Radioprotective Effect of Walnut Oligopeptides Against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice
Abstract
1. Introduction
2. Results
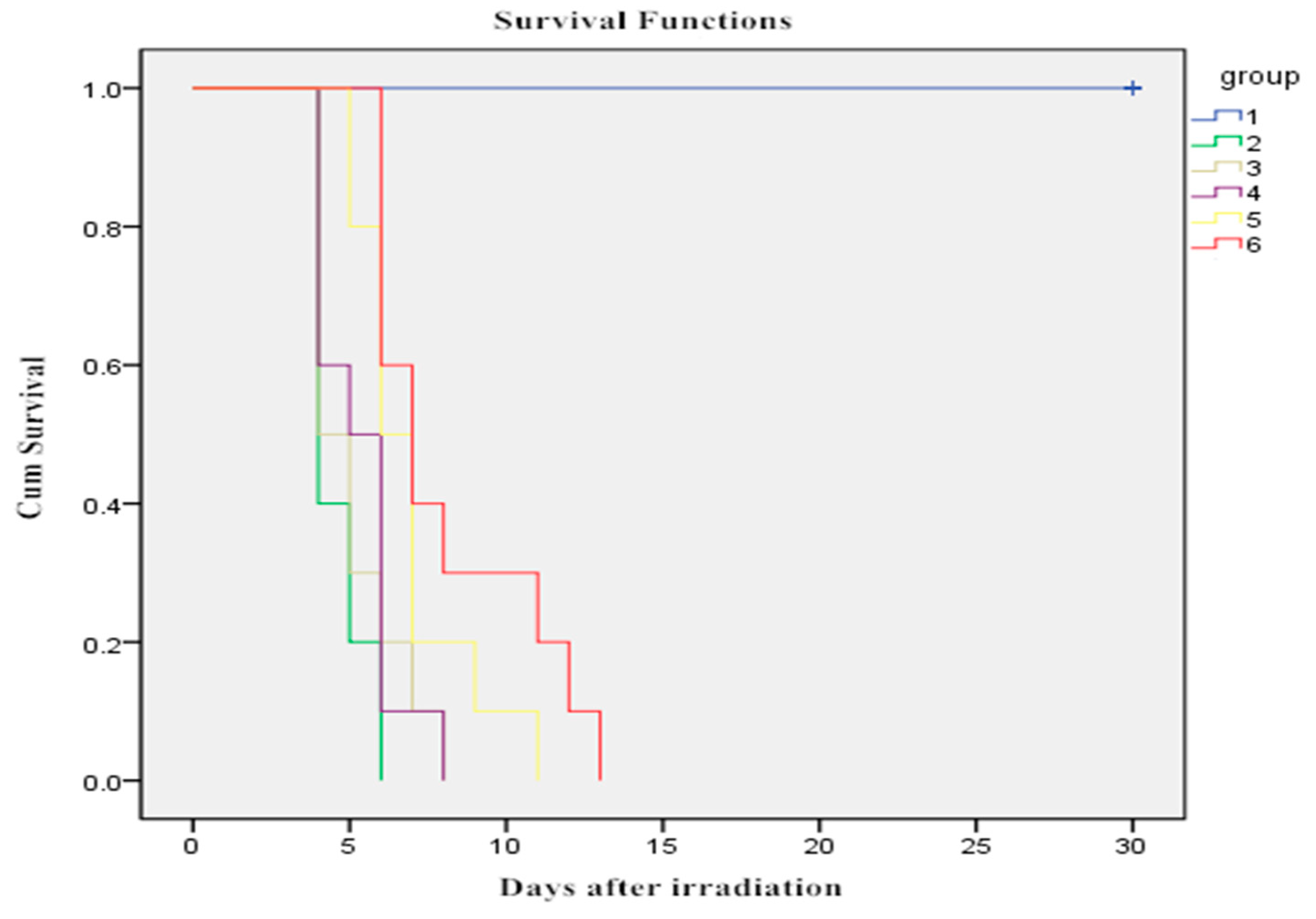
2.1. Effect of WOPs on Survival Time after Whole-Body Irradiation
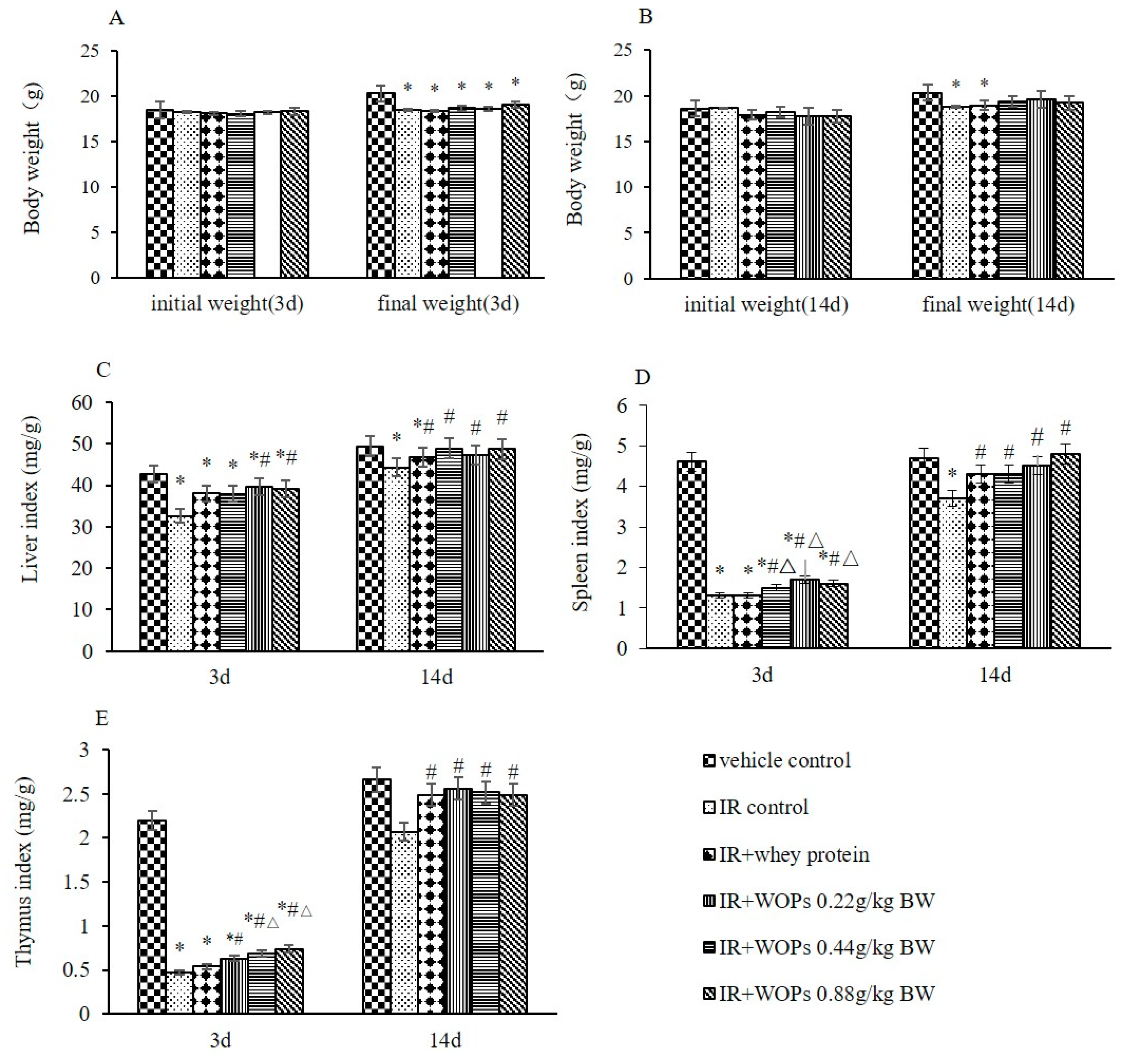
2.2. Effects of WOPs on Body Weight and Immune Organ Indices in Mice after Whole-Body Irradiation
2.3. Effect of WOPs on White Blood Cell Count and Bone Marrow Hematopoietic System Damage
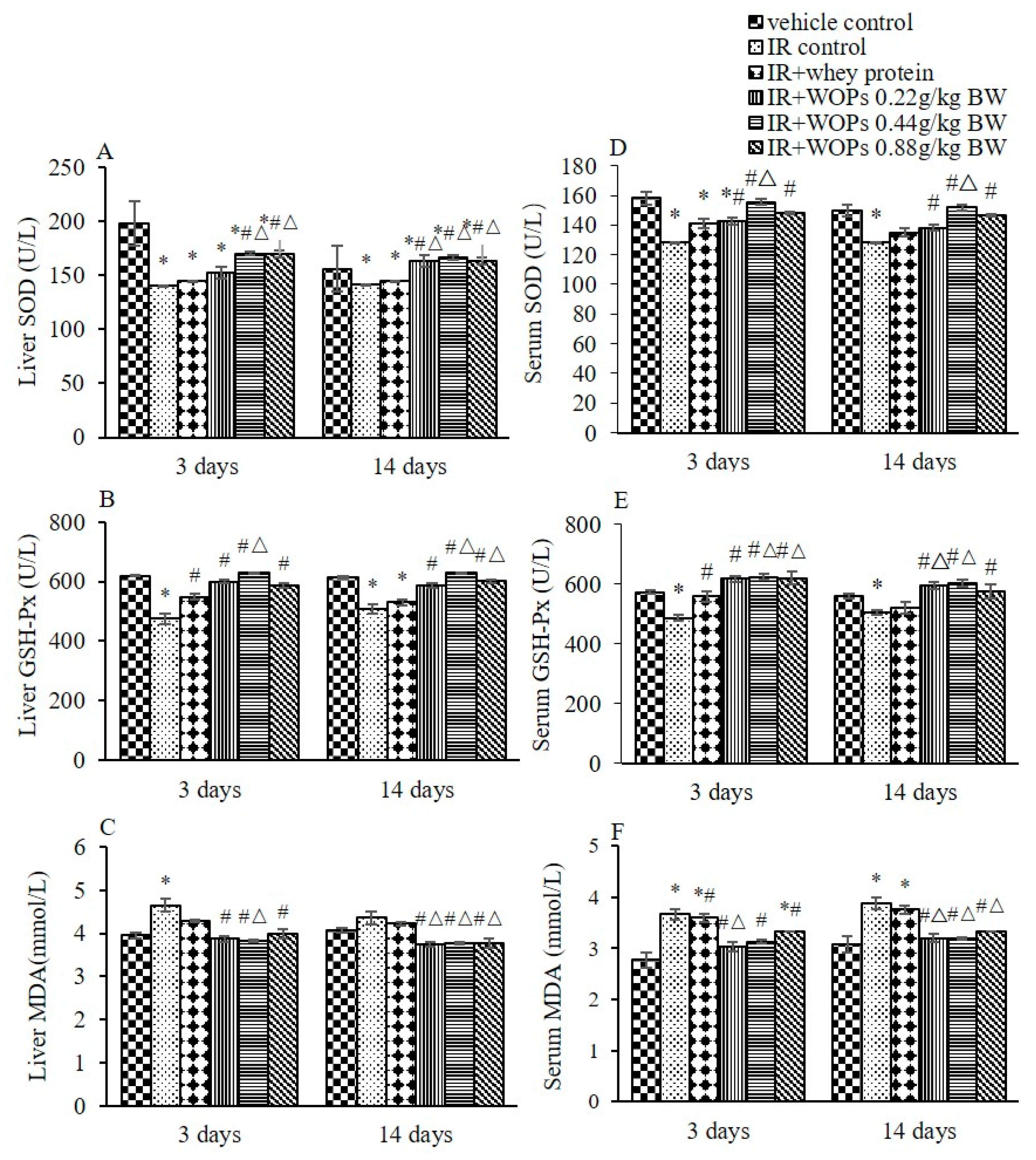
2.4. Effect of WOPs on Irradiation-Induced Depletion of Endogenous Antioxidant Defense Systems
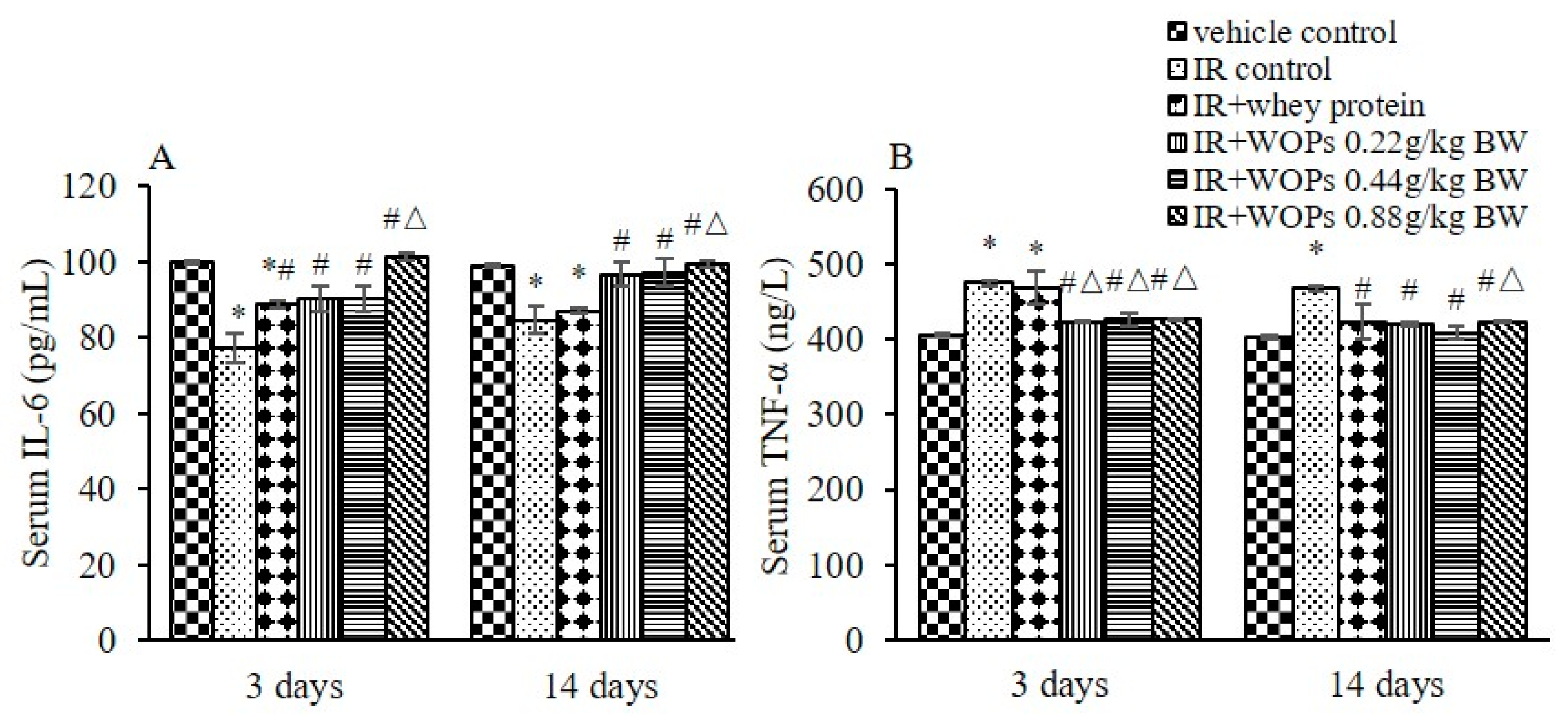
2.5. Effect of WOPs on Pro-Inflammatory Cytokine Levels in Serum
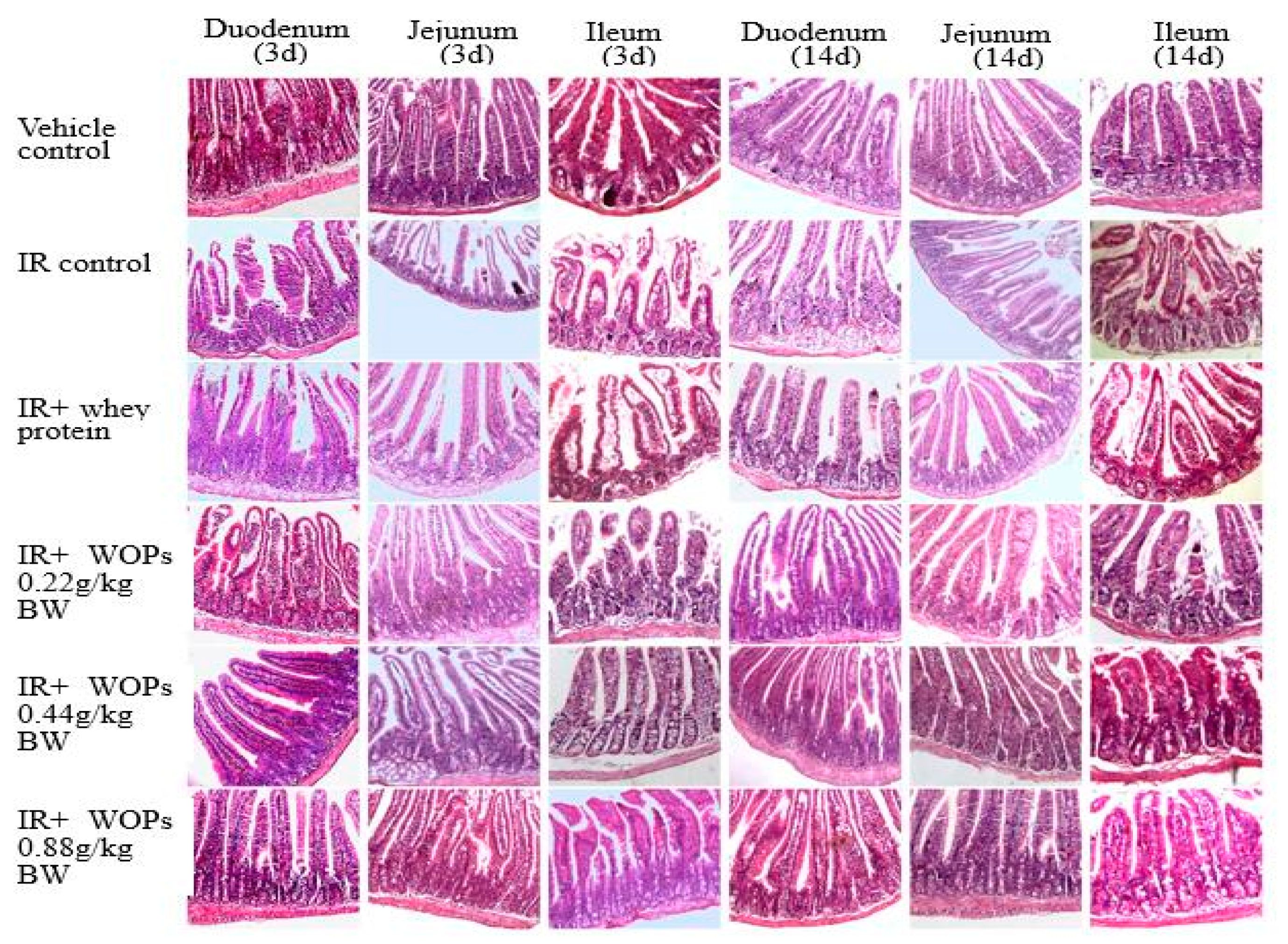
2.6. Effect of WOPs on Radiation-Induced Small Intestinal Mucosal Injury
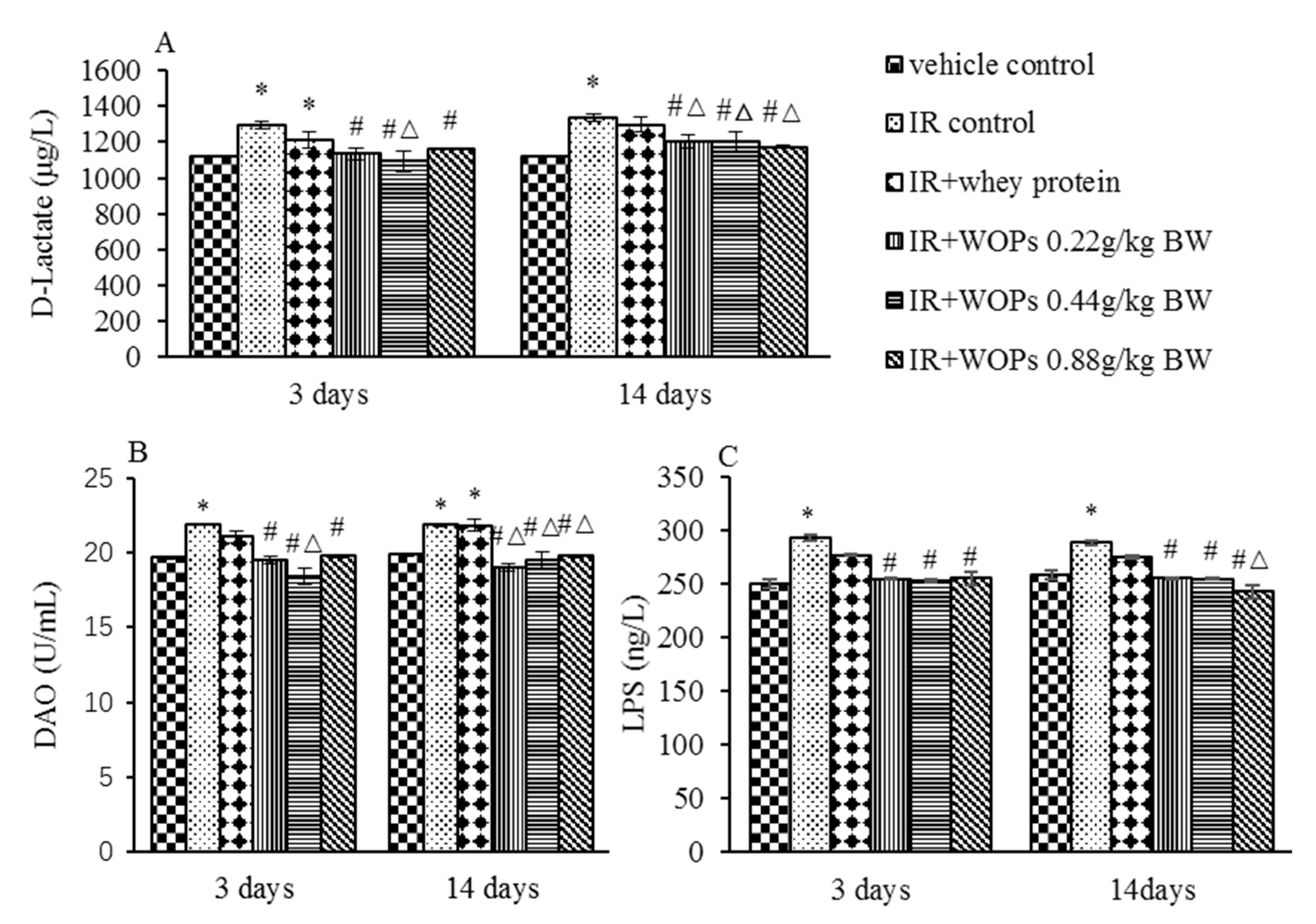
2.7. Effect of WOPs on Intestinal Permeability in Irradiated Mice
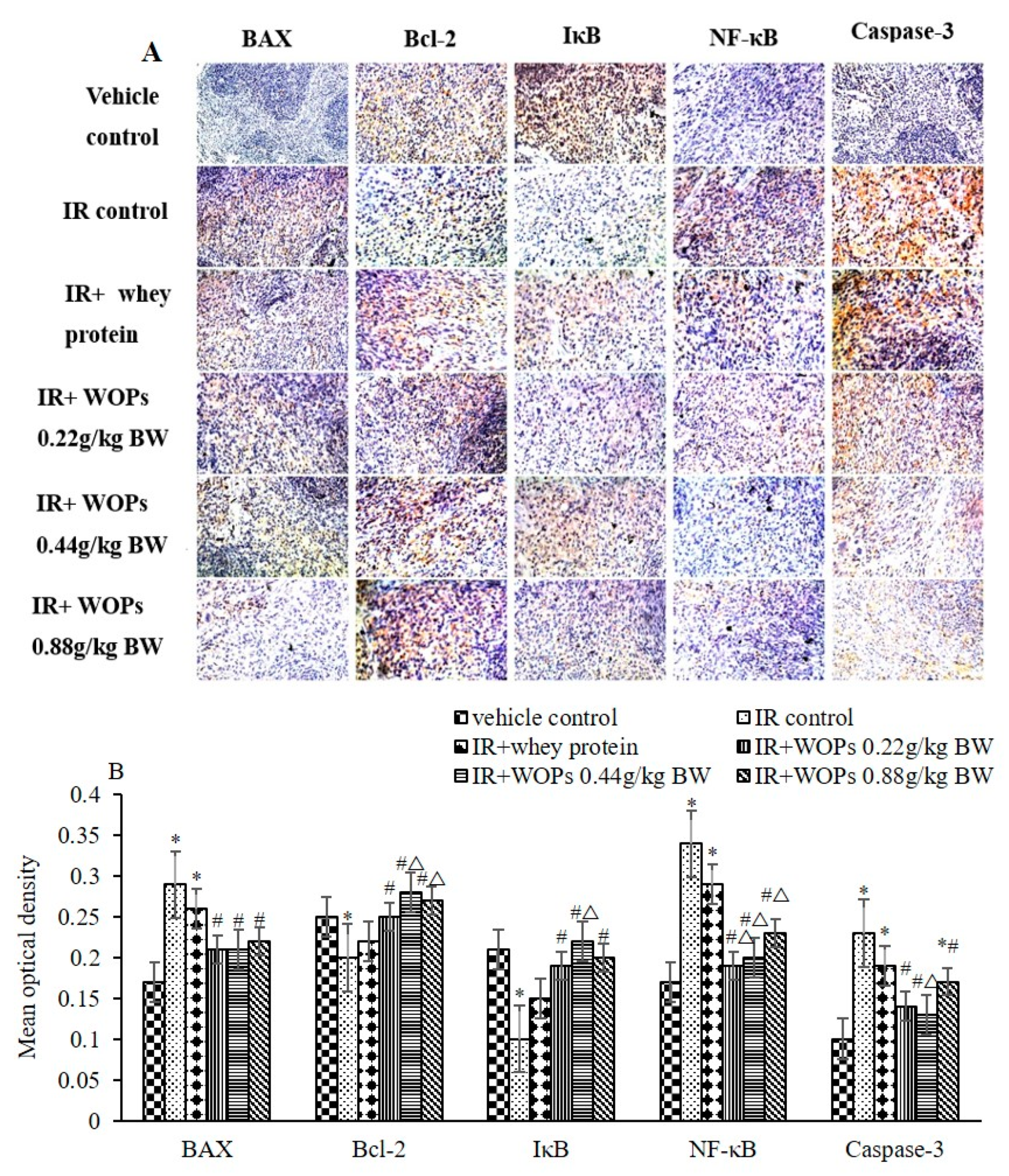
2.8. Effect of WOPs on the Expression of Spleen Apoptosis Related Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Treatments
4.3. Irradiation Exposure
4.4. Survival Assays
4.5. White Blood Cells Count and DNA Concentration in Bone Marrow Cells
4.6. Histopathology Analysis and Crypt and Villus Morphometry
4.7. Enzyme-linked Immunosorbent Assay
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sundaramoorthy, P.; Wang, Q.; Zheng, Z.; Jiao, Y.; Chen, B.J.; Doan, P.L.; Chao, N.J.; Kang, Y. Thioredoxin mitigates radiation-induced hematopoietic stem cell injury in mice. Stem Cell Res. Ther. 2017, 8, 263. [Google Scholar] [CrossRef]
