HBX-6, Standardized Cornus officinalis and Psoralea corylifolia L. Extracts, Suppresses Benign Prostate Hyperplasia by Attenuating E2F1 Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Sample Treatment
2.3. Cell Viability Assays
2.4. Preparation of the HBX-6
2.5. Chromatography Conditions
2.6. Calibration Curves, Limits of Detection, and Limits of Quantification
2.7. Animal Treatments
2.8. Histological Analysis
2.9. Western Blot Analysis
2.10. Statistical Analyses
3. Results
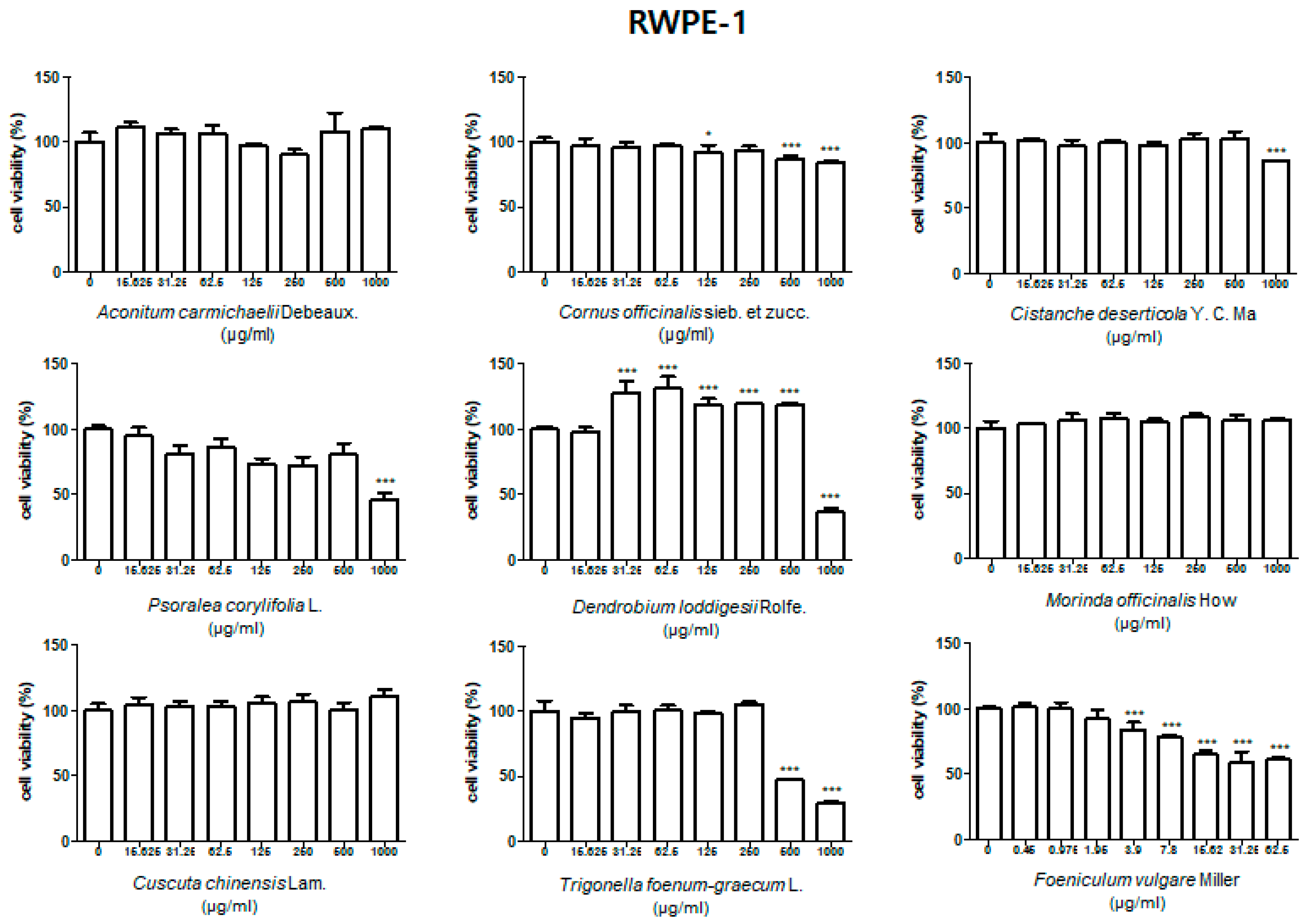
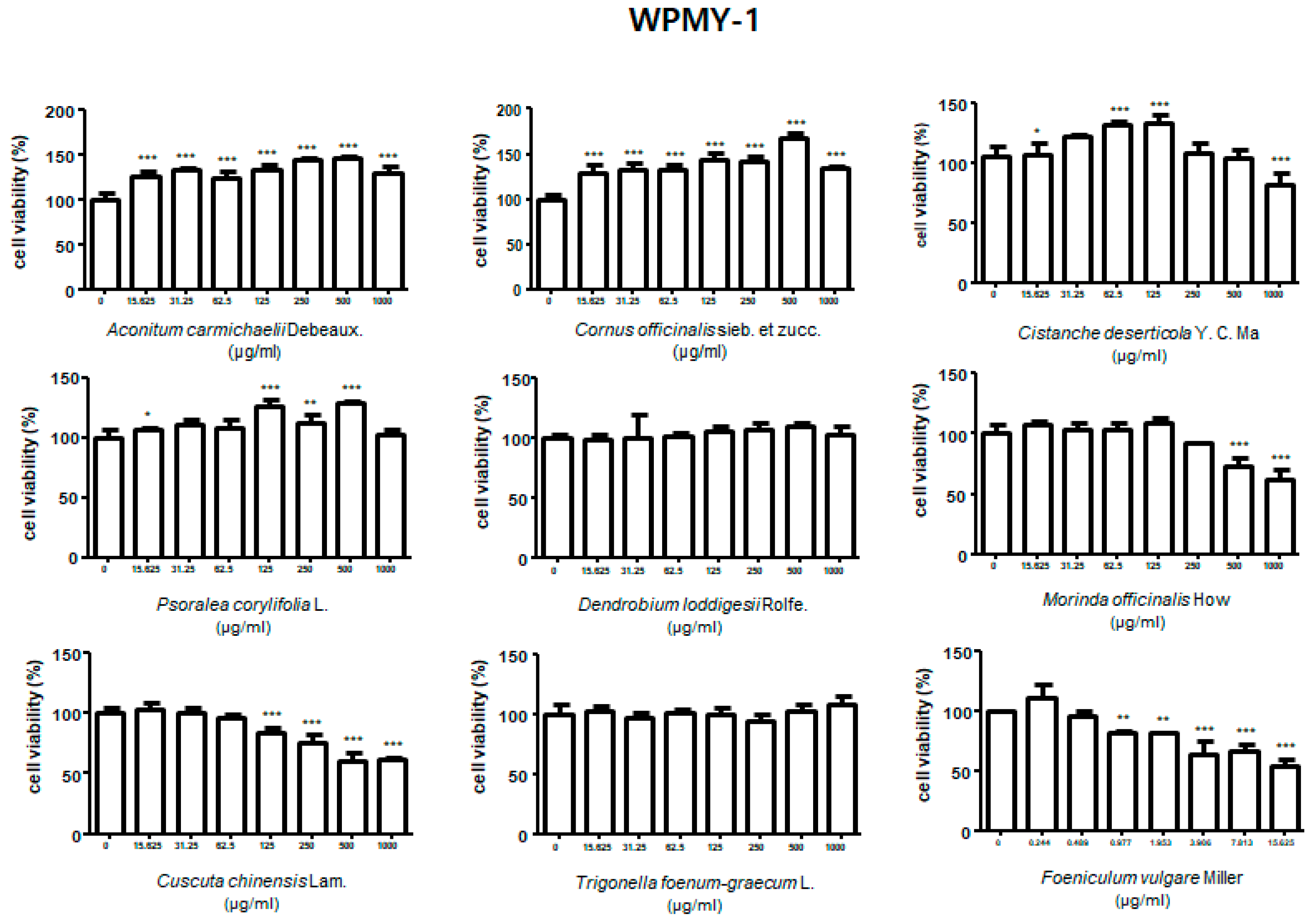
3.1. Effect of Treatment with HBX-5 Components on Viability of RWPE-1 and WPMY-1 Cells
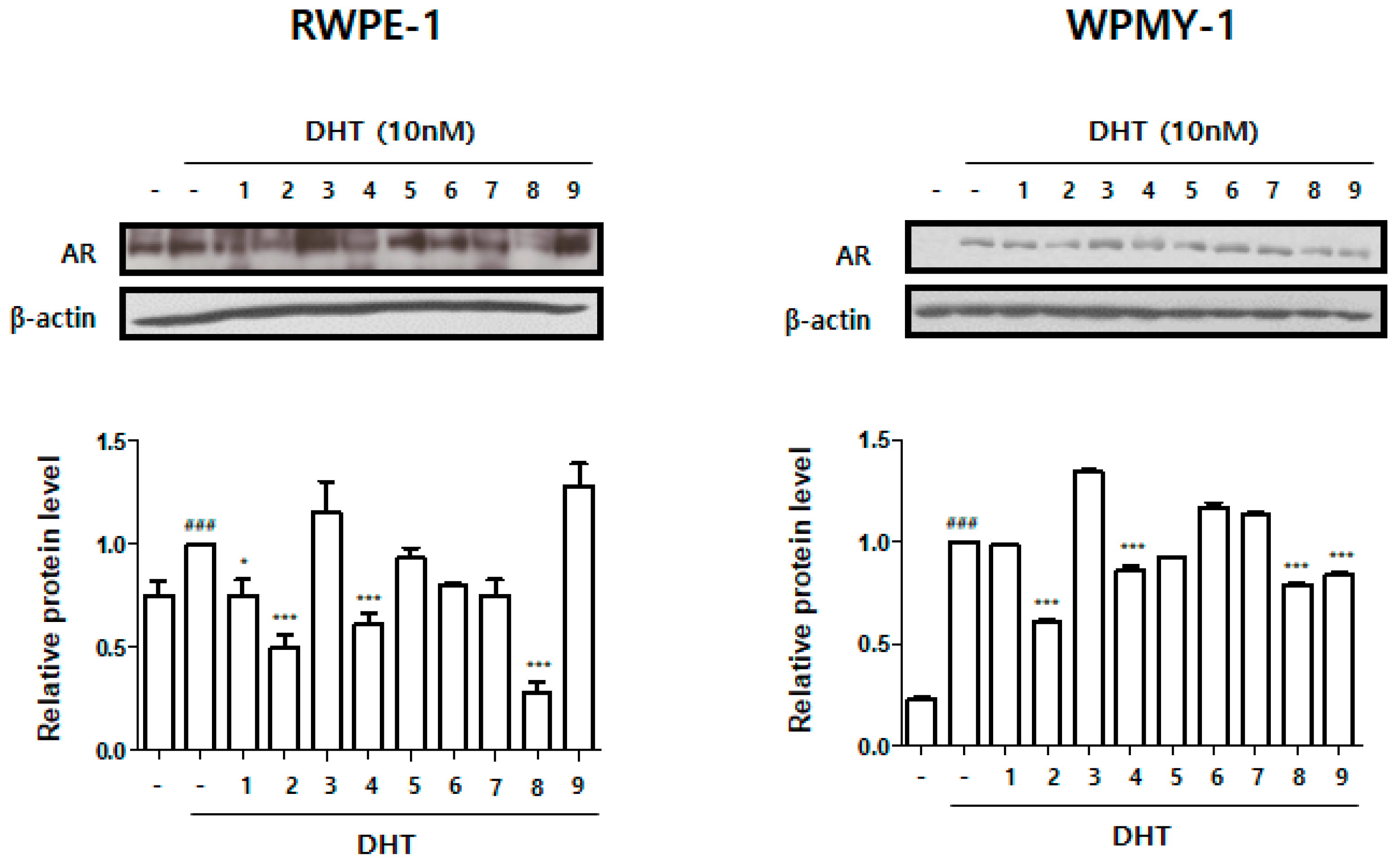
