Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy)
Abstract
1. Introduction
2. Results and Discussion
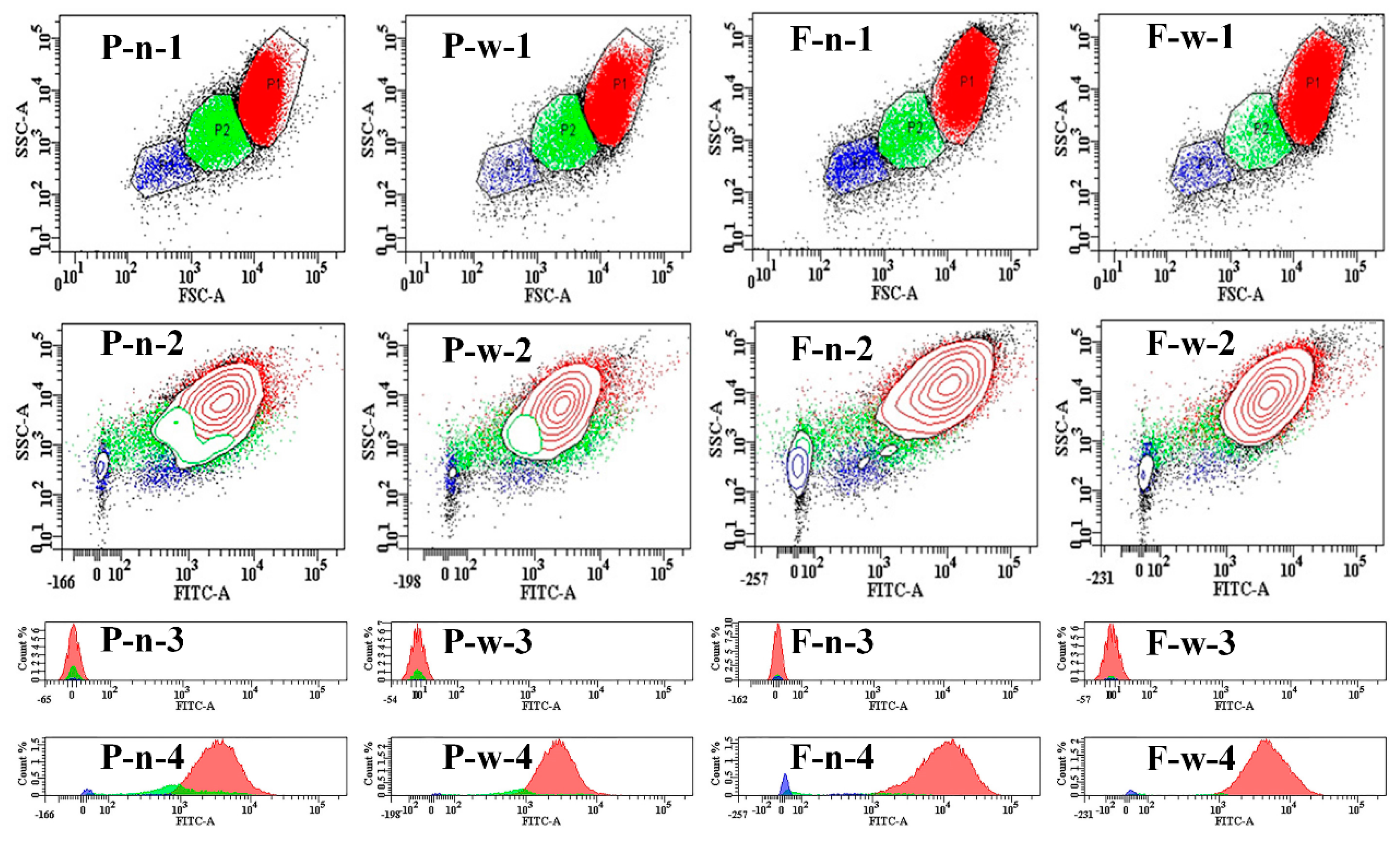
2.1. Morphology and Size of Starch
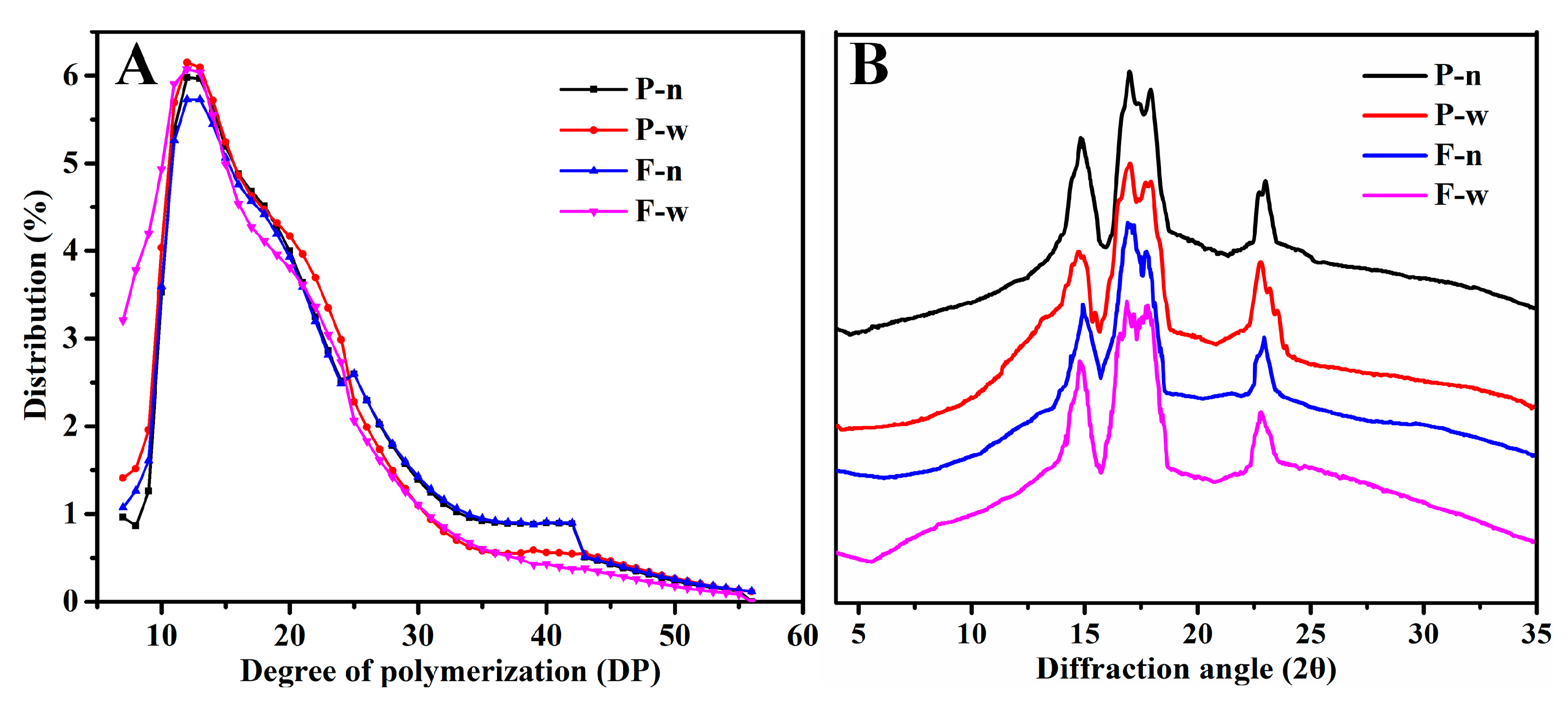
2.2. Molecular Weight of Starch and Chain Length Distribution of Amylopectin
2.3. Crystalline Structure
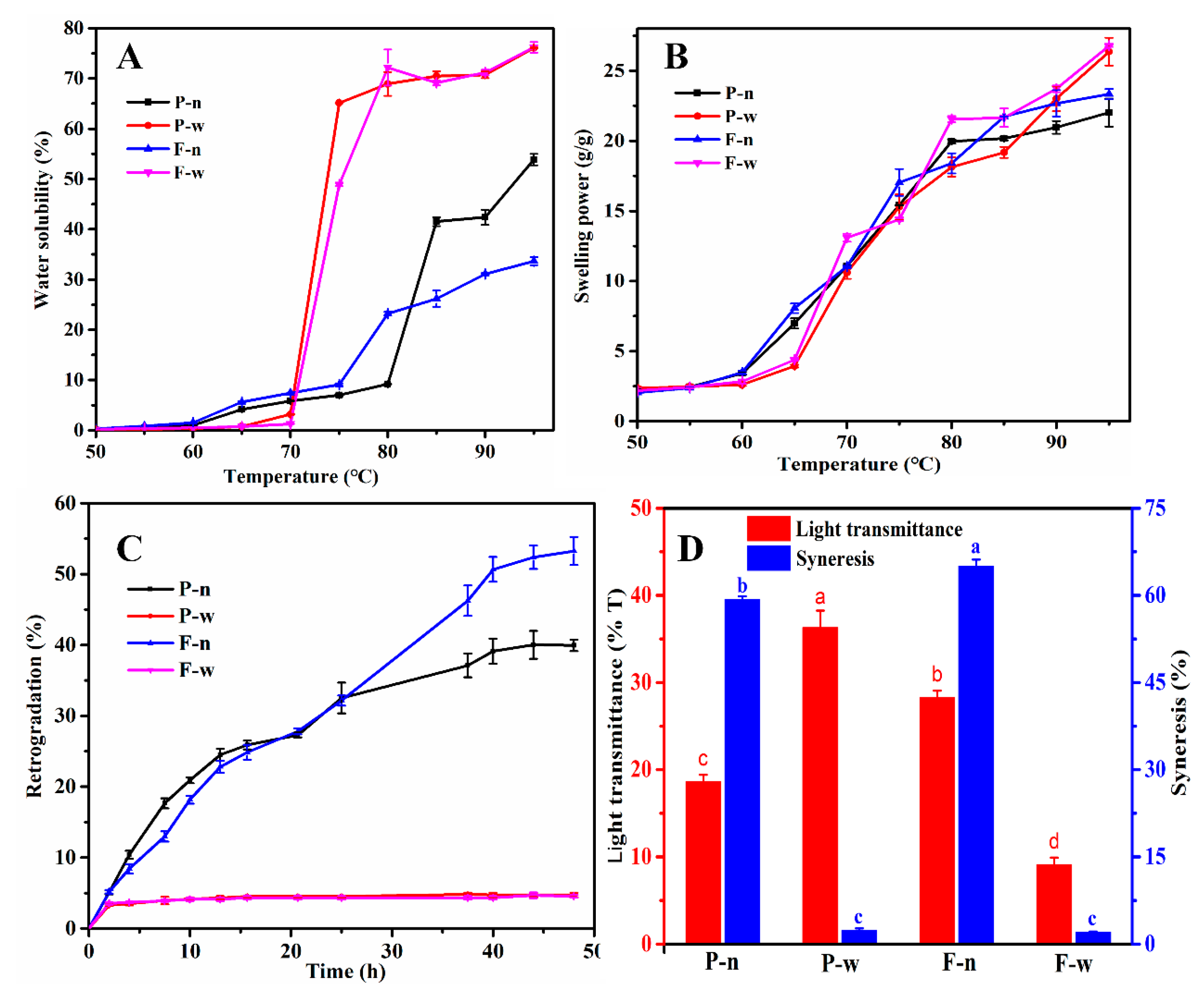
2.4. Physical Properties of Starches
2.5. Thermal Properties of Starch
2.6. Pasting Properties of Starch
3. Materials and Methods
3.1. Materials
3.2. Chemical Composition of Flour and Starch
3.3. Morphology Observation of Starch
3.4. Granule Size Analysis of Starch
3.5. Branching Degree of Starch
3.6. Molecular Weight Distribution of Starch
3.7. Chain Length Distribution of Amylopectin
3.8. Crystalline Structure
3.9. Physical Properties of Starch
3.10. Thermal Property Analysis of Starch
3.11. Pasting Property Analysis of Starch
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, F. Structure, physicochemical properties, and uses of millet starch. Food Res. Int. 2014, 64, 200–211. [Google Scholar] [CrossRef]
- Chao, G.; Gao, J.; Liu, R.; Wang, L.; Li, C.; Wang, Y.; Qu, Y.; Feng, B. Starch physicochemical properties of waxy proso millet (Panicum Miliaceum L.). Starch 2014, 66, 1005–1012. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Quan, Z.; Cheng, S.; Xu, X.; Pan, S.; Xie, M.; Zeng, P.; Yue, Z.; Wang, W.; et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 2012, 30, 549. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.H.; Zhang, P.P.; Qu, Y.; Gao, X.L.; Liang, J.B.; Yang, P.; Feng, B.L. Comparison of physicochemical properties and cooking edibility of waxy and non-waxy proso millet (Panicum miliaceum L.). Food Chem. 2018, 257, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Cai, C.H.; Gilbert, G.; Li, E.P.; Wang, J.; Wei, C.X. Relationships between amylopectin molecular structures and functional properties of different-sized fractions of normal and high-amylose maize starches. Food Hydrocolloid. 