- Bjerknes, M.; Cheng, H. Gastrointestinal stem cells. II. Intestinal stem cells. Am. J. Physiol. Gastrointest Liver Physiol. 2005, 289, G381–G387. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, S.; van den Brink, M.R.; Haas, T.; Poeck, H. The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front. Immunol. 2014, 5, 337. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- He, L.X.; Wang, J.B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.Q.; Ren, J.; Liu, R.; et al. Suppression of TNF-alpha and free radicals reduces systematic inflammatory and metabolic disorders: Radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Turan, R.; Yagmurdur, H.; Kavutcu, M.; Dikmen, B. Propofol and tourniquet induced ischaemia reperfusion injury in lower extremity operations. Eur. J. Anaesthesiol. 2007, 24, 185–189. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Raja, R.B. Arunachalam KD: Anti-genotoxic potential of casein phosphopeptides (CPPs): A class of fermented milk peptides against low background radiation and prevention of cancer in radiation workers. Toxicol. Ind. Health 2011, 27, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Kolivand, S.; Motevaseli, E.; Cheki, M.; Mahmoudzadeh, A.; Shirazi, A.; Fait, V. The Anti-apoptotic Mechanism of Metformin Against Apoptosis Induced by Ionizing Radiation in Human Peripheral Blood Mononuclear Cells. Klin. Onkol. 