3.2. Effect of Treatment with HBX-5 Components on AR Expression in RWPE-1 and WPMY-1 Cells
3.3. Chemical Profiling Analysis of the Newly Combined Herbal Formula, HBX-6
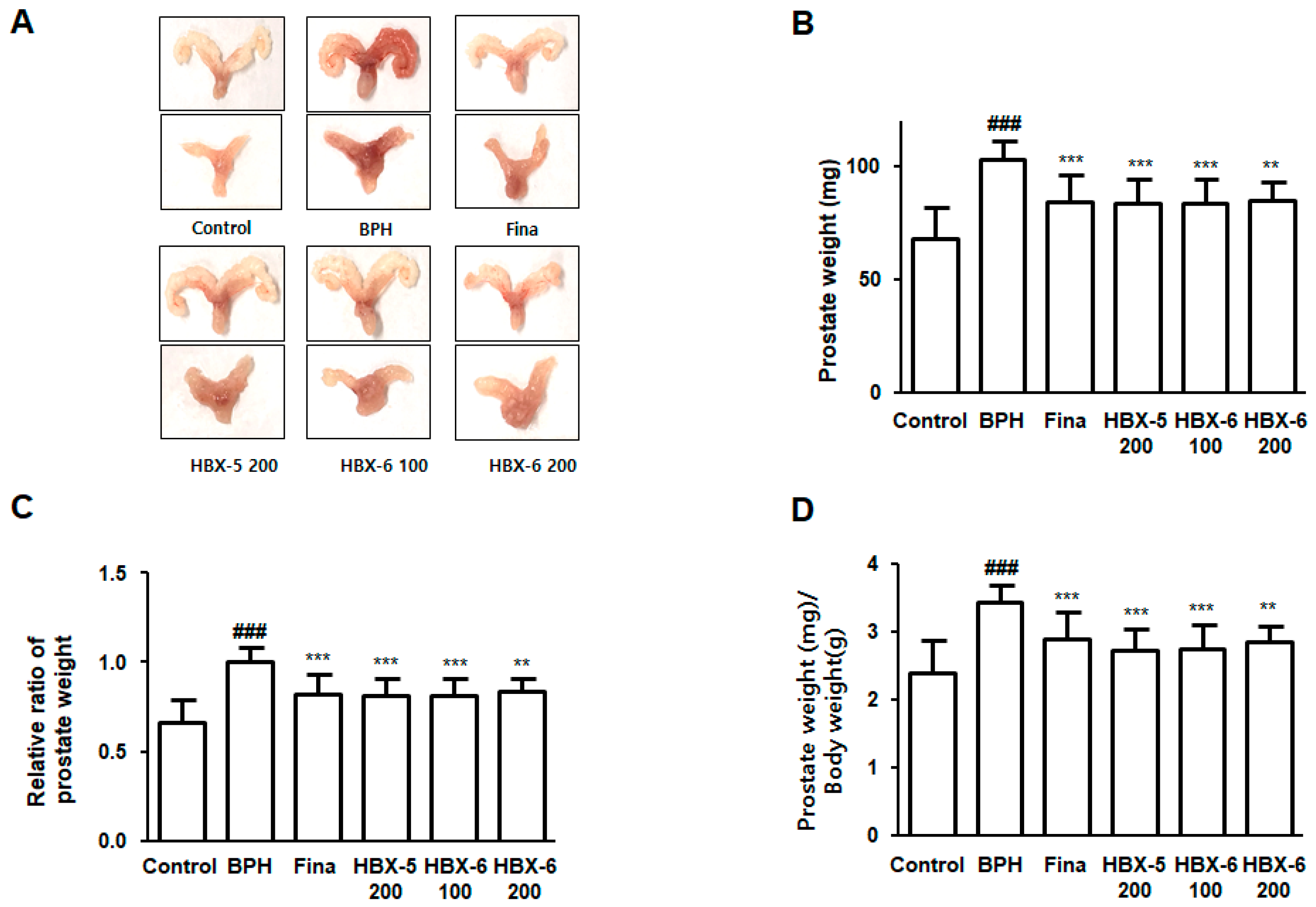
3.4. Effect of HBX-6 Treatment on Prostate Weight in BPH-Induced Mouse Model
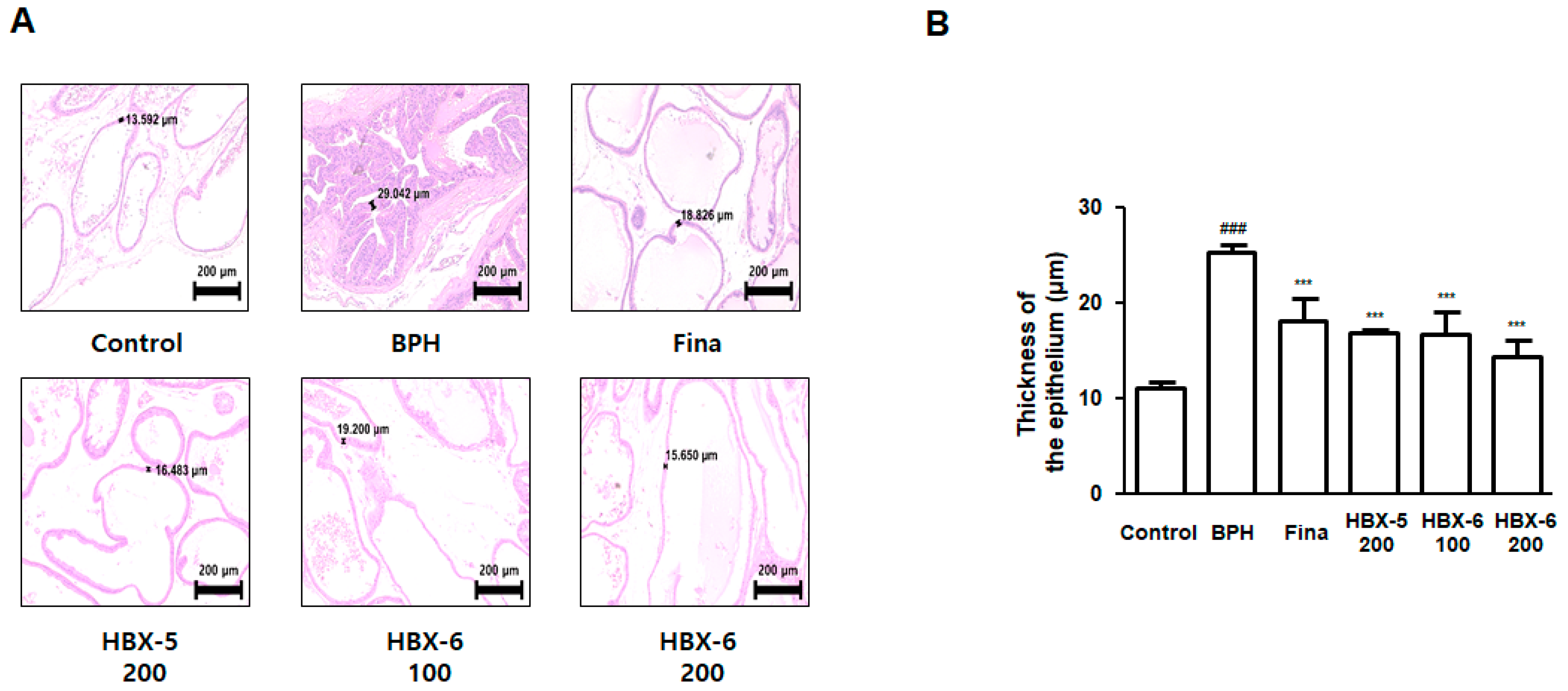
3.5. Effect of HBX-6 on Histological Changes in Prostate of BPH-Induced Mice
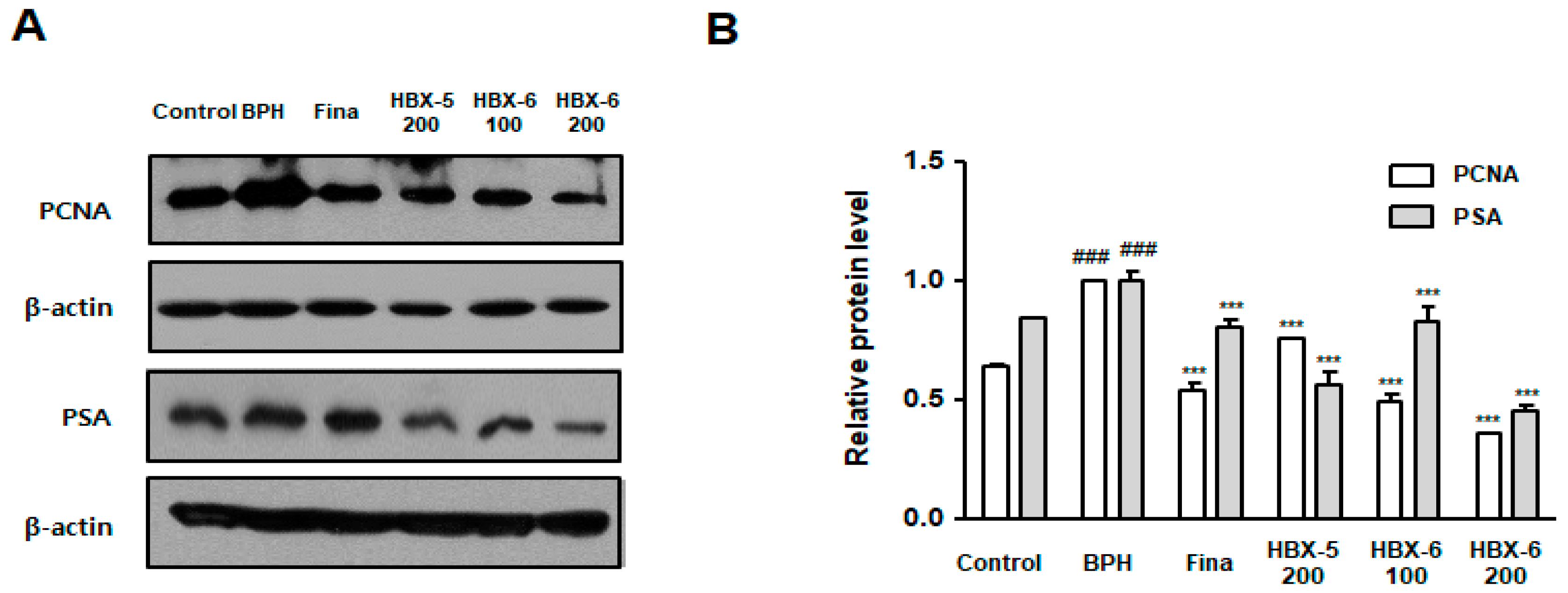
3.6. Effect of HBX-6 on Prostate Cell Proliferation in BPH-Induced Mice
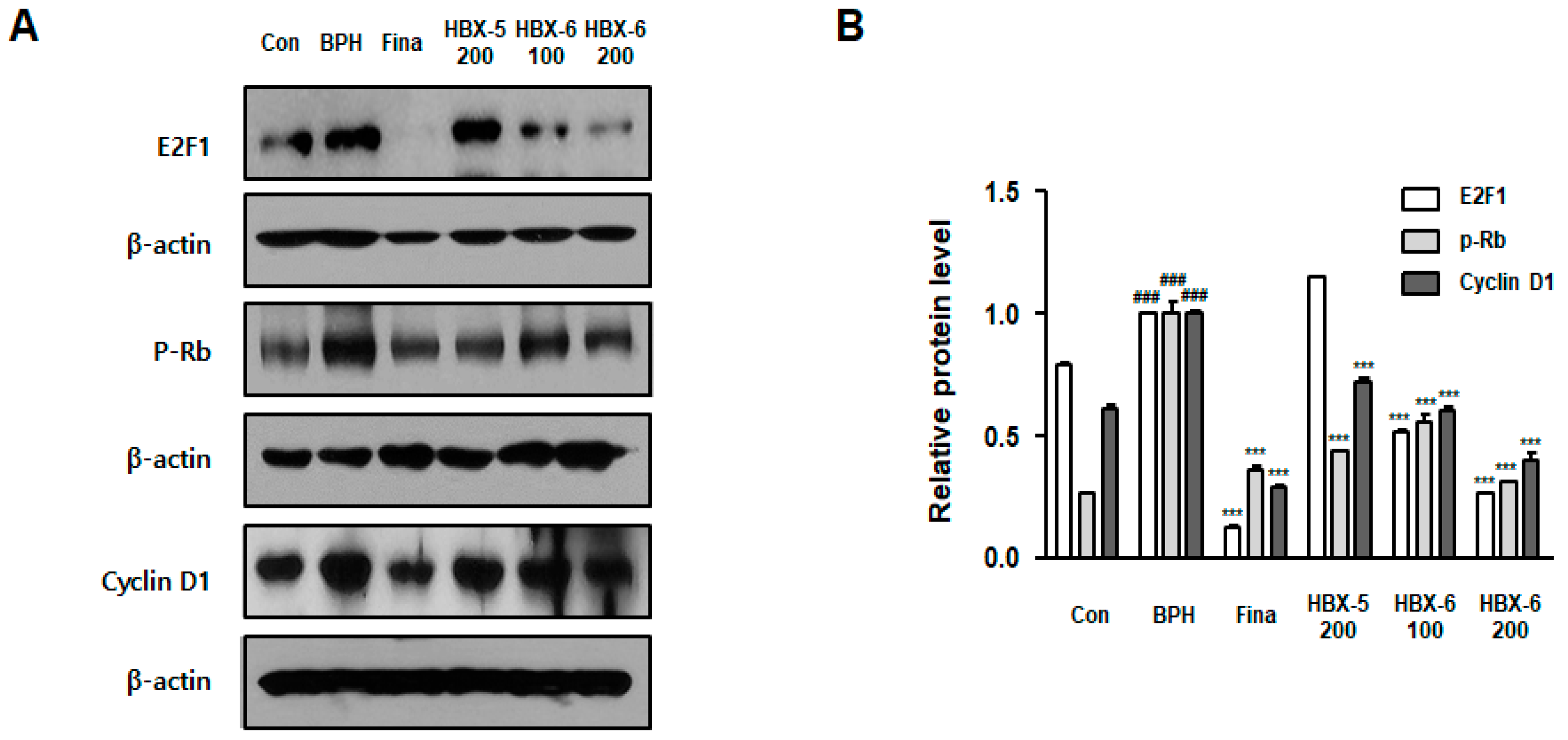
3.7. Effect of HBX-6 on Cell Cycle-Related Proteins in BPH-Induced Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BPH | Benign prostatic hyperplasia |
