Pyrimidine 2,4-Diones in the Design of New HIV RT Inhibitors
Abstract
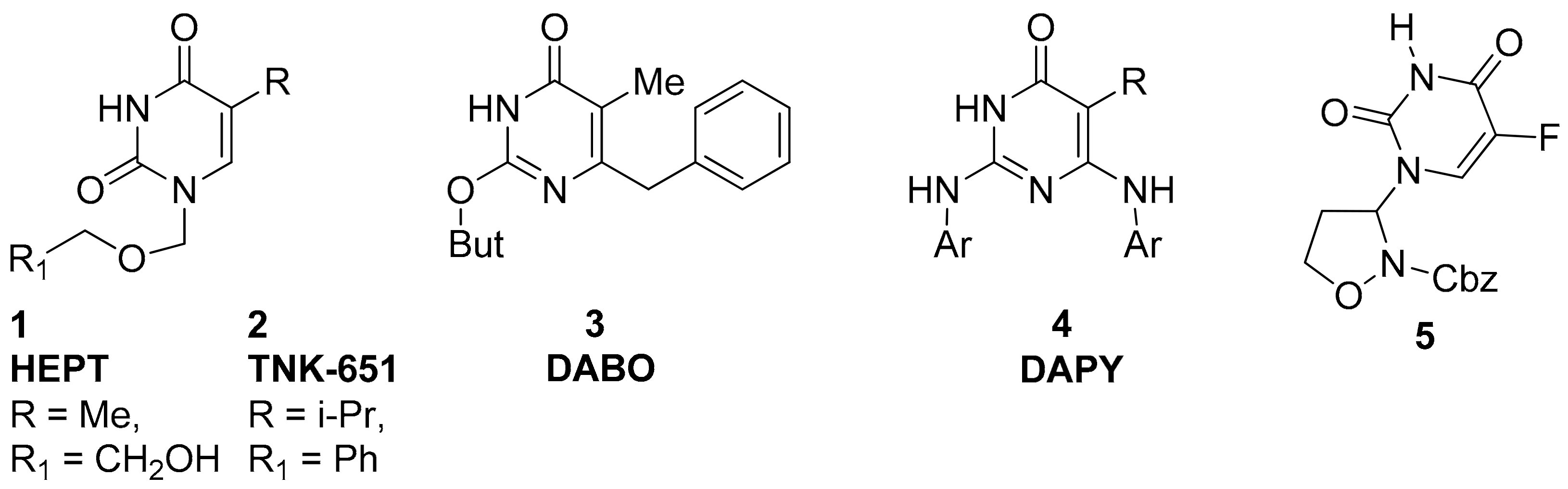
1. Introduction
2. Results and Discussion
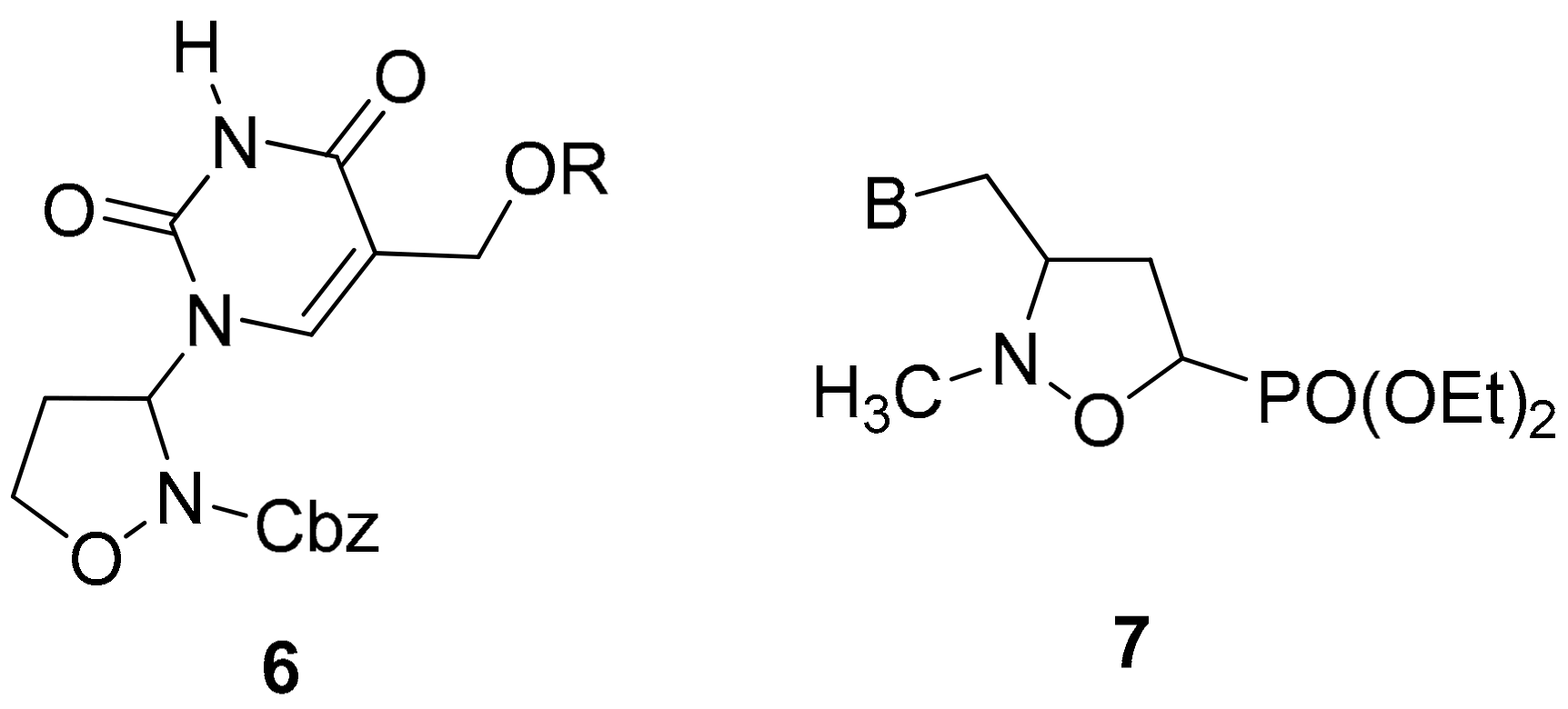
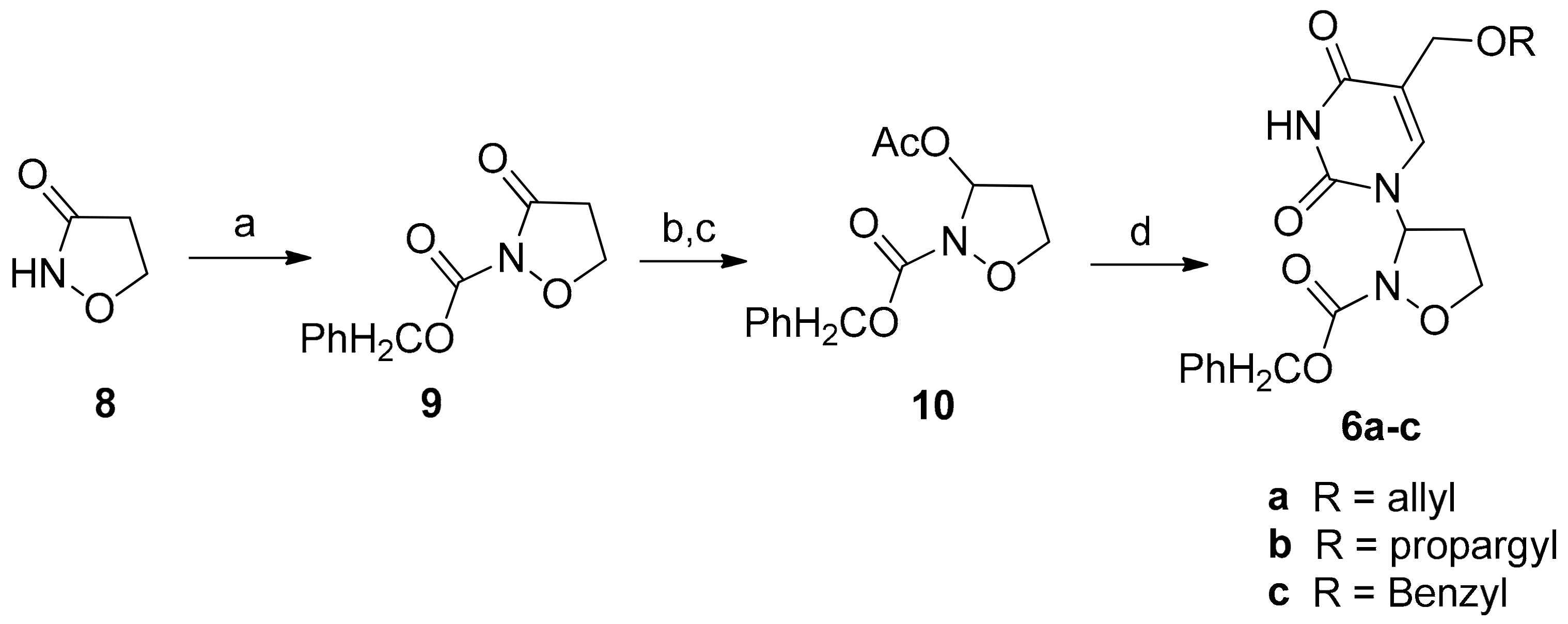
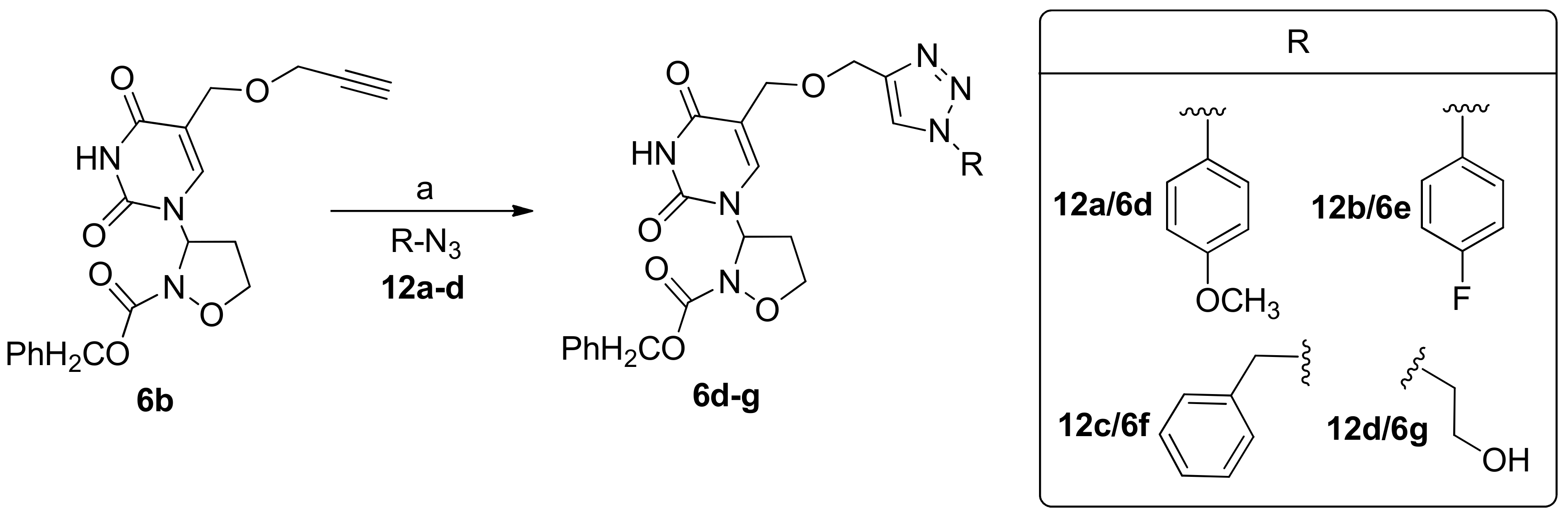
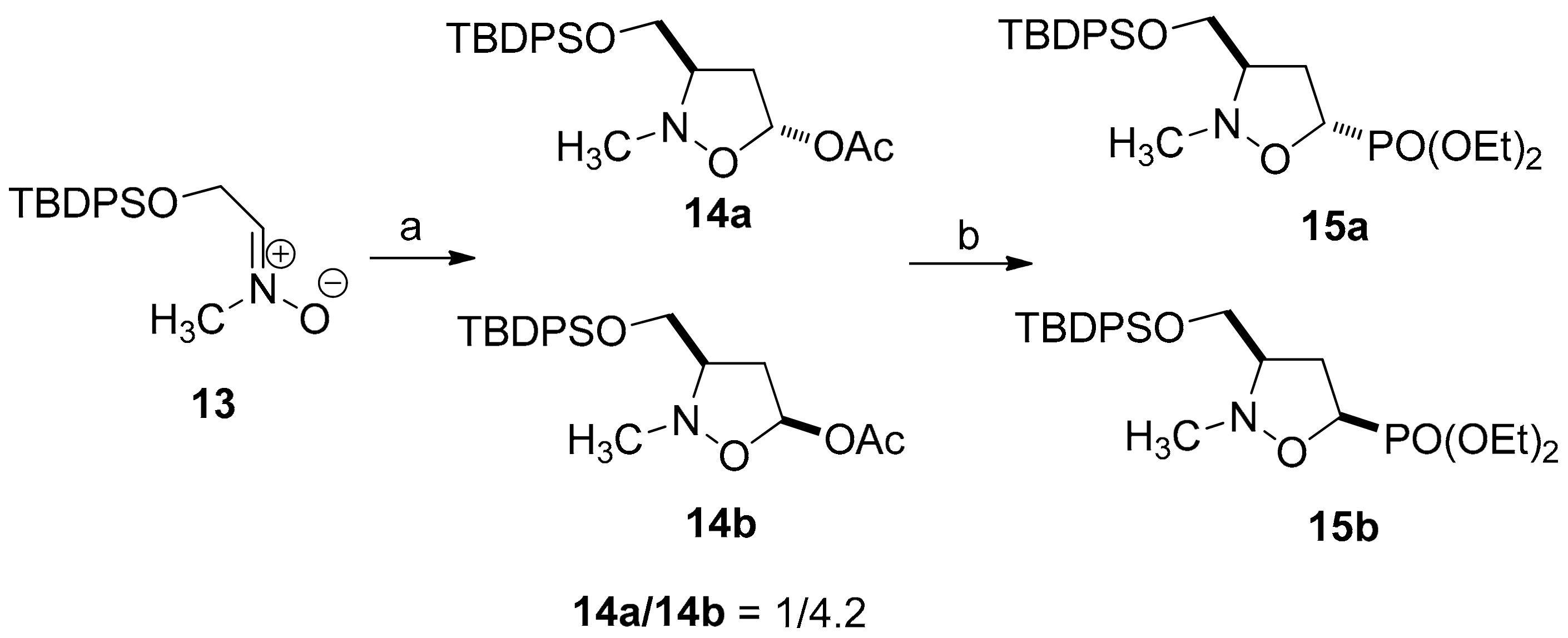
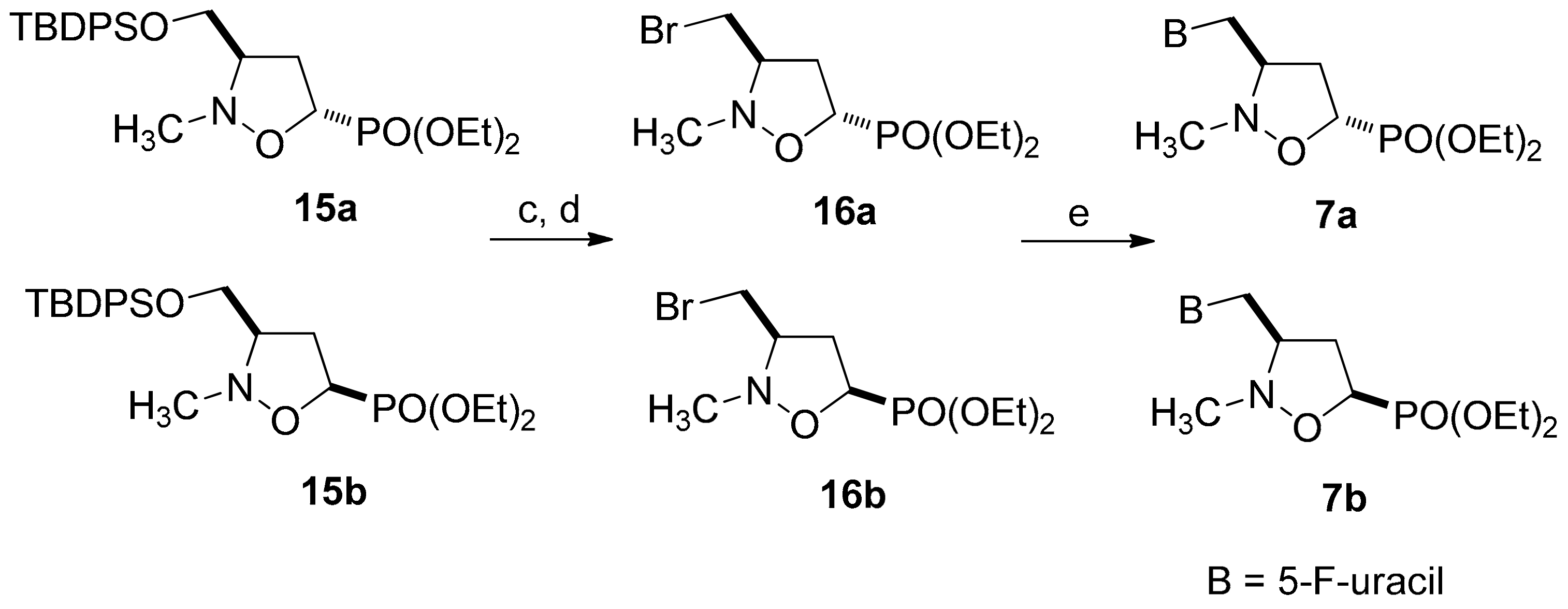
2.1. Chemistry
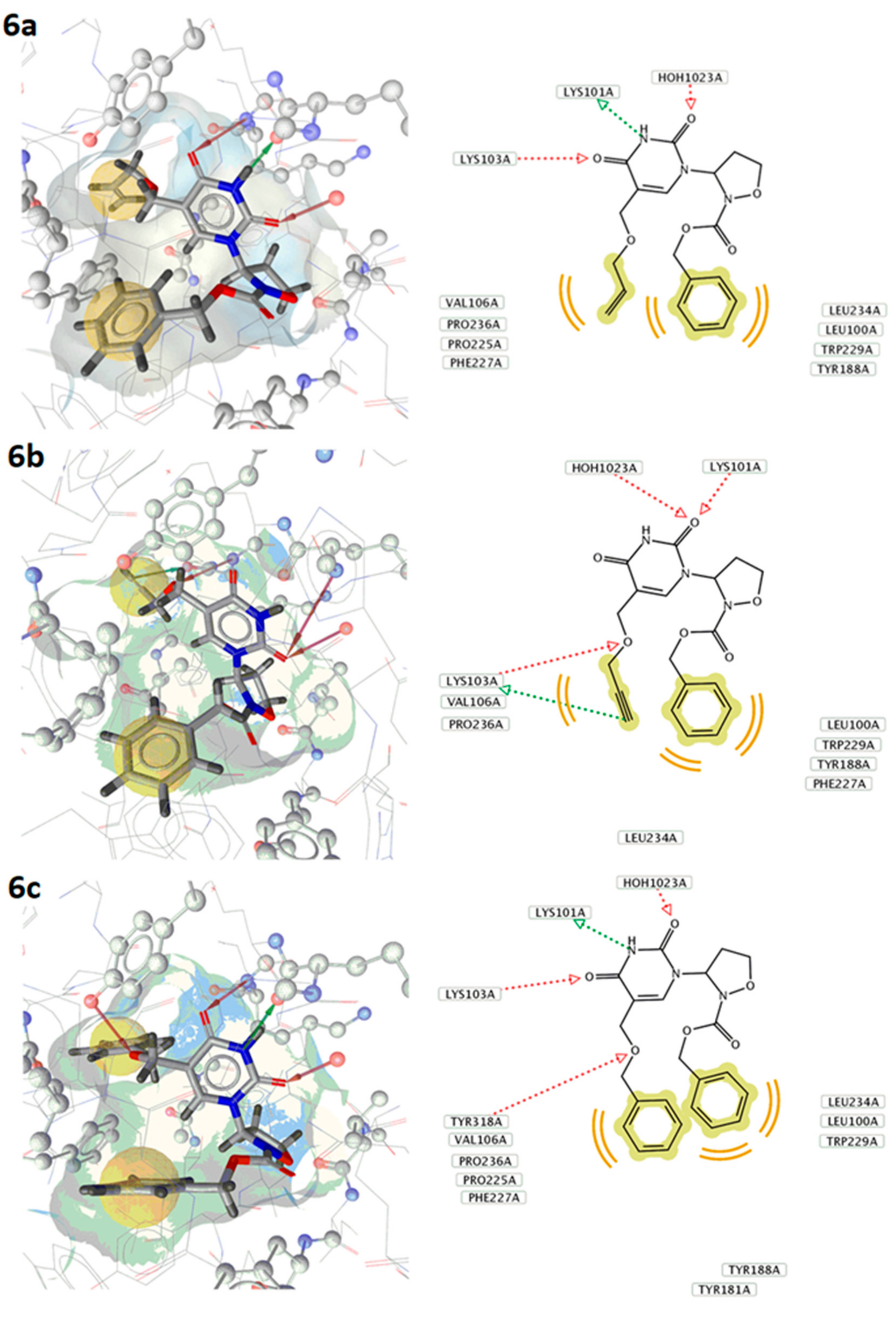
2.2. Biological Activity
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Preparation of 3-Pyrimidinyl-Isoxazolidines 6a–c
3.3. General Procedure for the Preparation of 3-Pyrimidinyl-Isoxazolidines 6d–g
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weber, R.; Ruppik, M.; Rickenbach, M.; Spoerri, A.; Furrer, H.; Battegay, M.; Cavassini, M.; Calmy, A.; Bernasconi, E.; Schmid, P.; et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013, 14, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, G.; Reiss, P.; Kowalska, J.; de Wit, S.; Law, M.; El Sadr, W.; et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014, 384, 241–248. [Google Scholar] [CrossRef]
- McManus, H.; O’Connor, C.C.; Boyd, M.; Broom, J.; Russell, D.; Watson, K.; Roth, N.; Read, P.J.; Petoumenos, K.; Law, M.G. Long-Term Survival in HIV Positive Patients with up to 15 Years of Antiretroviral Therapy. PLoS ONE 2012, 7, e48839. [Google Scholar] [CrossRef]
- Costagliola, D. Demographics of HIV and aging. Curr. Opin. HIV AIDS 2014, 9, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.N.; Grey, P.; Shaik, A.; Cooper, D.A.; Kelleher, A.D.; Petoumenos, K. Early Treatment of Primary HIV Infection Is Associated with Decreased Mortality. AIDS Res. Hum. Retrovir. 2018, 34, 936–946. [Google Scholar] [CrossRef]
- Heredia, A.; Le, N.; Gartenhaus, R.B.; Sausville, E.; Medina-Moreno, S.; Zapata, J.C.; Davis, C.; Gallo, R.C.; Redfield, R.R. Targeting of mTOR catalytic site inhibits multiple steps of the HIV-1 life cycle and suppresses HIV-1 viremia in humanized mice. Proc. Natl. Acad. Sci. USA 2003, 112, 9412–9417. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Amoroso, A.; Davis, C.; Le, N.; Reardon, E.; Dominique, J.K.; Klingebiel, E.; Gallo, R.C.; Redfield, R.R. Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV β-chemokines: An approach to suppress R5 strains of HIV-1. Proc. Natl. Acad. Sci. USA 2003, 100, 10411–10416. [Google Scholar] [CrossRef]