2017, 30, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, M.; Toledo, S.M.D.; Pain, D.; Azzam, E.I. Long-Term Consequences of Radiation-Induced Bystander Effects Depend on Radiation Quality and Dose and Correlate with Oxidative Stress. Radiat. Res. 2011, 175, 405. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Sharma, P. Protracted Oxidative Alterations in the Mechanism of Hematopoietic Acute Radiation Syndrome. Antioxidants (Basel) 2015, 4, 134–152. [Google Scholar] [CrossRef]
- Rezaeyan, A.; Fardid, R.; Haddadi, G.H.; Takhshid, M.A.; Hosseinzadeh, M.; Najafi, M.; Salajegheh, A. Evaluating Radioprotective Effect of Hesperidin on Acute Radiation Damage in the Lung Tissue of Rats. J. Biomed. Phys. Eng. 2016, 6, 165–174. [Google Scholar]
- Shaban, N.Z.; Ahmed Zahran, A.M.; El-Rashidy, F.H.; Abdo Kodous, A.S. Protective role of hesperidin against gamma-radiation-induced oxidative stress and apoptosis in rat testis. J. Biol. Res. 2017, 24, 5. [Google Scholar]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Radioprotective potential of melatonin against 60Co γ-ray-induced testicular injury in male C57BL/6 mice. J. Biomed. Sci. 2015, 22, 61. [Google Scholar] [CrossRef]
- Yousr, M.; Howell, N. Antioxidant and ACE Inhibitory Bioactive Peptides Purified from Egg Yolk Proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Isono, H.; Miyata, T. Potential antioxidant bioactive peptides from camel milk proteins. Anim. Nutr. 2018, 4, 273–280. [Google Scholar] [CrossRef]