2016, 52, 359–368. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. A comparative study of annealing of waxy, normal and high-amylose maize starches: The role of amylose molecules. Food Chem. 2014, 164, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural features and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef]
- Goldstein, A.; Annor, G.; Putaux, J.L.; Hebelstrup, K.H.; Blennow, A.; Bertoft, E. Impact of full range of amylose contents on the architecture of starch granules. Int. J. Biol. Macromol. 2016, 89, 305–318. [Google Scholar] [CrossRef]
- Li, H.; Wen, Y.; Wang, J.; Sun, B. Relations between chain-length distribution, molecular size, and amylose content of rice starches. Int. J. Biol. Macromol. 2018, 120, 2017–2025. [Google Scholar] [CrossRef]
- Zhou, Z.K.; Zhang, Y.; Chen, X.S.; Zhang, M.; Wang, Z.W. Multi-scale structural and digestion properties of wheat starches with different amylose contents. Int. J. Food Sci. Tech. 2014, 49, 2619–2627. [Google Scholar] [CrossRef]
- Wang, R.Y.; Wang, H.G.; Liu, X.H.; Ji, X.; Chen, L.; Lu, P.; Liu, M.X.; Teng, B.; Qiao, Z.J. Waxy allelic diversity in common millet (Panicum miliaceum L.) in China. Crop J. 2018, 6, 377–385. [Google Scholar] [CrossRef]
- Zhang, X.D.; Feng, J.J.; Wang, H.; Zhu, J.C.; Zhong, Y.Y.; Liu, L.S.; Xu, S.T.; Zhang, R.H.; Zhang, X.H.; Xue, J.Q. Bivariate flow cytometric analysis and sorting of different types of maize starch grains. Cytom. Part A 2018, 93, 213–221. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Shin, S.I.; Lee, C.J.; Kim, M.J.; Choi, S.J.; Choi, H.J.; Kim, Y.; Moon, T.W. Structural characteristics of low-glycemic response rice starch produced by citric acid treatment. Carbohyd. Polym. 2009, 78, 588–595. [Google Scholar] [CrossRef]