- Nicoletti, F.; Lapenta, C.; Donati, S.; Spada, M.; Ranazzi, A.; Cacopardo, B.; Mangano, K.; Belardelli, F.; Perno, C.; Aquaro, S. Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clin. Exp. Immunol. 2009, 155, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; McCubrey, J.A.; Bendtzen, K.; Nicoletti, F. Potential use of rapamycin in HIV infection. Br. J. Clin. Pharmacol. 2010, 70, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Fagone, P.; Meroni, P.; McCubrey, J.; Bendtzen, K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov. Today 2011, 16, 715–721. [Google Scholar] [CrossRef]
- Agnello, S.; Brand, M.; Chellat, M.F.; Gazzola, S.; Riedl, R. A structural view on medicinal chemistry strategy against drug resistance. Angew. Chem. Int. Ed. 2019, 53, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, C.; Grelli, S.; Balestrieri, E.; Minutolo, A.; Argaw-Denboba, A.; Macchi, B.; Sinibaldi-Vallebona, P.; Perno, C.F.; Mastino, A.; Garaci, E. Thymosin alpha 1 and HIV-1: Recent advances and future perspectives. Future Microbiol. 2017, 12, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Beyrer, C.; Pozniak, A. HIV drug resistance—An emerging threat to epidemic control. N. Engl. J. Med. 2017, 377, 1605–1607. [Google Scholar] [CrossRef]
- Lehman, D.A.; Wamelwa, D.C.; McCoy, C.O.; Matsen, F.A.; Langat, A.; Chohan, B.H.; Benki-Nugent, S.; Custers-Allen, R.; Bushman, F.D.; John-Stewart, G.C.; et al. Low-frequency Nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. J. Acq. Immun. Def. Synd. 2012, 60, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.; Eleftheriou, P.; Poroikov, V. Anti-HIV Agents: Current status and recent trends. In Communicable Diseases of the Developing World; Springer: Berlin, Germany, 2018; pp. 37–95. [Google Scholar]
- D’Ettorre, G.; Ceccarelli, G.; Pavone, P.; Vittozzi, P.; De Girolamo, G.; Schietroma, I.; Serafino, S.; Giustini, N.; Vullo, V. What happens to cardiovascular system behind the undetectablelevel of HIV viremia? AIDS Res. Ther. 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.S.; Chow, F.C. Neurologic Complications in Treated HIV-1 Infection. Curr. Neurol. Neurosci. Rep. 2016, 16, 62. [Google Scholar] [CrossRef]
- Haas, D.W.; Tarr, P.E. Perspectives on pharmacogenomics of antiretroviral medications and HIV-associated comorbidities. Curr. Opin. HIV AIDS 2015, 10, 116–122. [Google Scholar] [CrossRef]