| DHT | dihydrotestosterone |
| LUTS | lower urinary tract symptoms |
| IHC | immunohistochemistry |
| H & E | hematoxylin and eosin |
References
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7, S3–S14. [Google Scholar] [PubMed]
- Lim, K.B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef]
- Lu, S.-H.; Chen, C.-S. Natural history and epidemiology of benign prostatic hyperplasia. Formosan J. Surg. 2014, 47, 207–210. [Google Scholar] [CrossRef]
- Carson, C., 3rd; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 2011, 82, 184–199. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster Jr, H.E.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef]
- Patel, N.D.; Parsons, J.K. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J. Urol. 2014, 30, 170–176. [Google Scholar] [PubMed]
- Montorsi, F.; Moncada, I. Safety and tolerability of treatment for BPH. Eur. Urol. Suppl. 2006, 5, 1004–1012. [Google Scholar] [CrossRef]
- Jin, B.R.; Kim, H.J.; Park, S.K.; Kim, M.S.; Lee, K.H.; Yoon, I.J.; An, H.J. Anti-Proliferative Effects of HBX-5 on Progression of Benign Prostatic Hyperplasia. Molecules 2018, 23, 2638. [Google Scholar] [CrossRef]
- Dai, G.C.; Hu, B.; Zhang, W.F.; Peng, F.; Wang, R.; Liu, Z.Y.; Xue, B.X.; Liu, J.Y.; Shan, Y.X. Chemical characterization, anti-benign prostatic hyperplasia