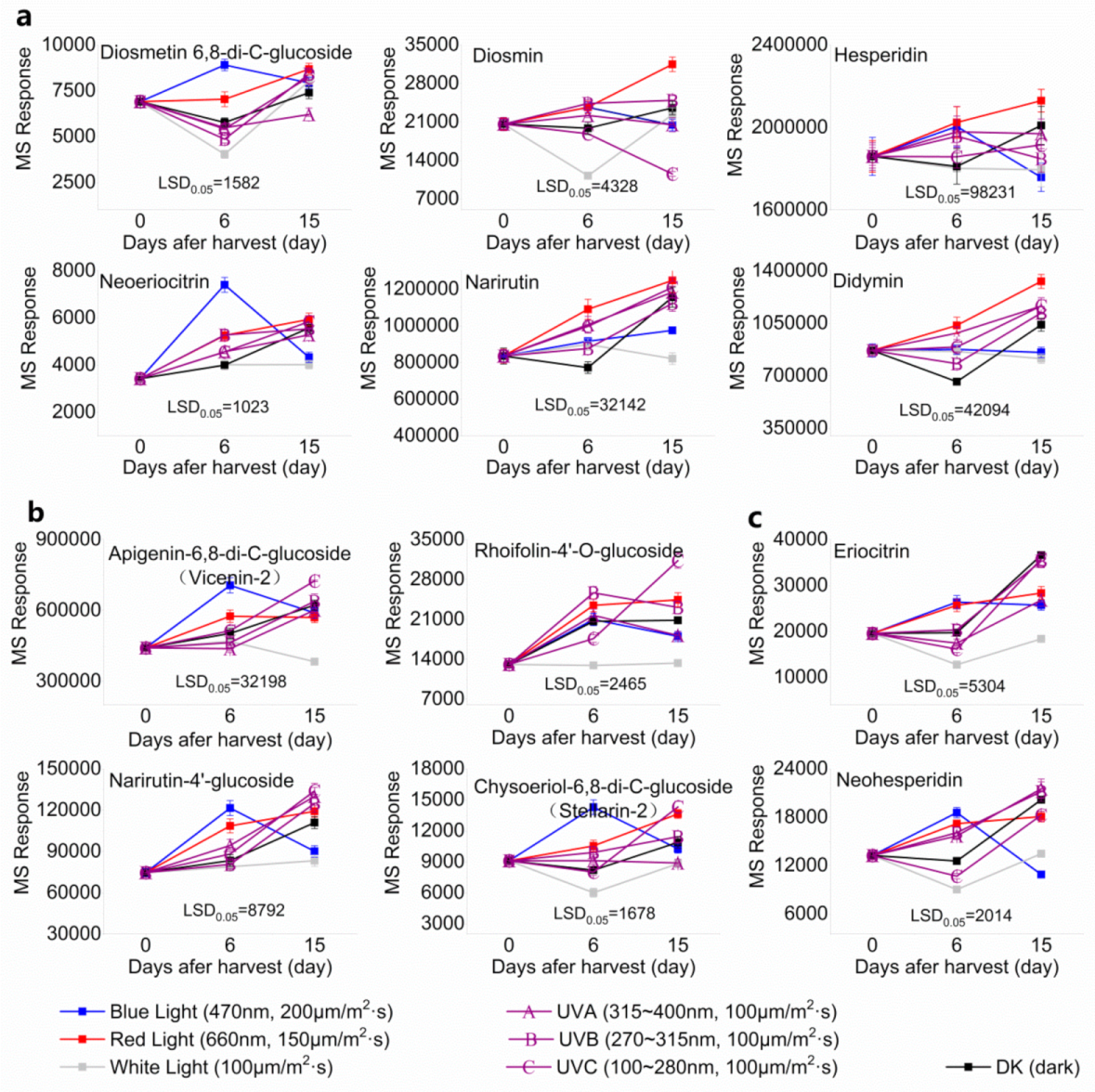
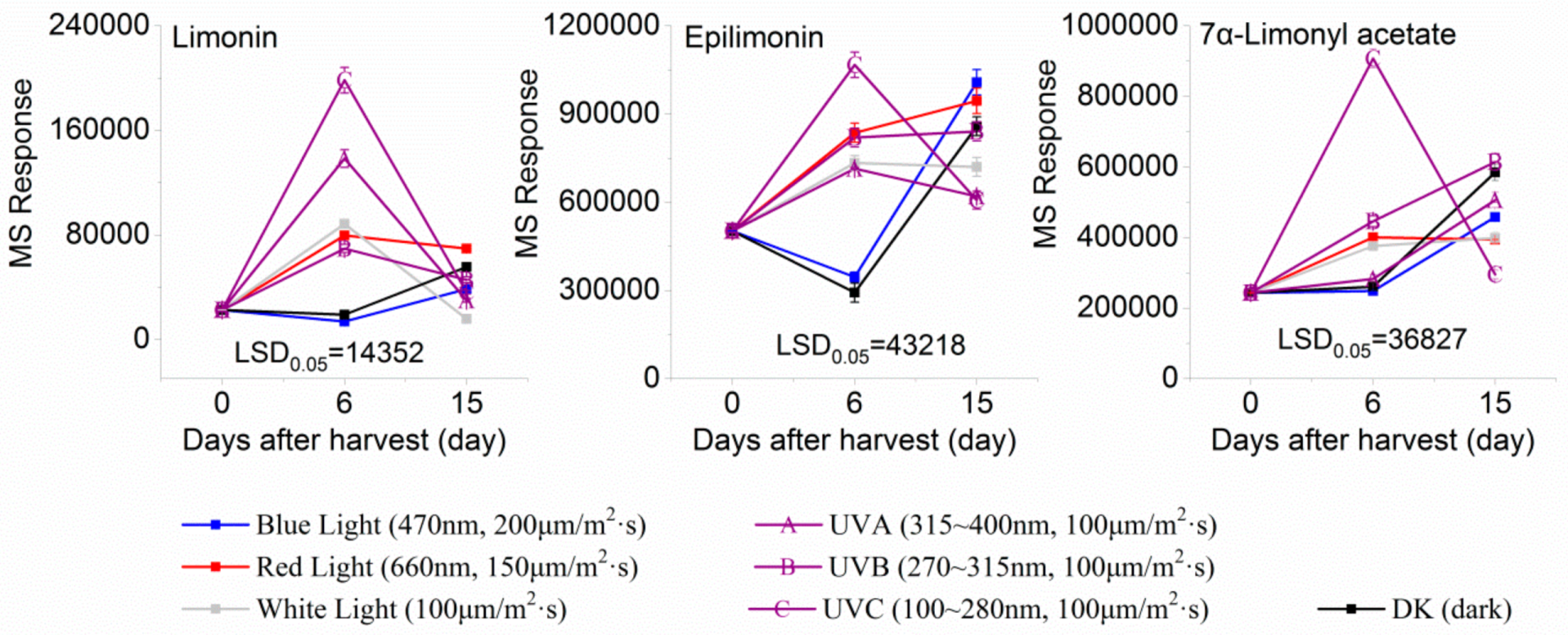
2.2. Effect of Light Irradiation on Flavonoids and Limonoids in the Segments of Newhall Navel Oranges
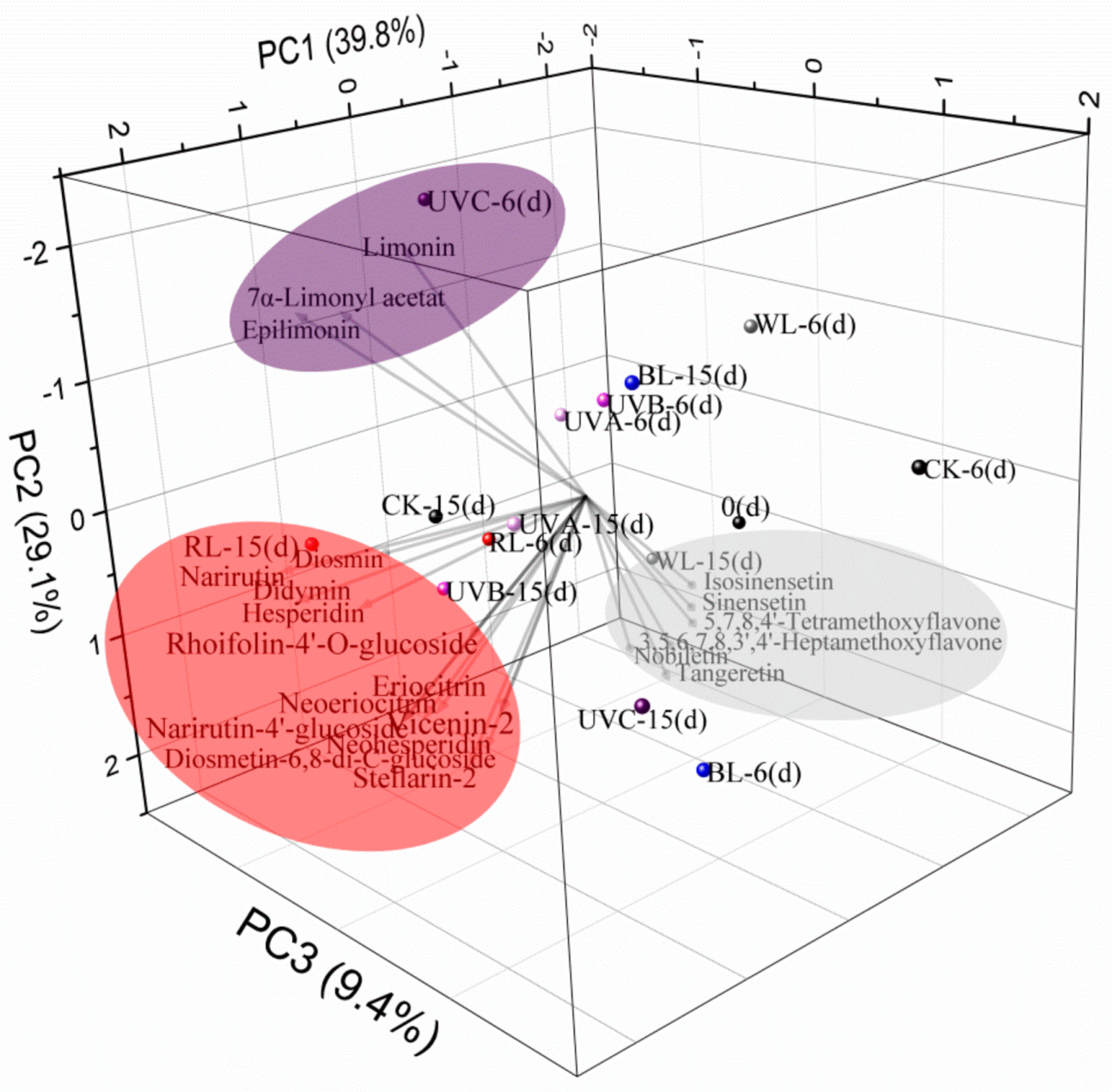
2.3. Principal Component Analysis of Flavonoid and Limonoid Responses
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Treatment
4.3. Extraction of Flavonoids and Limonoids
4.4. UPLC-qTOF-MS Analysis and MS Response Quantification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moradi, S.; Koushesh, S.M.; Mozafari, A.A.; Abdollahi, H. Antioxidant bioactive compounds changes in fruit of quince genotypes over cold storage. J. Food Sci. 2016, 81, H1833–H1839. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Gambourg, M.; Klein, J.D.; Lurie, S. Prestorage heat treatment reduces pathogenicity of Penicillium expansum in apple fruit. Plant Pathol. 2010, 45, 92–97. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in post-harvest broccoli. Postharvest Biol. Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef]
- Zheng, F.; Zheng, W.; Li, L.; Pan, S.; Liu, M.; Zhang, W.; Liu, H.; Zhu, C. Chitosan Controls Post-harvest Decay and Elicits Defense Response in Kiwifruit. Food Bioprocess Technol. 2017, 10, 1937–1945. [Google Scholar] [CrossRef]
- Cantos, E.; García-Viguera, C.; Pascual-Teresa, S.; De Tomás-Barberán, F.A. Effect of post-harvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Zavaleta-Gatica, R.; Tiznado-Hernández, M.E. Improving post-harvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biol. Technol. 2007, 45, 108–116. [Google Scholar]
- Pan, J.; Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Combined use of UV-C irradiation and heat treatment to improve post-harvest life of strawberry fruit. J. Sci. Food Agric. 2004, 84, 1831–1838. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous white–blue LED light exposition delays post-harvest senescence of broccoli. LWT Food Sci. Technol. 