- De Clercq, E. A 40-year journey in search of selective antiviral chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 1–24. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Ghosh, S.M.; Chawla, S. Recent advances in antiretroviral drugs. Expert Opin. Pharmacother. 2011, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Deeks, S.G. When to Start Antiretroviral Therapy. Curr. Hiv Aids Rep. 2010, 7, 60–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, E. Antiviral drug discovery: Ten more compounds, and ten more stories (part B). Med. Res. Rev. 2009, 29, 611–645. [Google Scholar] [CrossRef]
- De Clercq, E. The discovery of antiviral agents: Ten different compounds, ten different stories. Med. Res. Rev. 2008, 28, 929–953. [Google Scholar] [CrossRef]
- Dal Pozzo, F.; Andrei, G.; Lebeau, I.; Beadle, J.R.; Hostetler, K.Y.; De Clercq, E.; Snoeck, R. In vitro evaluation of the anti-orf virus activity of alkoxyalkyl esters of CDV, cCDV and (S)-HPMPA. Antivir. Res. 2007, 75, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Holy, A. Antiviral acyclic nucleoside phosphonates structure activity studies. Antivir. Res. 2006, 71, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Dash, C.; Ahmadibeni, Y.; Hanley, M.J.; Pandhare, J.; Gotte, M.; Le Grice, S.F.J.; Parang, K. Inhibition of multi-drug resistant HIV-1 reverse transcriptase by nucleoside β-triphosphates. Bioorg. Med. Chem. Lett. 2011, 21, 3519–3522. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Himmel, D.M.; Jiang, J.-K.; Wojtak, K.; Bauman, J.D.; Rausch, J.W.; Wilson, J.A.; Beutler, J.A.; Thomas, C.J.; Arnold, E.; et al. Synthesis, Activity, and Structural Analysis of Novel α-Hydroxytropolone Inhibitors of Human Immunodeficiency Virus Reverse Transcriptase-Associated Ribonuclease, H. J. Med. Chem. 2011, 54, 4462–4473. [Google Scholar] [CrossRef] [PubMed]
- Koczor, C.A.; Lewis, W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Exp. Opin. Drug Metab. Toxicol. 2010, 6, 1493–1504. [Google Scholar] [CrossRef]
- Martin, J.C.; Hitchcock, M.J.M.; De Clercq, E.; Prusoff, W.H. Early nucleoside reverse transcriptase inhibitors for the treatment of HIV: A brief history of stavudine (D4T) and its comparison with other dideoxynucleosides. Antivir. Res. 2010, 85, 34–38. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs): Past, Present, and Future. Chem. Biodivers. 2004, 1, 44–64. [Google Scholar] [CrossRef]
