Quality Evaluation of Gastrodia Elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker
Abstract
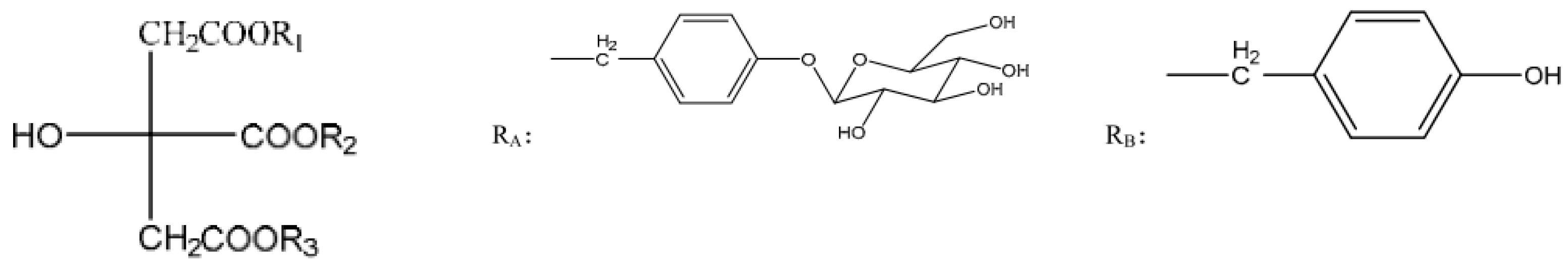
:1. Introduction
2. Results and Discussion
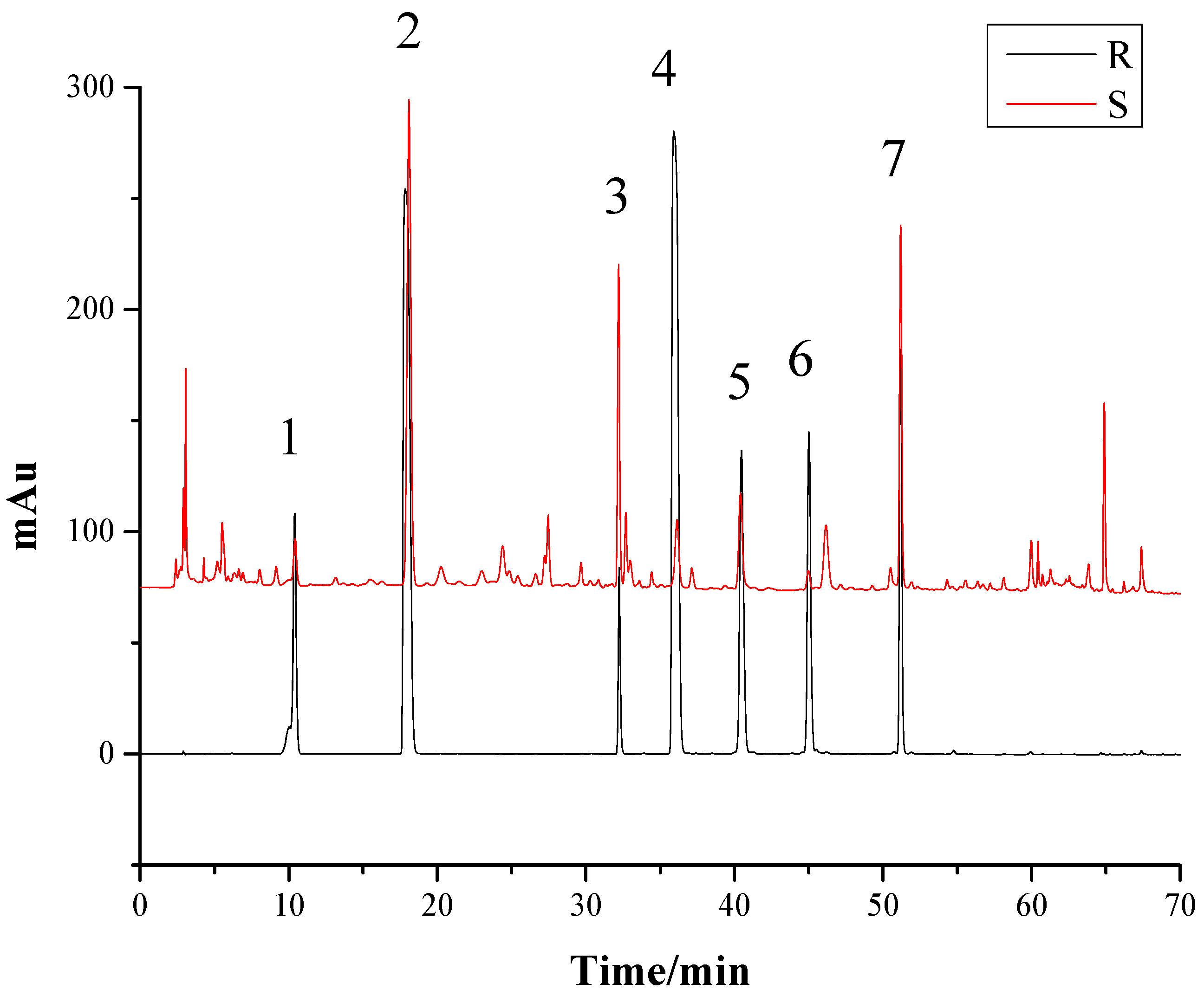
2.1. Optimization of the Chromatographic Conditions
2.2. Method Validation
2.2.1. Linearity
2.2.2. Precision, Stability, Repeatability, and Accuracy
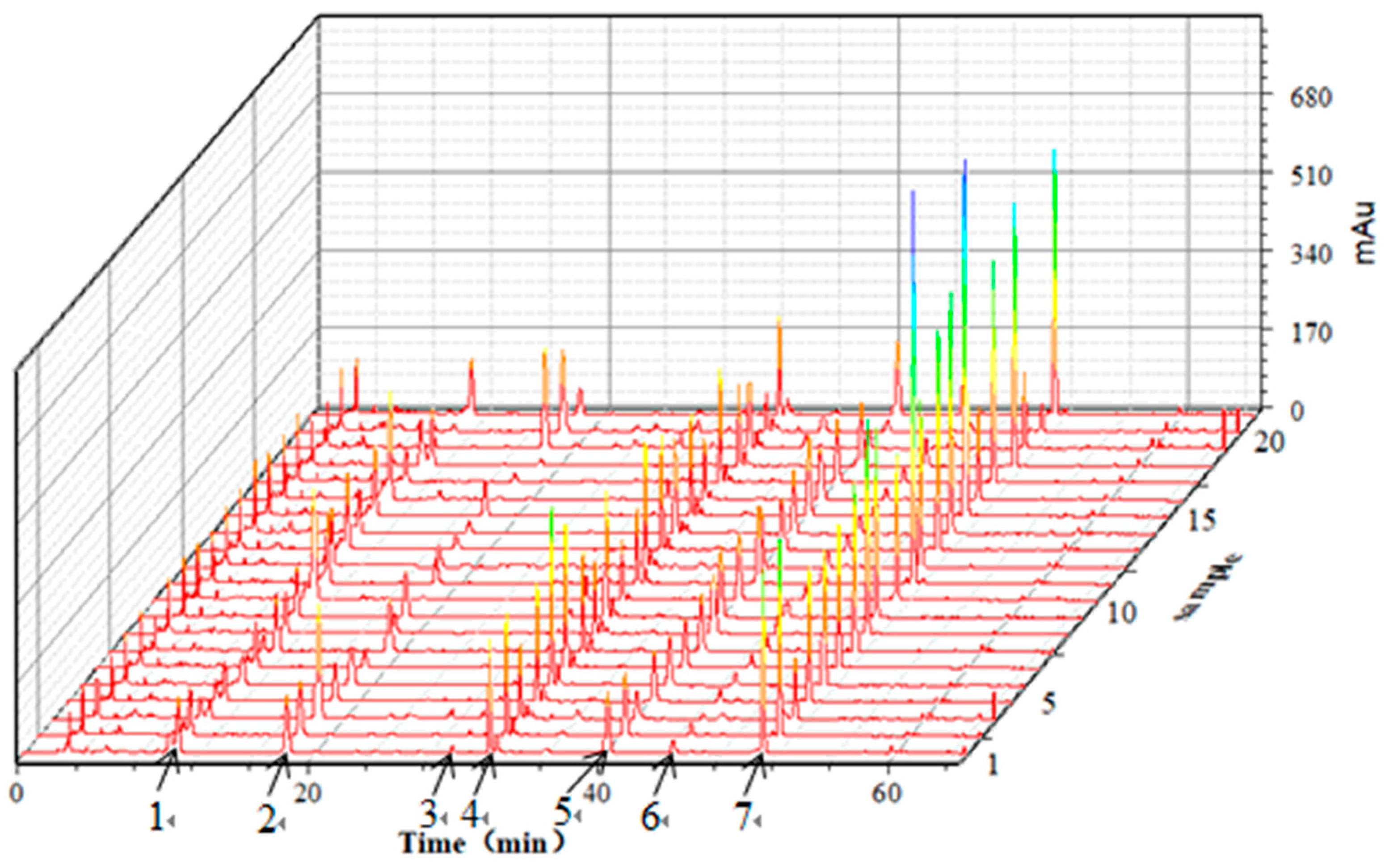
2.3. HPLC Fingerprints Analysis
2.4. Similarity Analysis
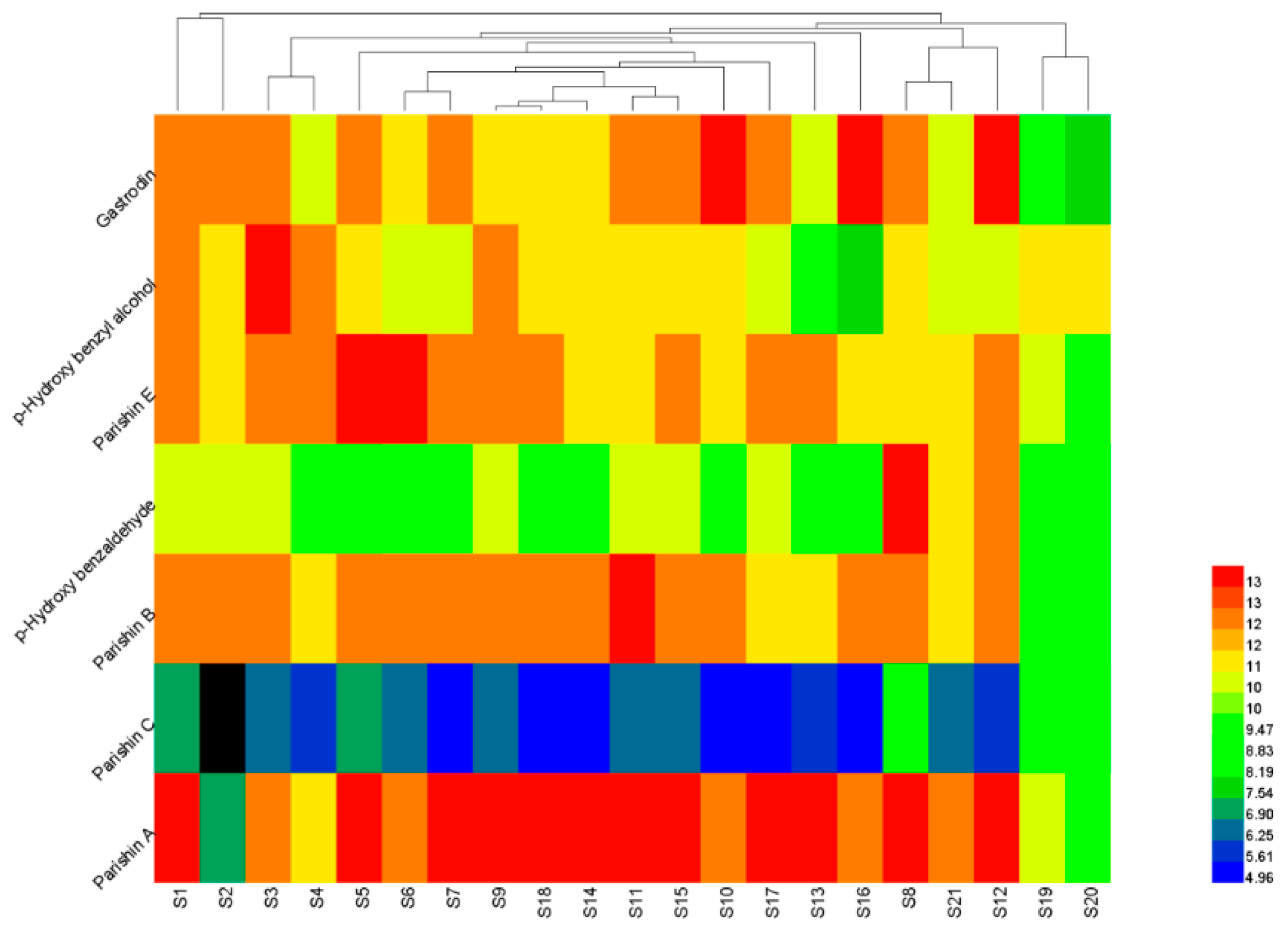
2.5. Hierarchical Cluster Analysis (HCA)
2.6. Quantitative Analysis of Multiple Components by Single Marker
2.6.1. Calculation of RCFs
2.6.2. Results from the QAMS Method
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Preparation of the Sample Solution
3.4. Reference Solution Preparation
3.5. Chromatographic Procedures
3.6. Theory of the QAMS Method
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPLC | High performance liquid chromatography |
| QAMS | Qualitative and quantitative analysis of multi-component by single marker |
| ESM | External standard method |
| RSD | Relative standard deviation |