effect and subchronic toxicity study of total flavonoid extract of Pteris multifida. Food Chem. Toxicol. 2017, 108, 524–531. [Google Scholar] [CrossRef]
- Zhong, W.; Peng, J.; He, H.; Wu, D.; Han, Z.; Bi, X.; Dai, Q. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin. Invest. Med. 2008, 31, E8–E15. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, S.; Awde, M.; Brock, G.; Casey, R.; Kozak, J.; Lee, J.; Nickel, J.C.; Saad, F. Diagnosis and management of benign prostatic hyperplasia in primary care. J. Gen. Intern. Med. 2009, 3, S92–S100. [Google Scholar] [CrossRef]
- Mega, S.; Miyamoto, M.; Ebihara, Y.; Takahashi, R.; Hase, R.; Li, L.; Shichinohe, T.; Kawarada, Y.; Uehara, H.; Kaneko, H.; et al. E2F1 expression levels are associated with characteristics and prognosis of esophageal squamous cell carcinoma. Dis. Esophagus 2005, 18, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Qian, H.N.; Zhao, Y.; Sun, K.; Wang, H.Q.; Liang, G.Q.; Li, F.H.; Li, Z. Relationship between age and prostate size. Asian J. Androl. 2013, 15, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Brasure, M.; MacDonald, R.; Dahm, P.; Olson, C.M.; Nelson, V.A.; Fink, H.A.; Risk, M.; Rwabasonga, B.; Wilt, T.J. Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostaic Hyperplasia: A Review; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, May 2016.