- De Clercq, E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antivir. Res. 1998, 38, 153–179. [Google Scholar] [CrossRef]
- Ding, J.; Das, K.; Moereels, H.; Koymans, L.; Andries, K.; Janssen, P.A.; Hughes, S.H.; Arnold, E. Structure of HIV-1 RT/TIBO R 86183 complex reveals similarity in the binding of diverse nonnucleoside inhibitors. Nat. Struct. Biol. 1995, 2, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Lewi, P.; Hughes, S.H.; Arnold, E. Crystallography and the design of anti-AIDS drugs: Conformational flexibility and positional adaptability are important in the design of non-nucleoside HIV-1 reverse transcriptase inhibitors. Prog. Biophys. Mol. Biol. 2005, 88, 209–231. [Google Scholar] [CrossRef]
- Romeo, G.; Chiacchio, U.; Corsaro, A.; Merino, P. ChemicalSynthesis of Heterocyclic−Sugar Nucleoside Analogues. Chem. Rev. 2010, 110, 3337–3370. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Carnovale, C.; Giofrè, S.V.; Macchi, B.; Frezza, A.; Marino-Merlo, C.; Pistarà, V.; Chiacchio, U. Truncatedphosphonated C-1′-branched N,O-nucleosides: A new class of antiviral agents. Bioorg. Med. Chem. 2012, 20, 3652–3657. [Google Scholar] [CrossRef]
- Balestrieri, E.; Matteucci, C.; Ascolani, A.; Piperno, A.; Romeo, R.; Romeo, G.; Chiacchio, U.; Mastino, A.; Macchi, B. Effect of Phosphonated Carbocyclic 2′-Oxa-3′-Aza-Nucleoside on Human T-Cell Leukemia Virus Type 1 Infection In Vitro. Antimicrob. Agents Chemother. 2008, 52, 54–64. [Google Scholar] [CrossRef]
- Chiacchio, U.; Iannazzo, D.; Piperno, A.; Romeo, R.; Romeo, G.; Rescifina, A.; Saglimbeni, M. Synthesis and biological evaluation of phosphonated carbocyclic 2′-oxa-3′-aza-nucleosides. Bioorg. Med. Chem. 2006, 14, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Guillemont, G.; Pasquier, E.; Palandjian, P.; Vernier, D.; Gaurrand, S.; Lewi, P.J.; Heeres, J.; de Jonge, L.M.; Koymans, F.F.; Daeyaert, M.H.; et al. Synthesis of Novel Diarylpyrimidine Analogues and Their Antiviral Activity against Human Immunodeficiency Virus Type 1. J. Med. Chem. 2005, 48, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhan, P.; Li, D.; De Clercq, E.; Liu, X. Recent advances in DAPYs and related analogues as HIV-1 NNRTIs. Curr. Med. Chem. 2011, 18, 359–376. [Google Scholar] [CrossRef]