- Li, X.; Miao, M.; Jiang, H.; Xue, J.; Jiang, B.; Zhang, T.; Gao, Y.; Jia, Y. Partial branching enzyme treatment increases the low glycaemic property and alpha-1,6 branching ratio of maize starch. Food Chem. 2014, 164, 502–509. [Google Scholar] [CrossRef]
- Ma, M.T.; Wang, Y.J.; Wang, M.X.; Jane, J.L.; Du, S.K. Physicochemical properties and in vitro digestibility of legume starches. Food Hydrocolloid. 2017, 63, 249–255. [Google Scholar] [CrossRef]
- Huang, J.; Shang, Z.Q.; Man, J.M.; Liu, Q.Q.; Zhu, C.J.; Wei, C.X. Comparison of molecular structures and functional properties of high-amylose starches from rice transgenic line and commercial maize. Food Hydrocolloid. 2015, 46, 172–179. [Google Scholar] [CrossRef]
- Genkina, N.; Kozlov, S.; Martirosyan, V.; Kiseleva, V. Thermal behavior of maize starches with different amylose/amylopectin ratio studied by DSC analysis. Starch 2014, 66, 700–706. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Amante, E.R.; Demiate, I.M.; Vieira, F.; Delgadillo, I.; Maraschin, M. Physicochemical, thermal, and pasting properties of flours and starches of eight Brazilian maize landraces (Zea mays L.). Food Hydrocolloid. 2013, 30, 614–624. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, C.L.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Gao, H.M.; Cai, J.W.; Han, W.L.; Huai, H.Y.; Chen, Y.F.; Wei, C.X. Comparison of starches isolated from three different Trapa species. Food Hydrocolloid. 2014, 37, 174–181. [Google Scholar] [CrossRef]