- Naslund, M.J.; Miner, M. A review of the clinical efficacy and safety of 5alpha-reductase inhibitors for the enlarged prostate. Clin. Ther. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.; Saitz, T.R.; Hellstrom, W.J. Side Effects of 5-Alpha Reductase Inhibitors: A Comprehensive Review. Sex. Med. Rev. 2013, 1, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mizokami, A.; Lin, W.J.; Lai, K.P.; Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 2013, 182, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef]
- Kang, D.G.; Choi, D.H.; Lee, J.K.; Lee, Y.J.; Moon, M.K.; Yang, S.N.; Kwon, T.O.; Kwon, J.W.; Kim, J.S.; Lee, H.S. Endothelial NO/cGMP-dependent vascular relaxation of cornuside isolated from the fruit of Cornus officinalis. Planta Med. 2007, 73, 1436–1440. [Google Scholar] [CrossRef]
- Bensky, D.; Gamble, A.; Kaptchuk, T.J. Chinese Herbal Medicine: Materia Medica; Eastland Press Seattle: Seattle, WA, USA, 2004. [Google Scholar]
- Yang, W.M.; Chang, M.S.; Park, S.K. Effects of Psoralea corylifolia on the cAMP-responsive element modulator (CREM) expression and spermatogenesis in rats. J. Ethnopharmacol. 2008, 117, 503–506. [Google Scholar] [CrossRef]
- Zhang, H.; Jing, Y.; Wu, G. Inhibitory effects of crude polysaccharides from Semen vaccariae on benign prostatic hyperplasia in mice. J. Ethnopharmacol. 2013, 145, 667–669. [Google Scholar] [CrossRef]
- Guo, Q.L.; Ding, Q.L.; Wu, Z.Q. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol. Pharm. Bull. 2004, 27, 333–337. [Google Scholar] [CrossRef]
- Kyprianou, N.; Tu, H.; Jacobs, S.C. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Human Pathol. 1996, 27, 668–675. [Google Scholar] [CrossRef]
- Te, A.E.; Malloy, T.R.; Stein, B.S.; Ulchaker, J.C.; Nseyo, U.O.; Hai, M.A. Impact of prostate-specific antigen level and prostate volume as predictors of efficacy in photoselective vaporization prostatectomy: Analysis and results of an ongoing prospective multicentre study at 3 years. BJU Int. 2006, 97, 1229–1233. [Google Scholar] [CrossRef]
- Ishida, S.; Huang, E.; Zuzan, H.; Spang, R.; Leone, G.; West, M.; Nevins, J.R. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol. Cell. Biol. 2001, 21, 4684–4699. [Google Scholar] [CrossRef]
- Ogawa, H.; Ishiguro, K.; Gaubatz, S.; Livingston, D.M.; Nakatani, Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 2002, 296, 1132–1136. [Google Scholar] [CrossRef]
- DeGregori, J.; Kowalik, T.; Nevins, J.R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 1995, 15, 4215–4224. [Google Scholar] [CrossRef]
- Lukas, J.; Petersen, B.O.; Holm, K.; Bartek, J.; Helin, K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol. Cell. Biol. 1996, 16, 1047–1057. [Google Scholar] [CrossRef]