- Li, W.; Zhao, T.; Zhang, J.; Xu, J.; Sunwaterhouse, D.; Zhao, M.; Su, G. Effect of walnut protein hydrolysate on scopolamine-induced learning and memory deficits in mice. J. Food Sci. Technol. 2017, 54, 1–9. [Google Scholar] [CrossRef]
- Zou, J.; Cai, P.S.; Xiong, C.M.; Ruan, J.L. Neuroprotective effect of peptides extracted from walnut (Juglans Sigilata Dode) proteins on Abeta25-35-induced memory impairment in mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Liu., R.; Wu, L.; Du, Q.; Ren, J.W.; Chen, Q.H.; Li, D.; Mao, R.X.; Liu, X.R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef]
- Kim, J.H.; Thimmulappa, R.K.; Kumar, V.; Cui, W.; Kumar, S.; Kombairaju, P.; Zhang, H.; Margolick, J.; Matsui, W.; Macvittie, T.; et al. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J. Clin. Invest. 2014, 124, 730–741. [Google Scholar] [CrossRef]
- Bing, S.J.; Kim, M.J.; Park, E.; Ahn, G.; Kim, D.S.; Kyeong, R.; Lee, N.H.; Shin, T.; Park, J.W.; Jee, Y. 1,2,3,4,6-Penta-0-galloyl-fi-o-glucose Protects Splenocytes against Radiation-Induced Apoptosis in Murine Splenocytes. Biol. Pharm. Bull. 2010, 33, 1122–1127. [Google Scholar] [CrossRef]
- Dainiak, N.; Waselenko, J.K.; Armitage, J.O.; Macvittie, T.J.; Farese, A.M. The hematologist and radiation casualties. Hematol. Am. Soc. Hematol. Educ. Program. 2003, 2003, 473–496. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Liu, W.; Xuan, Z.; Wu, S.; Yu, S.; Mei, K.; Huang, Y.; Zhang, P.; Cai, J. Protective effect of hydrogen-rich saline against radiation-induced immune dysfunction. J. Cell Mol. Med. 2014, 18, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Augustin, O.; Rivero-Gutierrez, B.; Mascaraque, C.; Sanchez de Medina, F. Food derived bioactive peptides and intestinal barrier function. Int. J. Mol. Sci. 2014, 15, 22857–22873. [Google Scholar] [CrossRef] [PubMed]