- Wang, W.W.; Guan, L.; Seib, P.A.; Shi, Y.C. Settling volume and morphology changes in cross-linked and unmodified starches from wheat, waxy wheat, and waxy maize in relation to their pasting properties. Carbohyd. Polym. 2018, 196, 18–26. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Q.; Hu, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Physicochemical properties, structure and in vitro digestibility on complex of starch with lotus (Nelumbo nucifera Gaertn.) leaf flavonoids. Food Hydrocolloid. 2018, 81, 191–199. [Google Scholar] [CrossRef]
- Gao, J.F.; Kreft, I.; Chao, G.M.; Wang, Y.; Liu, X.J.; Wang, L.; Wang, P.K.; Gao, X.L.; Feng, B.L. Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chem. 2016, 190, 552–558. [Google Scholar] [CrossRef]
- Cai, C.H.; Lin, L.S.; Man, J.M.; Zhao, L.X.; Wang, Z.F.; Wei, C.X. Different structural properties of high-amylose maize starch fractions varying in granule size. J. Agric. Food Chem. 2014, 62, 11711–11721. [Google Scholar] [CrossRef]
- Zeng, F.; Gao, Q.Y.; Han, Z.; Zeng, X.A.; Yu, S.J. Structural properties and digestibility of pulsed electric field treated waxy rice starch. Food Chem. 2016, 194, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Lin, L.S.; Fan, X.X.; Zhang, L.; Wei, C.X. Comparison of structural and functional properties of starches from five fruit kernels. Food Chem. 2018, 257, 75–82. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Woo, K.S.; Chung, H.J. Starch characteristics of cowpea and mungbean cultivars grown in Korea. Food Chem. 2018, 263, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Arunyanart, T.; Charoenrein, S. Effect of sucrose on the freeze–thaw stability of rice starch gels: Correlation with microstructure and freezable water. Carbohyd. Polym. 2008, 74, 514–518. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Varieties | Flour | Starch | ||||||
|---|---|---|---|---|---|---|---|---|
| Fat Content (%) | Protein Content (%) | Starch Content (%) | Amylose Content (%) | Fat Content (%) | Protein Content (%) | Starch Content (%) | Amylose Content (%) | |