- Schrijvers, R. Etravirine for the treatment of HIV/AIDS. Expert Opin. Pharmacother. 2013, 2014, 1087–1096. [Google Scholar] [CrossRef]
- Minudo, J.J.; Haubrich, R. Etravirine: A second-generation NNRTI for treatment-experienced adults with resistant HIV-1 infection. Futur. HIV Ther. 2008, 2, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Pannecouque, C.; De Clercq, E.; Liu, X. Anti-HIV Drug Discovery and Development: Current Innovations and Future Trends. J. Med. Chem. 2016, 59, 2849–2878. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhan, P.; De Clercq, E.; Liu, X. Strategies for the Design of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors: Lessons from the Development of Seven Representative Paradigms. J. Med. Chem. 2012, 55, 3595–3613. [Google Scholar] [CrossRef]
- Marino-Merlo, F.; Macchi, B.; Armenia, D.; Bellocchi, M.C.; Ceccherini-Silberstein, F.; Mastino, A.; Grelli, S. Focus on recently developed assays for detection of resistance/sensitivity to reverse transcriptase inhibitors. Appl. Microbiol. Biotechnol. 2018, 102, 9925–9936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, P.; Huang, B.; Zhou, Z.; Yu, Z.; Yuan, Z.; Liu, H.; Pannecouque, C.; Daelemans, D.; De Clercq, E.; et al. Discovery of novel piperidine-substituted indolylarylsulfones as potent HIV NNRTIs via structure-guided scaffold morphing and fragment rearrangement. Eur. J. Med. Chem. 2017, 126, 190–201. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Tien, Y.; Song, Y.; Zhan, P.; Liu, X. Novel HIV-1 non-nucleoside reverse transcriptaseinhibitors: A patentreview (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1199–1227. [Google Scholar] [CrossRef]
- Zhan, P.; Chen, X.; Li, D.; Fang, Z.; De Clercq, E.; Liu, X. HIV NNRTIs: Structural diversity, pharmacophore similarity, and implications for drug design. Med. Res. Rev. 2013, 33, E1–E72. [Google Scholar] [CrossRef]
- De Bethune, M.P. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in treatment of HIV infections: A review of the last 20 years (1989–2009). Antivir. Res. 2010, 85, 75–90. [Google Scholar] [CrossRef]