- Iademarco, M.F.; Mcquillan, J.J.; Rosen, G.D.; Dean, D.C. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J. Biol. Chem. 1992, 267, 16323–16329. [Google Scholar] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Hellweg, C.E. The Nuclear Factor κB pathway: A link to the immune system in the radiation response. Cancer Lett. 2015, 368, 275–289. [Google Scholar] [CrossRef]
- Medina, D.F.S.; Daddaoua, A.; Requena, P.; CapitánCañadas, F.; Zarzuelo, A.; Martínez-Augustin, O. New insights into the immunological effects of food bioactive peptides in animal models of intestinal inflammation. Proc. Nutr. Soc. 2010, 69, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Turbay, M.B.E.; Leblanc, A.D.M.D.; Perdigón, G.; Giori, G.S.D.; Hebert, E.M. β-Casein hydrolysate generated by the cell envelope-associated proteinase of Lactobacillus delbrueckii ssp. lactis CRL 581 protects against trinitrobenzene sulfonic acid-induced colitis in mice. J. Dairy Sci. 2012, 95, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.C.; Bscheider, M.; Eisenkolb, G.; Lin, C.C.; Wintges, A.; Otten, V.; Lindemans, C.A.; Heidegger, S.; Rudelius, M.; Monette, S.; et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med. 2017, 9, eaag2513. [Google Scholar] [CrossRef]