| HCA | Hierarchical cluster analysis |
| TCM | Traditional Chinese medicine |
| RCF | Relative correction factor |
References
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume–An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Wang, Y.W.; Ouyang, H.; Lu, Y.; Qiu, Y.; Feng, Y.L.; Jiang, H.L.; Zhou, X.; Yang, S.L. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodia elata using UHPLC/Q-TOF-MS/MS. J. Chromatogr. B 2015, 988, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yan, H.; Fu, Y.; Jiang, Y.; Yao, W.; Yu, S.; Zhang, L.; Wu, Q.; Ding, A.; Shan, M. Optimal extraction study of gastrodin-type components from Gastrodia Elata tubers by response surface design with integrated phytochemical and bioactivity evaluation. Molecules 2019, 24, 547. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.C.; Woo, S.U.; Son, M.; Park, J.Y.; Choi, W.S.; Chang, K.T.; Kim, S.U.; Yoon, E.K.; Shin, S.H.; Lee, S.H. Anti-tumor activity of Gastrodia elata Blume is closely associated with a GTP-Ras-dependent pathway. Oncol. Rep. 2007, 18, 849–853. [Google Scholar] [CrossRef]
- Hu, Y.H.; Li, C.Y.; Shen, W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology 2014, 34, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Deng, X.; Wu, Q. Discrimination of gastrodia elata from different geographical origin for quality evaluation using newly-build near infrared spectrum coupled with multivariate analysis. Molecules 2018, 23, 1088. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, Z.J.; Zhou, X.; Yang, S.L. An edible medicinal plant-Gastrodia elata Bl. J. Guizhou Norm. Univ. 2013, 31, 9–12. [Google Scholar]
- Kang, C.; Lai, C.J.; Zhao, D.; Zhou, T.; Liu, D.H.; Lv, C.; Wang, S.; Kang, L.; Yang, J.; Zhan, Z.L.; et al. A practical protocol for comprehensive evaluation of sulfur-fumigation of Gastrodia Rhizoma using metabolome and health risk assessment analysis. J. Hazard. Mater. 2017, 340, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus raw olive mill waste as substrates for the production of medicinal mushrooms: An assessment of selected cultivation and quality parameters. Biomed. Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.S.; Lu, K.H.; Lin, S.H.; Liao, L.Y.; Ho, C.T.; Sheen, L.Y. Method development and validation for the high-performance liquid chromatography assay of gastrodin in water extracts from different sources of Gastrodia elata Blume. J. Food Drug Anal. 2015, 23, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Part I; Chinese Medical Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Ojemann, L.M.; Nelson, W.L.; Shin, D.S.; Rowe, A.O.; Buchanan, R. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006, 8, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sha, M.; Zhai, Q.C.; Yu, H.; Guo, L.; Wei, Y.Q.; Li, Y. Research Progress on Parishins from Gastrodia Elata. Guangdong Chem. Ind. 2016, 22, 93–95. [Google Scholar]
- FDA. Guidance for Industry-Botanical Drug Products; U. S. Food and Drug Administration: Silver Spring, MD, USA, 2004.
- SFDA. Technical Requirements for Studying Fingerprint of Traditional Chinese Medicine Injections (Draft); Drug Administration Bureau of China: Beijing, China, 2000.
- EMEA. Guidance on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products; European Medicines Agency: London, UK, 2006.