2008, 65, 495–502. [Google Scholar]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of Blue and Red LED Light Irradiation on β-Cryptoxanthin Accumulation in the Flavedo of Citrus Fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Regulation of ascorbic acid metabolism by blue LED light irradiation in citrus juice sacs. Plant Sci. 2015, 233, 134–142. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Chun, W.J.; Kim, T.Y.; Sun, J.H.; Yu, C.Y. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010, 120, 539–543. [Google Scholar] [CrossRef]
- Cazal, C.M.; Choosang, K.; Severino, V.G.; Soares, M.S.; Sarria, A.L.; Fernandes, J.B.; Silva, M.F.; Vieira, P.C.; Pakkong, P.; Almeida, G.M. Evaluation of effect of triterpenes and limonoids on cell growth, cell cycle and apoptosis in human tumor cell line. Anticancer Agents Med. Chem. 2010, 10, 769–776. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Alexandre, C. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, M.; Wu, R.; Niu, Q.; Teng, Y.; Zhang, D. Post-harvest pigmentation in red Chinese sand pears (Pyrus pyrifolia Nakai) in response to optimum light and temperature. Postharvest Biol. Technol. 2014, 91, 64–71. [Google Scholar] [CrossRef]
- Awad, M.A.; Wagenmakers, P.S.; Jager, A.D. Effects of light on flavonoid and chlorogenic acid levels in the skin of ‘Jonagold‘ apples. Sci. Hortic. 2001, 88, 289–298. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts post-harvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Xie, L.; He, Y.; Luo, T.; Sheng, L.; Luo, Y.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Regulation of cuticle formation during fruit development and ripening in ‘Newhall’ navel orange (Citrus sinensis Osbeck) revealed by transcriptomic and metabolomic profiling. Plant Sci. 2016, 243, 131–144. [Google Scholar] [CrossRef]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2018, 1–18. [Google Scholar] [CrossRef]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Feng, C.; Cheng, Y.; Deng, X. Comparative analysis of surface wax in mature fruits between Satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef]