- Mordant, C.; Schmitt, B.; Pasquier, E.; Demestre, C.; Queguiner, L.; Masungi, C.; Peeters, A.; Smeulders, L.; Bettern, E.; Hertogs, K.; et al. Synthesis of novel diarylpyrimidine analogues of TMC278 and their antiviral activity against HIV-1 wild-type and mutant strains. Eur. J. Med. Chem. 2007, 42, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-Q.; Liang, Y.-H.; Zeng, Z.-S.; Chen, F.-E.; Balzarini, J.; Pannecouque, C.; De Clercq, E. Structural modifications of DAPY analogues with potent HIV activity. Chem. Med. Chem. 2009, 4, 219–224. [Google Scholar] [CrossRef]
- Liang, Y.-H.; He, Q.-Q.; Zeng, Z.-S.; Liu, Z.-Q.; Feng, X.-Q.; Chen, F.-E.; Pannecouque, C.; De Clercq, E. Synthesis and anti-HIV activity of 2-naphtyl substituted DAPY analogues as non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2010, 18, 4601–4605. [Google Scholar] [CrossRef] [PubMed]
- Rotili, D.; Tarantino, D.; Artico, M.; Nawrozkij, M.B.; Gonzalez-Ortega, E.; Clotet, B.; Samuele, A.; EstØ, J.A.; Maga, G.; Mai, A. Diarylpyrimidine-dihydrobenzyloxopyrimidine hybrids: New, wide-spectrumanti-HIV-1 agents active at (sub)nanomolar level. J. Med. Chem. 2011, 54, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Nawrozkij, M.B.; Rotili, D.; Tarantino, D.; Botta, G.; Eremiychuk, A.S.; Musmuca, I.; Ragno, R.; Samuele, A.; Zanoli, S.; Armand-Ugòn, M.; et al. 5-Alkyl-6-benzyl-2-(2-oxo-2-phenylethylsulfonyl)pyrimidine-4(3H)-ones, a series of anti-HIV-1 agents of the dihydro-alkoxy-benzyl-oxopyrimidine family with peculiar structure-activity relationship profile. J. Med. Chem. 2008, 51, 4641–4652. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Guo, Y.; Liu, Z.; Shi, X.; Xu, Y.; Wang, X.; Zhang, Z.; Liu, J. The design and synthesis of N-1-alkylated-5-aminoarylalkylsubstituted-6-methyluracils as potential non-nucleoside HIV-1 RT inhibitors. Bioorg. Med. Chem. 2007, 15, 7399–7407. [Google Scholar] [CrossRef]
- Danel, K.; Larsen, L.M.; Pedersen, E.B.; Sanna, G.; La Colla, P.; Loddo, R. Synthesis and antiviral activity of new dimeric inhibitors against HIV-1. Bioorg. Med. Chem. 2008, 16, 511–517. [Google Scholar] [CrossRef]
- Campiani, G.; Ramunno, A.; Maga, G.; Nacci, V.; Fattorusso, C.; Catalanotti, B.; Morelli, E.; Novellino, E. Non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors: Past, present, and future perspectives. Curr. Pharm. Des. 2002, 8, 615–657. [Google Scholar] [CrossRef]