- Cui, L.L.; Zhang, Y.; Shao, W.; Gao, D. Analysis of the HPLC fingerprint and QAMS from Pyrrosia species. Ind. Crop. Prod. 2016, 85, 29–37. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Crockett, S.; Khan, I.A. The role of chemical fingerprinting: Application to Ephedra. Phytochemistry 2003, 62, 911–918. [Google Scholar] [CrossRef]
- Bauer, R. Quality criteria and phytopharmaceuticals: Can acceptable drug standards be achieved? Drug Inf. J. 1998, 31, 101–110. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Wang, D.J.; Qing, D.S.; Yao, L.C.; Li, L.Y.; Luo, W.Z. Application and advance of QAMS in quality control of traditional Chinese medicines. Chin. Tradit. Pat. Med. 2016, 38, 395–402. [Google Scholar]
- Li, D.W.; Zhu, M.; Shao, Y.D.; Shen, Z.; Weng, C.C.; Yan, W.D. Determination and quality evaluation of green tea extracts through qualitative and quantitative analysis of multi-components by single marker (QAMS). Food Chem. 2016, 197, 1112–1120. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Zhu, J.; Yan, L.; Zhang, Q.; Gong, M.; Wang, W. Assay of evodin, evodiamine and rutaecarpine in Fructus Evodiae by QAMS. Zhongguo Zhong Yao Za Zhi 2009, 34, 2781–2785. [Google Scholar] [PubMed]
- Lei, Y.C. Authenticity Identification and Quality Assessment of Gastrodia tuber (Tianma) Based on Chemical Characteristics; Chengdu University of TCM: Chengdu, China, 2015; p. 5. [Google Scholar]
- Gao, X.Y.; Jiang, Y.; Lu, J.Q.; Tu, P.F. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J. Chromatogr. A 2009, 1216, 2118–2123. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, M.H.; Zou, J.; Liu, Z.H. Analysis of the HPLC Fingerprint and QAMS for Sanhuang Gypsum Sou. J. Anal. Methods Chem. 2018. [Google Scholar] [CrossRef]
- Wang, N.; Li, Z.Y.; Zheng, X.L.; Li, Q.; Yang, X.; Xu, H. Quality Assessment of Kumu Injection, a Traditional Chinese Medicine Preparation, Using HPLC Combined with Chemometric Methods and Qualitative and Quantitative Analysis of Multiple Alkaloids by Single Marker. Molecules 2018, 23, 856. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Liu, Y.; Tian, J.; Li, C.H.; Yang, S.Y. Study on quality control of Houttuynia Cordata, a tradtional Chinese medicine by fingerprint combined with quantitative analysis of multi-components by single marker. China J. Chin. Mater. Med. 2013, 38, 2682–2689. [Google Scholar]
- Ding, L.Y.; Zhou, L.; Wang, L.N.; Guo, Y.H.; Wang, H. Multi-components quantitation by one marker for simultaneous content determination of four components in Psoralea corylifolia. Chin. J. Exp. Tradit. Med Formulae 2013, 19, 152–154. [Google Scholar]
- Dou, Z.H.; Qiao, J.; Bian, L.; Hou, J.Y.; Mao, C.F.; Chen, Z.X.; Shi, Z. Combinational quality control method of Rhei Radix et Rhizoma based on fingerprint and QAMS. J. Chin. Pharm. Sci. 2015, 50, 442–448. [Google Scholar]
- Yan, D.M.; Chang, Y.X.; Kang, L.Y.; Gao, X.M. Quality evaluation and regional analysis of Psoraleae Fructus by HPLC-DAD-MS/MS plus chemometrics. Chin. Herb. Med. 2010, 2, 216–223. [Google Scholar]
- Chen, W.H.; Luo, D. Research Development on Pharmacological Action in Gastrodia elata blume and Gastrodia Elata Polysaccharide. China J. Drug Eval. 2013, 30, 132–141. [Google Scholar]