- Lokesh Dalasanur, N.; Rit, V.; Jyotsana, S.; Spence, F.; Rhonda, R.; Sanjay, A.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109. [Google Scholar]
- Miyake, Y.; Yamamoto, K.; Morimitsu, Y. Isolation of C -Glucosylflavone from Lemon Peel and Antioxidative Activity of Flavonoid Compounds in Lemon Fruit. J. Agric. Food. Chem. 1998, 45, 1203–1208. [Google Scholar] [CrossRef]
- Subramani, S.; Leelavinothan, P. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem. Biol. Interact. 2012, 195, 43–51. [Google Scholar]
- Fu, Q.; Zhang, C.; Lin, Z. Rapid screening and identification of compounds with DNA-binding activity from Folium Citri Reticulatae using on-line HPLC–DAD–MSn coupled with a post column fluorescence detection system. Food Chem. 2016, 192, 250–259. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Kumquat (Fortunella japonica Swingle) juice: Flavonoid distribution and antioxidant properties. Food Res. Int. 2011, 44, 2190–2197. [Google Scholar] [CrossRef]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci Rep. 2014, 4, 3708. [Google Scholar] [CrossRef]
- Miyake, Y.; Suzuki, E.; Ohya, S.; Fukumoto, S.; Hiramitsu, M.; Sakaida, K.; Osawa, T.; Furuichi, Y. Lipid-Lowering Effect of Eriocitrin, the Main Flavonoid in Lemon Fruit, in Rats on a High-Fat and High-Cholesterol Diet. J. Food Sci. 2010, 71, S633–S637. [Google Scholar] [CrossRef]
- Funaguchi, N.; Ohno, Y.; La, B.L.; Asai, T.; Yuhgetsu, H.; Sawada, M.; Takemura, G.; Minatoguchi, S.; Fujiwara, T.; Fujiwara, H. Narirutin inhibits airway inflammation in an allergic mouse model. Clin. Exp. Pharmacol. Physiol. 2010, 34, 766–770. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H.; Kim, Y.S.; Jeong, C.S. Protective effects of neohesperidin and poncirin isolated from the fruits of Poncirus trifoliata on potential gastric disease. Phytother. Res. 2009, 23, 1748–1753. [Google Scholar] [CrossRef]