| P-n | 2.83 ± 0.12 c | 11.04 ± 0.05 a | 76.43 ± 1.21 a | 28.00 ± 0.70 a | 0.01 ± 0.01 a | 1.30 ± 0.06 a | 87.23 ± 0.56 b | 32.80 ± 0.70 b |
| P-w | 4.37 ± 0.20 b | 11.09 ± 0.09 a | 75.37 ± 0.94 a | 2.00 ± 0.20 b | 0.01 ± 0.00 a | 1.07 ± 0.26 a b | 94.60 ± 1.30 a | 2.80 ± 0.20 c |
| F-n | 3.85 ± 0.21 b | 8.83 ± 0.05 c | 71.79 ± 0.92 b | 29.10 ± 1.30 a | 0.02 ± 0.01 a | 0.89 ± 0.06 b | 87.15 ± 0.96 b | 36.70 ± 0.40 a |
| F-w | 4.54 ± 0.50 a | 10.07 ± 0.19 b | 72.04 ± 0.93 b | 2.00 ± 0.30 b | 0.01 ± 0.01 a | 0.80 ± 0.18 b | 83.03 ± 1.31 c | 2.50 ± 0.10 c |
| Varieties | Granule Size (μm) | Particle Percentage of Subgroups in Different Starches (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| d (0.1) | d (0.5) | d (0.9) | D (3, 2) | D (4, 3) | P1 | P2 | P3 | |
| P-n | 4.49 ± 0.01 d | 8.93 ± 0.08 b | 22.47 ± 0.04 d | 8.07 ± 0.07 c | 14.82 ± 0.03 c | 72.5 | 18.6 | 3.7 |
| P-w | 4.70 ± 0.04 c | 8.94 ± 0.08 b | 18.62 ± 0.03 c | 8.11 ± 0.06 c | 12.95 ± 0.04 d | 79.6 | 13.0 | 2.0 |
| F-n | 6.58 ± 0.01 a | 12.31 ± 0.03 a | 28.29 ± 0.06 b | 11.36 ± 0.01 a | 17.64 ± 0.01 b | 86.8 | 4.7 | 2.6 |
| F-w | 6.20 ± 0.07 b | 12.19 ± 0.07 a | 36.16 ± 0.06 a | 11.11 ± 0.01 b | 18.71 ± 0.06 a | 79.8 | 7.7 | 6.4 |
| Mw (×107, g/mol) | Average Chain Length of Amylopectin (%) | Branching Degree (%) | Relative Crystallinity (%) | Chain Length Distribution (%) | ||||
|---|---|---|---|---|---|---|---|---|
| DP 6–12 | DP 13–24 | DP 25–37 | DP ≥37 | |||||
| P-n | 2.4 ± 0.1 c | 20.1 ± 0.3 a | 2.8 ± 0.1 b | 37.6 ± 0.1 b | 18.0 ± 0.5 c | 45.4 ± 0.4 b | 17.8 ± 0.3 a | 9.1 ± 0.4 a |
| P-w | 17.0 ± 0.5 b | 19.2 ± 0.2 b | 4.2 ± 0.2 a | 38.4 ± 0.4 a | 20.8 ± 0.3 b | 47.4 ± 0.8 a | 14.1 ± 0.6 b | 7.7 ± 0.4 b |