- Liang, Y.X.; Lu, J.M.; Mo, R.J.; He, H.C.; Xie, J.; Jiang, F.N.; Lin, Z.Y.; Chen, Y.R.; Wu, Y.D.; Luo, H.W.; et al. E2F1 promotes tumor cell invasion and migration through regulating CD147 in prostate cancer. Int. J. Oncol. 2016, 48, 1650–1658. [Google Scholar] [CrossRef]
- Miah, S.; Catto, J. BPH and prostate cancer risk. Indian J Urol. 2014, 30, 214–218. [Google Scholar] [CrossRef]
- Dai, X.; Fang, X.; Ma, Y.; Xianyu, J. Benign Prostatic Hyperplasia and the Risk of Prostate Cancer and Bladder Cancer: A Meta-Analysis of Observational Studies. Medicine 2016, 95, e3493. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
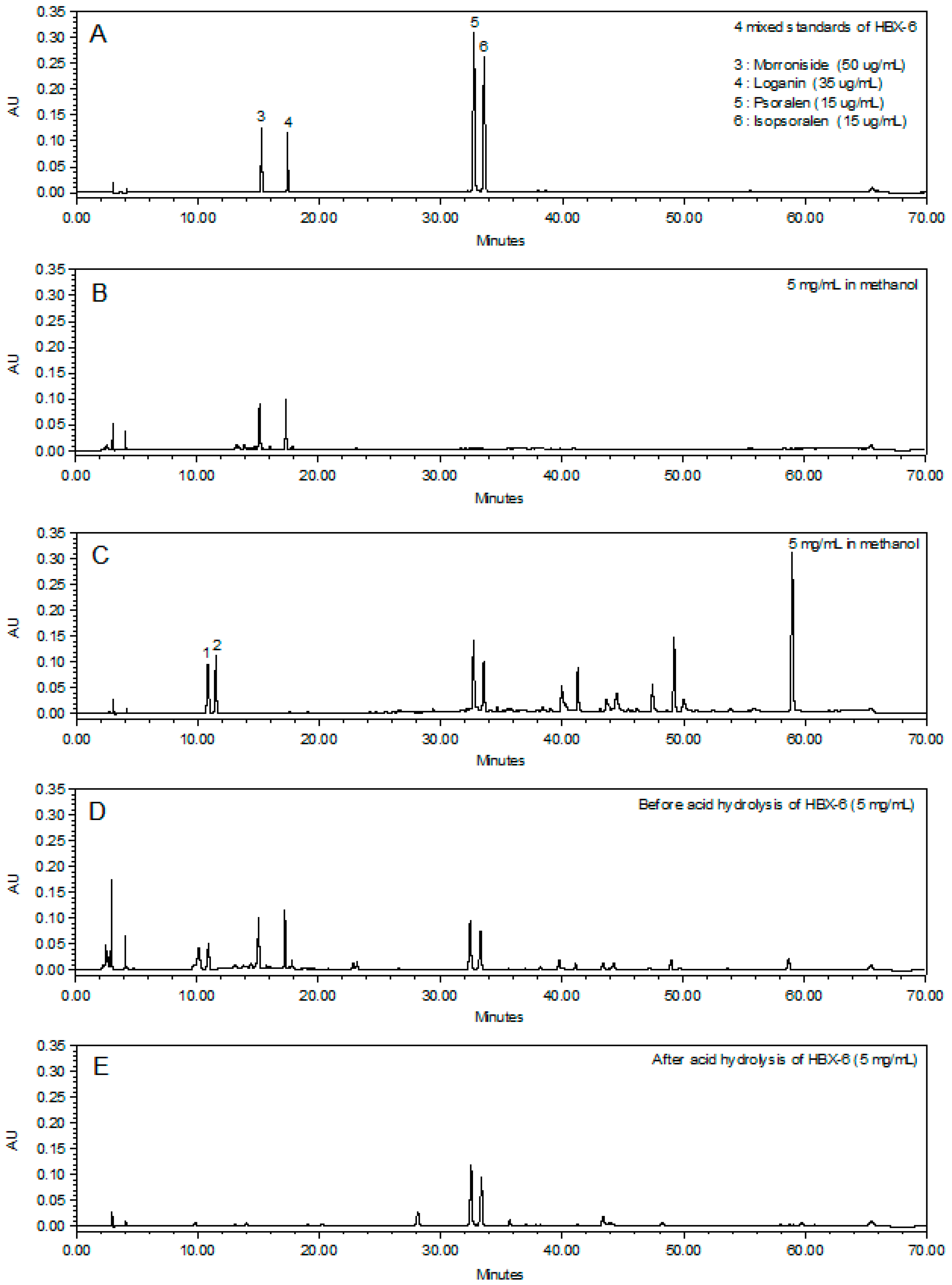
| Analyte | Regression Equation | r2 | Linear Ranage (μg/mL) | LOD a (μg/mL) | LOQ b (μg/mL) |
|---|---|---|---|---|---|
| Morroniside | y = 17572x − 9299.20 | 0.9981 | 25.00–125.00 | 2.75 | 0.91 |
| Loganin | y = 16559x + 9546.56 | 0.9994 | 20.00–80.00 | 0.22 | 0.65 |
| Psoralen | y = 85821x + 21320.47 | 0.9997 | 10.00–30.00 | 0.50 | 1.51 |
| Isopsoralen (Angelicin) | y = 77196x − 61110.20 | 0.9986 | 10.00–30.00 | 0.30 | 0.10 |
| No. | Retension Time (min) | Analyte | Cornus officinalis Sieb. et Zucc. | Psoralea corylifolia L. | HBX-6 |
|---|---|---|---|---|---|
| Content (mg/g) | |||||
| (3) | 15.197 | Morroniside | 7.490 ± 0.143 | - | 11.511 ± 0.015 |
| (4) | 17.368 | Loganin | 4.821 ± 0.082 | - | 8.001 ± 0.013 |
| (5) | 32.761 | Psoralen | - | 3.072 ± 0.004 | 3.457 ± 0.003 a |
| (6) | 33.613 | Isopsoralen (Angelicin) | - | 2.473 ± 0.002 | 3.110 ± 0.008 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.-R.; Kim, H.-J.; Seo, J.-H.; Kim, M.-S.; Lee, K.-H.; Yoon, I.-J.; An, H.-J. HBX-6, Standardized Cornus officinalis and Psoralea corylifolia L. Extracts, Suppresses Benign Prostate Hyperplasia by Attenuating E2F1 Activation. Molecules 2019, 24, 1719. https://doi.org/10.3390/molecules24091719
Jin B-R, Kim H-J, Seo J-H, Kim M-S, Lee K-H, Yoon I-J, An H-J. HBX-6, Standardized Cornus officinalis and Psoralea corylifolia L. Extracts, Suppresses Benign Prostate Hyperplasia by Attenuating E2F1 Activation. Molecules. 2019; 24(9):1719. https://doi.org/10.3390/molecules24091719
Chicago/Turabian StyleJin, Bo-Ram, Hyo-Jung Kim, Jong-Hwan Seo, Myoung-Seok Kim, Kwang-Ho Lee, Il-Joo Yoon, and Hyo-Jin An. 2019. "HBX-6, Standardized Cornus officinalis and Psoralea corylifolia L. Extracts, Suppresses Benign Prostate Hyperplasia by Attenuating E2F1 Activation" Molecules 24, no. 9: 1719. https://doi.org/10.3390/molecules24091719
APA StyleJin, B.-R., Kim, H.-J., Seo, J.-H., Kim, M.-S., Lee, K.-H., Yoon, I.-J., & An, H.-J. (2019). HBX-6, Standardized Cornus officinalis and Psoralea corylifolia L. Extracts, Suppresses Benign Prostate Hyperplasia by Attenuating E2F1 Activation. Molecules, 24(9), 1719. https://doi.org/10.3390/molecules24091719