- Chini, M.G.; Malafronte, N.; Vaccaro, M.C. Identification of Limonol Derivatives as Heat Shock Protein?90 (Hsp90) Inhibitors through a Multidisciplinary Approach. Chem. Eur. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-Y.; Hsu, Y.-L.; Ko, Y.-C.; Tsai, Y.-M.; Yang, C.-J.; Huang, M.-S.; Kuo, P.-L. Didymin, a dietary flavonoid glycoside from citrus fruits, induces Fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer 2010, 68, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.; Sonowal, H.; Saxena, A.; Ramana, K.V. Didymin prevents hyperglycemia-induced human umbilical endothelial cells dysfunction and death. Biochem. Pharmacol. 2018, 152, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Chen, H. The methoxyflavones in Citrus reticulata Blanco cv. ponkan and their antiproliferative activity against cancer cells. Food Chem. 2010, 119, 567–572. [Google Scholar] [CrossRef]
- Battinelli, L.; Mengoni, F.; Lichtner, M.; Mazzanti, G.; Saija, A.; Mastroianni, C.M.; Vullo, V. Effect of limonin and nomilin on HIV-1 replication on infected human mononuclear cells. Planta Med. 2003, 69, 910–913. [Google Scholar] [PubMed]
- Tian, Q.; Miller, E.G.; Ahmad, H.; Tang, L.; Patil, B.S. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr. Cancer 2001, 40, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.R.; Lafuente, M.T. LED Blue Light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chem. 2017, 218, 575–583. [Google Scholar] [CrossRef]
- Arcas, M.C.; Botía, J.M.; Ortuño, A.M.; Río, J.A.D. UV irradiation alters the levels of flavonoids involved in the defence mechanism of citrus aurantium fruits against penicillium digitatum. Eur. J. Plant Pathol. 2000, 106, 617–622. [Google Scholar] [CrossRef]
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, Y.G.; Yan, Q.; Liu, C.Z. Spectral composition of irradiation regulates the cell growth and flavonoids biosynthesis in callus cultures of Saussurea medusa Maxim. Plant Growth Regul. 2007, 52, 259–263. [Google Scholar] [CrossRef]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I. Expression analysis of anthocyanin regulatory genes in response to different light qualities in arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef]