| F-n | 2.8 ± 0.2 c | 20.2 ± 0.3 a | 2.8 ± 0.2 b | 34.1 ± 0.6 c | 18.5 ± 0.2 c | 44.4 ± 0.2 b | 18.1 ± 0.4 a | 9.4 ± 0.5 a |
| F-w | 22.3 ± 0.4 a | 17.9 ± 0.1 c | 3.9 ± 0.1 a | 36.5 ± 0.4 b | 28.1 ± 1.0 a | 44.0 ± 0.3 b | 13.7 ± 0.5 b | 5.4 ± 0.2 c |
| Varieties | Pasting Properties | Thermal Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PV | TV | BD | FV | SB | PTM | PT | To (°C) | Tp (°C) | Tc (°C) | ΔH(J/g) | |
| P-n | 2110 ± 4 c | 997 ± 26 d | 1114 ± 30 d | 1275 ± 8 d | 1478 ± 4 b | 80.9 ± 0.0 a | 4.5 ± 0.0 a | 64.6 ± 0.5 c | 70.5 ± 0.1 c | 77.4 ± 0.1 c | 9.6 ± 0.2 b |
| P-w | 3286 ± 37 ab | 1097 ± 13 c | 2189 ± 24 a | 2575 ± 9 b | 279 ± 35 d | 77.8 ± 0.0 b | 4.2 ± 0.0 c | 71.1 ± 0.6 a | 77.9 ± 0.2 a | 82.3 ± 0.0 a | 10.8 ± 0.0 a |
| F-n | 3237 ± 16 b | 1348 ± 43 b | 1768 ± 6 c | 3599 ± 8 a | 2070 ± 8 a | 77.8 ± 0.0 b | 4.3 ± 0.0 b | 65.1 ± 0.3 c | 71.0 ± 0.4 c | 76.3 ± 0.5 d | 6.6 ± 0.2 d |
| F-w | 3299 ± 4 a | 1509 ± 28 a | 1890 ± 28 b | 1816 ± 7 c | 469 ± 36 c | 76.7 ± 0.0 c | 4.1 ± 0.1 c | 68.3 ± 0.3 b | 73.0 ± 0.3 b | 78.7 ± 0.1 b | 8.5 ± 0.4 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhang, W.; Li, J.; Gong, X.; Feng, B. Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy). Molecules 2019, 24, 1743. https://doi.org/10.3390/molecules24091743
Yang Q, Zhang W, Li J, Gong X, Feng B. Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy). Molecules. 2019; 24(9):1743. https://doi.org/10.3390/molecules24091743
Chicago/Turabian StyleYang, Qinghua, Weili Zhang, Jing Li, Xiangwei Gong, and Baili Feng. 2019. "Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy)" Molecules 24, no. 9: 1743. https://doi.org/10.3390/molecules24091743
APA StyleYang, Q., Zhang, W., Li, J., Gong, X., & Feng, B. (2019). Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy). Molecules, 24(9), 1743. https://doi.org/10.3390/molecules24091743