- Lu, X.; Nurmemet, D.; Bolduc, D.L.; Elliott, T.B.; Kiang, J.G. Radioprotective effects of oral 17-dimethylaminoethylamino-17-demethoxygeldanamycin in mice: Bone marrow and small intestine. Cell Biosci. 2013, 3, 36. [Google Scholar] [CrossRef][Green Version]
- Bie, T.; Yin, Y.L.; Kong, X.F.; Li, P.; Li, X.L.; Gao, H.J.; Li, X.G.; Huang, R.L.; Wu, G.Y. L-arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 2010, 38, 1227–1235. [Google Scholar]
- Yin, J.; Ren, W.; Duan, J.; Wu, L.; Chen, S.; Li, T.; Yin, Y.; Wu, G. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 2014, 46, 883. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef]
- Tan, B.; Xiao, H.; Xiong, X.; Wang, J.; Li, G.; Yin, Y.; Huang, B.; Hou, Y.; Wu, G. L-arginine improves DNA synthesis in LPS-challenged enterocytes. Front Biosci. 2015, 20, 989. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Jiang, Z.; Zheng, C.; Zhou, G.; Yu, D.; Cao, T.; Wang, J.; Chen, F. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 2010, 38, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Bounous, G.; Kongshavn, P.A. The effect of dietary amino acids on immune reactivity. Immunology 1978, 35, 257. [Google Scholar] [PubMed]
- Li, W.; Feng, L.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of dietary phenylalanine on growth, digestive and brush border enzyme activities and antioxidant capacity in the hepatopancreas and intestine of young grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 2015, 21, 913–925. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Tang, L.; Wu, P.; Tang, W.N.; Zhang, Y.A. Dietary phenylalanine-improved intestinal barrier health in young grass carp (Ctenopharyngodon idella) is associated with increased immune status and regulated gene expression of cytokines, tight junction proteins, antioxidant enzymes and related signall. Fish Shellfish Immunol. 2015, 45, 495–509. [Google Scholar] [CrossRef]
- Mohagheghpour, N.; Waleh, N.; Garger, S.J.; Dousman, L.; Grill, L.K.; Tusé, D. Synthetic Melanin Suppresses Production of Proinflammatory Cytokines. Cell. Immunol. 2000, 199, 25–36. [Google Scholar] [CrossRef]
- Borlongan, I.G. Dietary requirement of milkfish ( Chanos chanos Forsskal) juveniles for total aromatic amino acids. Aquaculture 1992, 102, 309–317. [Google Scholar] [CrossRef]
- Xia, C.; Fukushi, K.; Moeller, L.C.; Samuel, R.; Hisao, S. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol. Endocrinol. 2005, 19, 102–112. [Google Scholar]
- Weichhart, T.; Costantino, G.; Poglitsch, M.; Rosner, M.; Zeyda, M.; Stuhlmeier, K.M.; Kolbe, T.; Stulnig, T.M.; Hörl, W.H.; Hengstschläger, M. The TSC-mTOR Signaling Pathway Regulates the Innate Inflammatory Response. Immunity 2008, 29, 565–577. [Google Scholar] [CrossRef]
- Proietti, E.; Greco, G.; Garrone, B.; Baccarini, S.; Mauri, C.; Venditti, M.; Carlei, D.; Belardelli, F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J. Clin. Invest. 1998, 100, 429–441. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Amino Acid | Content (g/100g) | Amino Acid | Content (g/100g) |
|---|---|---|---|
| Aspartic Acid | 0.085 | Cystine | 0.001 |