- Jairam, V.; Greg, C.; Turner, N.D.; Lupton, J.R.; Kil Sun, Y.; Pike, L.M.; Patil, B.S. Bioactive compounds of grapefruit (Citrus paradisi Cv. Rio Red) respond differently to post-harvest irradiation, storage, and freeze drying. J. Agric. Food Chem. 2005, 53, 3980–3985. [Google Scholar]
- Lau, O.S.; Deng, X.W. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 2012, 17, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.M.; Arvai, A.S.; Baxter, K.J.; Heilmann, M.; Pratt, A.J.; O’Hara, A.; Kelly, S.M.; Hothorn, M.; Smith, B.O.; Hitomi, K.; Jenkins, G.I.; Getzoff, E.D. Plant UVR8 Photoreceptor Senses UV-B by Tryptophan-Mediated Disruption of Cross-Dimer Salt Bridges. Science 2012, 335, 1492–1496. [Google Scholar] [CrossRef]
- Li, S. Transcriptional control of flavonoid biosynthesis. Plant Signal Behav. 2014, 9, e27522. [Google Scholar] [CrossRef]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of citrus using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615. [Google Scholar] [CrossRef]
- Zhao, X.J.; Xing, T.T.; Li, Y.F.; Jiao, B.N.; Jiang, D. Efficient analysis of phytochemical constituents in the peel of Chinese wild citrus Mangshanju (Citrus reticulata Blanco) by ultra high performance liquid chromatography–quadrupole time-of-flight-mass spectrometry. J. Sep. Sci. 2018. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
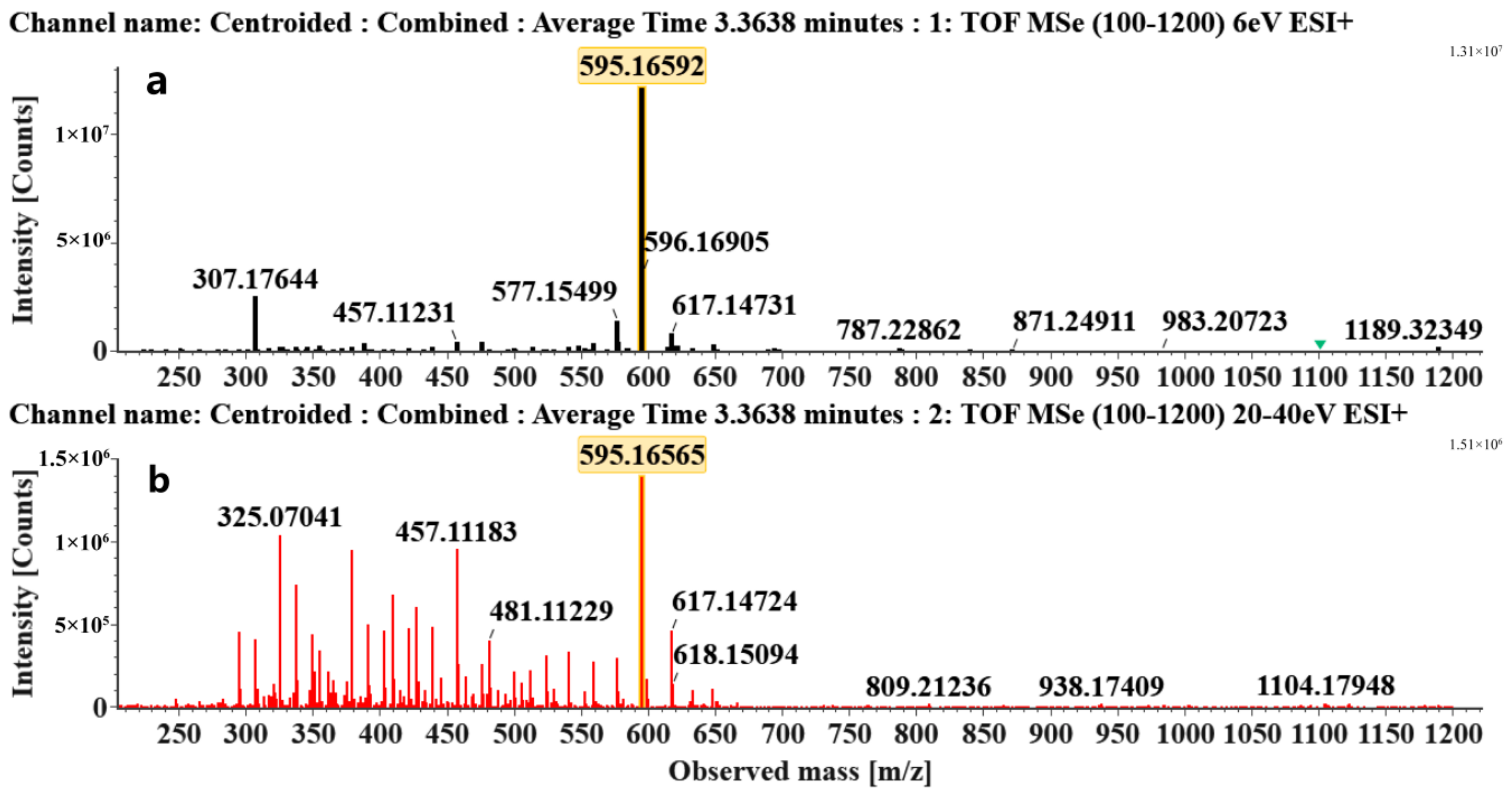
| Retention Time (min) | Component Name | Formula | [M + H]+ (Error, ppm) | Diagnostic MS2 Ion (%) | Structure | CAS Registry Number | Health-Promoting Properties |
|---|---|---|---|---|---|---|---|
| 3.36 | Apigenin-6,8-di-C-glucoside (Vicenin-2) (Flavone C-glycoside) | C27H30O15 | 595.16592 (0.29) | 595.16565 (100), 325.07041 (74.91), 457.11183 (68.54), 379.08087 (67.98), 409.09118 (48.64), 477.11946 (2.90) | 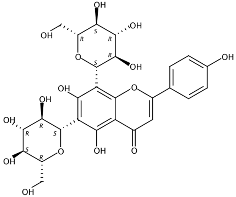 | 23666-13-9 | Anti-prostate cancer [22] |
| 3.59 | Diosmetin 6,8-di-C-glucoside (Flavone C-glycoside) | C28H32O16 | 625.17556 (−1.20) | 625.17448 (100), 355.07895 (97.31), 487.12160 (76.16), 409.09206 (75.73), 457.11214 (63.68), 367.08299 (55.83), 607.16286 (25.34) | 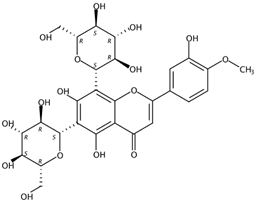 | 98813-28-6 | Antioxidant; Antihypertensive effect [23] |