- Romeo, R.; Giofrè, S.V.; Macchi, B.; Balestrieri, E.; Mastino, A.; Merino, P.; Romeo, G.; Chiacchio, U. Truncated reverse isoxazolidinyl nucleosides: A new class of allosteric HIV-1 reverse transcriptase inhibitors. Chem. Med. Chem. 2012, 7, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Macchi, B.; Romeo, G.; Chiacchio, U.; Frezza, C.; Marino Merlo, F.; Mastino, A. Phosphonated nucleoside analoguesasantiviral agents. In Topics in Medicinal Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; Volume 9, pp. 53–92. [Google Scholar]
- Reddy, A.S.; Kumar, M.S.; Reddy, G.R. A convenient method for the preparation of hydroxamic acids. Tetrahedron Lett. 2000, 41, 6285–6288. [Google Scholar] [CrossRef]
- Piperno, A.; Chiacchio, U.; Iannazzo, D.; Giofrè, S.V.; Romeo, G.; Romeo, R. First example of direct RuO4-catalyzed oxidation of isoxazolidines to 3-oxazolidones. J. Org. Chem. 2007, 72, 3958–3960. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Balzarini, J.; Andrei, G.; Schols, D.; Snoeck, R.; Wroblewski, A.E.; Gatkowska, J. Novel isoxazolidine analogues of homonuclemosides and homonucleotides. Tetrahedron 2016, 72, 8294–8308. [Google Scholar] [CrossRef]
- Romeo, R.; Giofrè, S.V.; Iaria, D.; Sciortino, M.T.; Ronsisvalle, S.; Chiacchio, M.A.; Scala, A. Synthesis of 5-alkynyl isoxazolidinyl nucleosides. Eur. J. Org. Chem. 2011, 28, 5690–5695. [Google Scholar] [CrossRef]
- Romeo, R.; Giofrè, S.V.; Garozzo, A.; Bisignano, B.; Corsaro, A.; Chiacchio, M.A. Synthesis and biological evaluation of furopyrimidine N,O-nucleosides. Bioorg. Chem. Med. 2013, 21, 5688–5693. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Balestrieri, E.; Marino-Merlo, F.; Mastino, A.; Macchi, B. A novel, cell-free PCR-based assay for evaluating the inhibitor activity of antiretroviral compounds against HIV reverse transcriptase. J. Med. Virol. 2014, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Grelli, S.; Federico, M.; Marino-Merlo, F.; Mastino, A.; Macchi, B. Testing anti-HIV activity of antiretroviral agents in vitro using flow cytometry analysis of CEM-GFP cells infected with transfection-derived HIV-1 NL4-3. J. Med. Virol. 2016, 88, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.A.; Sciortino, M.T.; Perri, D.; Amici, C.; Avitabile, E.; Ciotti, M.; Balestrieri, E.; De Smaele, E.; Franzoso, G.; Mastino, A. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: Role of nuclear factor kappaB. J. Biol. Chem. 2003, 19, 36059–36067. [Google Scholar] [CrossRef]