| Glutamic Acid | 0.124 | Valine | 0.069 |
| Serine | 0.112 | Methionine | 0.020 |
| Histidine | 0.016 | Phenylalanine | 0.477 |
| Glycine | 0.058 | Isoleucine | 0.041 |
| Threonine | 0.150 | Leucine | 0.279 |
| Arginine | 1.015 | Lysine | 0.053 |
| Alanine | 0.132 | Proline | 0.057 |
| Tyrosine | 0.298 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Liu, R.; He, L.-X.; Mao, R.-X.; Liu, X.-R.; Zhang, T.; Hao, Y.-T.; Fan, R.; Xu, M.-H.; Li, Y. Radioprotective Effect of Walnut Oligopeptides Against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice. Molecules 2019, 24, 1582. https://doi.org/10.3390/molecules24081582
Zhu N, Liu R, He L-X, Mao R-X, Liu X-R, Zhang T, Hao Y-T, Fan R, Xu M-H, Li Y. Radioprotective Effect of Walnut Oligopeptides Against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice. Molecules. 2019; 24(8):1582. https://doi.org/10.3390/molecules24081582
Chicago/Turabian StyleZhu, Na, Rui Liu, Li-Xia He, Rui-Xue Mao, Xin-Ran Liu, Ting Zhang, Yun-Tao Hao, Rui Fan, Mei-Hong Xu, and Yong Li. 2019. "Radioprotective Effect of Walnut Oligopeptides Against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice" Molecules 24, no. 8: 1582. https://doi.org/10.3390/molecules24081582
APA StyleZhu, N., Liu, R., He, L.-X., Mao, R.-X., Liu, X.-R., Zhang, T., Hao, Y.-T., Fan, R., Xu, M.-H., & Li, Y. (2019). Radioprotective Effect of Walnut Oligopeptides Against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice. Molecules, 24(8), 1582. https://doi.org/10.3390/molecules24081582