| 3.69 | Rhoifolin-4′-O-glucoside(Flavone O-glycoside) | C33H40O19 | 741.22360 (−0.07) | 433.11258 (71.19), 271.05979 (68.12), 595.16594 (59.59), 153.01818 (9.23), 163.03854 (6.68) |  | 31498-83-6 | Not found |
| 3.71 | Neoeriocitrin(Flavanone O-glycoside) | C27H32O15 | 597.17996 (−2.41) | 289.07002 (35.39), 153.01818 (9.23), 435.12045 (6.36), 451.12259 (4.27) |  | 13241-32-2 | Anti-osteoporosis [24] |
| 3.76 | Chysoeriol-6,8-di-C-glucoside(Stellarin-2) (Flavone C-glycoside) | C28H32O16 | 625.17571 (−0.96) | 285.07510 (3.29), 457.10814 (3.07), 355.07804 (2.69), 367.07961 (1.65), 487.12086 (1.53), 607.16347 (0.48) | 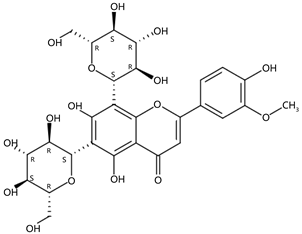 | 63975-58-6 | DNA-binding activity [25] |
| 3.78 | Narirutin-4′-glucoside(Flavanone O-glycoside) | C33H42O19 | 743.23932 (−0.02) | 273.07568 (100), 765.22083 (35.37), 153.01801 (18.88), 147.04375 (6.36), 435.12831 (18.31) | 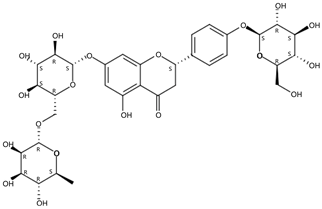 | 17257-22-6 | Antioxidant [26] |
| 4.34 | Epilimonin(Limonoid) | C26H30O8 | 471.20201 (1.41) | 471.20151(100), 425.19591 (75.41), 161.05961 (32.74), 409.20043 (12.39), 315.15814 (3.13), 273.12685 (1.79) | 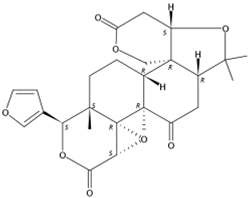 | 1180-71-8 | Not found |
| 4.68 | Eriocitrin(Flavanone O-glycoside) | C27H32O15 | 597.18124 (−0.26) | 289.07048 (100), 153.01781 (34.16), 163.03866 (19.72), 417.12444 (7.56), 435.12720 (7.19) | 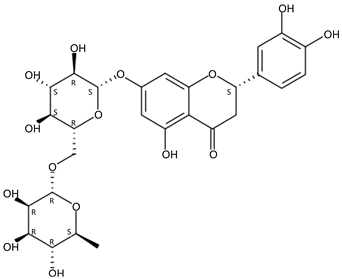 | 13463-28-0 | Anti-obesity [27]; Reducing blood fat [28] |
| 5.65 | Narirutin(Flavanone O-glycoside) | C27H32O14 | 581.18661 (0.22) | 273.07588 (100), 329.15998 (0.04), 153.01794 (29.66), 493.04405 (0.49), 419.13333 (18.54) | 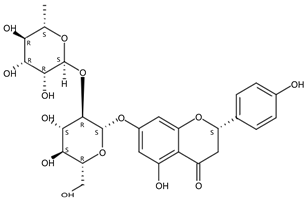 | 15822-82-9 | Anti-inflammation [29]; |
| 6.26 | Diosmin(Flavone O-glycoside) | C28H32O15 | 609.18126 (−0.22) | 301.07027(100), 463.12343(18.13), 286.04676(11.08), 258.05273(10.79) | 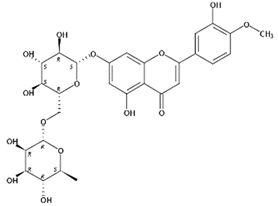 | 520-27-4 | Antidiabetic [24] |