- Romeo, R.; Giofrè, S.V.; Carnovale, C.; Chiacchio, M.A.; Campisi, A.; Mancuso, R.; Cirmi, S.; Navarra, M. Synthesis and Biological Activity of Triazole-Appended N,O-Nucleosides. Eur. J. Org. Chem. 2014, 25, 5442–5447. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 6a–g and 7a,b are available from the authors. |
| RT Inhibition Assay 1 MRTIC (μM) | Cytotoxicity CC50 ± SD (μM) 2 | ||
|---|---|---|---|
| Compound | HIV-RT | MOLT-3 | Pearson’s 3 |
| 6a | 0.1 | >1000 | 0.94 |
| 6b | 1 | >1000 | 0.95 |
| 6c | 0.01 | >1000 | 0.97 |
| 6d | >100 | 594 ± 0.8 | - |
| 6e | >100 | 579 ± 2 | - |
| 6f | >100 | 552 ± 3 | - |
| 6g | 100 | 670 ± 1 | - |
| 7a | n.d. | >1000 | - |
| 7b | 90 4 | >1000 | - |
| NVP | 0.001 | >1000 | 0.97 |
| RPV | 0.038 | n.d. | n.d. |
| EVF | 0.0000094 | n.d. | n.d. |
| IC50 ± SD (µM) 1 | Cytotoxicity CC50 ± SD (µM) 2 | Selectivity Index 3 | |
|---|---|---|---|
| Compound | % GFP + cells | CEM/GFP | SI |
| 6a | 10.1 | 747 ± 10.7 | 73.9 |
| 6b | 0.69 | 1053 ± 8.2 | 1526 |
| 6c | 15.7 | 1127 ± 6.6 | 71.8 |
| 6g | 103 | 427 ± 0.1 | 4 |
| NVP | 0.14 | 980 ± 9.9 | 7000 |
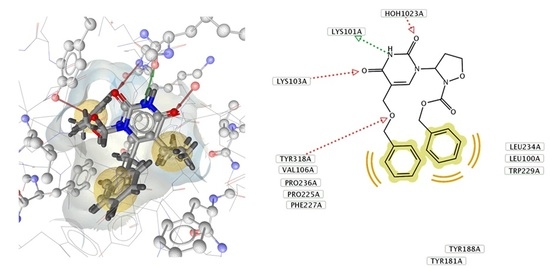
| Compound | ΔG Kcal/mol |
|---|---|
| 6a | −8.51 |
| 6b | −7.45 |
| 6c | −8.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, R.; Iannazzo, D.; Veltri, L.; Gabriele, B.; Macchi, B.; Frezza, C.; Marino-Merlo, F.; Giofrè, S.V. Pyrimidine 2,4-Diones in the Design of New HIV RT Inhibitors. Molecules 2019, 24, 1718. https://doi.org/10.3390/molecules24091718
Romeo R, Iannazzo D, Veltri L, Gabriele B, Macchi B, Frezza C, Marino-Merlo F, Giofrè SV. Pyrimidine 2,4-Diones in the Design of New HIV RT Inhibitors. Molecules. 2019; 24(9):1718. https://doi.org/10.3390/molecules24091718
Chicago/Turabian StyleRomeo, Roberto, Daniela Iannazzo, Lucia Veltri, Bartolo Gabriele, Beatrice Macchi, Caterina Frezza, Francesca Marino-Merlo, and Salvatore V. Giofrè. 2019. "Pyrimidine 2,4-Diones in the Design of New HIV RT Inhibitors" Molecules 24, no. 9: 1718. https://doi.org/10.3390/molecules24091718
APA StyleRomeo, R., Iannazzo, D., Veltri, L., Gabriele, B., Macchi, B., Frezza, C., Marino-Merlo, F., & Giofrè, S. V. (2019). Pyrimidine 2,4-Diones in the Design of New HIV RT Inhibitors. Molecules, 24(9), 1718. https://doi.org/10.3390/molecules24091718