| 6.41 | Hesperidin(Flavanone O-glycoside) | C28H34O15 | 611.19777 (1.18) | 303.08637 (100), 449.14389 (27.63), 153.01803 (23.49), 177.05451 (17.19), 465.13864 (13.85) |  | 520-26-3 | Antioxidant; Anti-inflammation; Anticancer; Anti-atherosclerotic effects; Vasodilatation effects [20] |
| 6.75 | Neohesperidin(Flavanone O-glycoside) | C28H34O15 | 611.19631 (−1.20) | 303.086432 (100), 359.01192 (4.22), 153.01814 (41.50), 345.10040 (9.97) | 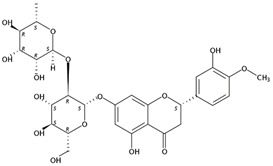 | 13241-33-3 | Anti-gastric disease [30] |
| 6.85 | 7α-Limonyl acetate(Limonoid) | C28H34O9 | 515.22775 (0.37) | 515.22780 (100), 161.05954 (22.10), 303.08669 (2.38), 487.23253 (28.24), 469.22182 (17.67) | 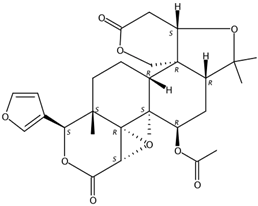 | 1110-03-8 | Hsp90 inhibition activity [31] |
| 8.99 | Didymin(Flavanone O-glycoside) | C28H34O14 | 595.20210 (−0.05) | 287.09139 (100), 153.01808 (29.95), 389.12180 (0.26), 161.05957 (12.80), 433.14904 (16.67) | 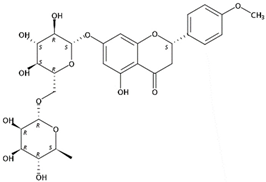 | 14259-47-3 | Anticancer [32]; Antioxidant; Anti-inflammation; prevention of cardiovascular complications in diabetes [33] |
| 10.41 | 5,7,8,3′,4′-Pentamethoxyflavone(Isosinensetin) (Polymethoxylated flavone) | C20H20O7 | 373.12780 (−1.01) | 343.08079 (100), 373.12755 (41.38), 315.08636 (19.74), 357.09485 (15.26), 153.06825 (11.84), 181.08895 (8.36) |  | 17290-70-9 | Anticancer [34] |
| 11.06 | 5,6,7,3′,4′-Pentamethoxyflavone(Sinensetin) (Polymethoxylated flavone) | C20H20O7 | 373.12819 (0.02) | 343.08115 (100), 373.12778 (86.23), 312.09888 (58.65), 358.10324 (25.10), 153.01835 (9.06), 163.07473 (5.57) | 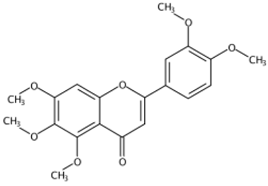 | 2306-27-6 | Anti-inflammation; Anticancer [20] |
| 11.18 | Limonin(Limonoid) | C26H30O8 | 471.20110 (−0.51) | 343.08084 (100), 328.05373 (6.03), 161.06004 (7.74), 395.10636 (8.58), 425.19653 (6.58) | 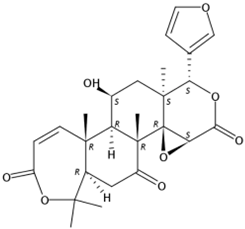 | 23885-43-0 | Inhibit HIV-1 replication [35]; Anticancer [36] |
| 11.95 | 5,6,7,8,3′,4′-Hexamethoxyflavone(Nobiletin) (Polymethoxylated flavone) | C21H22O8 | 403.13818 (−1.40) | 373.09128 (100), 403.13793 (30.35), 388.11432 (12.31), 327.08555 (10.22), 211.02312 (4.26), 183.02844 (3.77) | 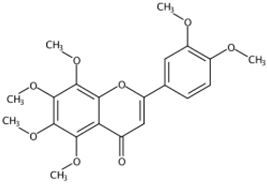 | 478-01-3 | Anti-inflammation; Anticancer; Anti-atherosclerotic effects; Anti-diabetic effects [20] |
| 12.03 | 5,7,8,4’-Tetramethoxyflavone(Polymethoxylated flavone) | C19H18O6 | 343.11751 (−0.30) | 313.07010 (100), 282.08813 (68.95), 343.11731 (54.57), 153.01802 (13.31), 181.01279 (6.96), 133.06470(6.08) | 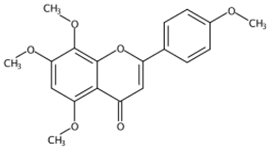 | 6601-66-7 | Not found |
| 12.58 | 3,5,6,7,8,3′,4′-Heptamethoxyflavone(Polymethoxylated flavone) | C22H24O9 | 433.14855 (−1.75) | 403.10148 (100), 433.14821 (44.27), 373.05410 (5.31), 404.10479 (23.30), 418.12464 (15.15), 385.09043(10.56) | 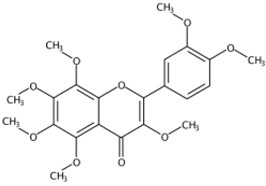 | 1178-24-1 | Anti-inflammation; Anti-atherosclerotic effects [20] |
| 13.02 | 5,6,7,8,4′-Pentamethoxyflavone(Tangeretin) (Polymethoxylated flavone) | C20H20O7 | 373.12765 (−1.42) | 343.08087 (100), 395.10987 (3.85), 373.12802 (20.26), 344.08433 (20.73), 297.07575 (10.69), 211.02316 (6.07) | 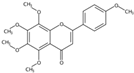 | 481-53-8 | Antioxidant; Anti-inflammation; Anticancer; Anti-atherosclerotic effects; Anti-diabetic effects [20]; neuroprotective effects [14] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Hu, L.; Jiang, D.; Xi, W. Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck). Molecules 2019, 24, 1755. https://doi.org/10.3390/molecules24091755
Liu S, Hu L, Jiang D, Xi W. Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck). Molecules. 2019; 24(9):1755. https://doi.org/10.3390/molecules24091755
Chicago/Turabian StyleLiu, Shengyu, Linping Hu, Dong Jiang, and Wanpeng Xi. 2019. "Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck)" Molecules 24, no. 9: 1755. https://doi.org/10.3390/molecules24091755
APA StyleLiu, S., Hu, L., Jiang, D., & Xi, W. (2019). Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck). Molecules, 24(9), 1755. https://doi.org/10.3390/molecules24091755