- Lu, G.W.; Zou, Y.J.; Mo, Q.Z. Kinetic aspects of absorption, distribution, metabolism and excretion of ~3H-gastrodin in rats. Yao Xue Xue Bao 1985, 20, 167–172. [Google Scholar] [PubMed]
- Choi, S.R.; Jang, I.; Kim, C.S.; You, D.H.; Kim, J.Y.; Kim, Y.G.; Ahn, Y.S.; Kim, J.M.; Kim, Y.S.; Seo, K.W. Changes of components and quality in gastrodiae rhizoma by different dry methods. Korean J. Med. Crop Sci. 2011, 19, 354–361. [Google Scholar] [CrossRef]
- KPC Pharmaceuticals, Inc. The Invention Relates to Gastrodin Compound, Preparation Method, Preparation and Application. CN107056853A, 2017. [Google Scholar]
- Wang, W.Y. Study on Solubility of p-Hydroxybenzaldehyde, m-Hydroxybenzaldehyde and Their Mixture in Supercritical Carbon Dioxide; Beijing University of Chemical Technology: Beijing, China, 2014. [Google Scholar]
- Wang, W.J. Study on the Aerobic Oxidation of o/p-Cresol to o/p-Hydroxybenzaldehyde Catalyzed by Metallopoprhyrinns; Beijing University of Chemical Technology: Beijing, China, 2013. [Google Scholar]
- Hou, J.J.; Wu, W.Y.; Da, J.; Yao, S.; Long, H.L.; Yang, Z.; Cai, L.Y.; Yang, M.; Liu, X.; Jiang, B.H.; et al. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J. Chromatogr. A 2011, 1218, 5618–5627. [Google Scholar] [CrossRef]
- Huang, J.; Yin, L.; Dong, L.; Quan, H.F.; Chen, R.; Hua, S.Y.; Ma, J.H.; Guo, D.Y.; Fu, X.Y. Quality evaluation for Radix Astragali based on fingerprint, indicative components selection and QAMS. Biomed. Chromatogr. 2018, 32, e4343. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

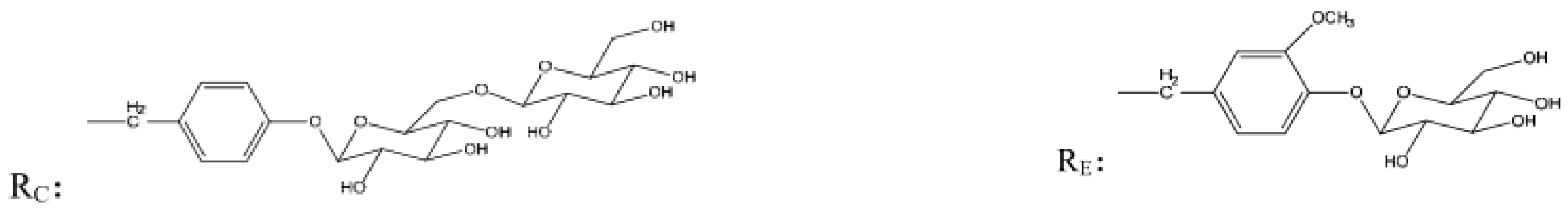
| Analytes | Regression Equations | Linear Ranges (mg/mL) | R2 | LOD (mg/mL) | LOQ (mg/mL) |
|---|---|---|---|---|---|
| Gastrodin | Y = 18634X − 264.07 | 1.906~6.483 | 0.9997 | 0.042 | 0.139 |
| p-Hydroxybenzyl alcohol | Y = 39300X + 42.955 | 0.075~1.773 | 0.9995 | 0.001 | 0.003 |
| Parishin E | Y = 14141X + 142.93 | 2.273~7.052 | 0.9997 | 0.037 | 0.122 |
| p-Hydroxy benzaldehyde | Y = 52536X + 7.9174 | 0.079~2.588 | 1.0000 | 0.001 | 0.005 |
| Parishin B | Y = 20791X + 6.7746 | 1.450~5.190 | 1.0000 | 0.004 | 0.015 |
| Parishin C | Y = 31240X − 335.24 | 0.286~0.356 | 0.9997 | 0.005 | 0.015 |
| Parishin A | Y = 11769X − 100.83 | 0.181~19.301 | 0.9995 | 0.020 | 0.070 |
| Analyte | Precision | Stability | Repeatability | Accuracy | |
|---|---|---|---|---|---|
| RSD (%) | RSD (%) | RSD (%) | RSD (%) | Mean (%) | RSD (%) |
| Gastrodin | 1.93 | 1.15 | 1.25 | 92.05% | 2.02% |
| p-Hydroxybenzyl alcohol | 1.10 | 2.04 | 2.15 | 95.78% | 1.09% |
| Parishin E | 1.29 | 1.51 | 1.60 | 98.05% | 2.90% |
| p-Hydroxy benzaldehyde | 2.30 | 2.37 | 1.81 | 92.44% | 0.25% |
| Parishin B | 2.03 | 2.10 | 1.72 | 93.33% | 1.32% |
| Parishin C | 2.63 | 1.12 | 1.84 | 92.91% | 2.10% |
| Parishin A | 0.89 | 2.25 | 1.60 | 91.80% | 1.36% |
| No. | Peak Area of Seven Characteristic Peaks | ||||||
|---|---|---|---|---|---|---|---|
| Gastrodin | p-Hydroxy Benzyl Alcohol | Parishin E | p-Hydroxy Benzaldehyde | Parishin B | Parishin C | Parishin A | |
| S1 | 1797.1 | 2249.5 | 2337.8 | 217.4 | 2263.6 | 420.7 | 4340.3 |
| S2 | 1470.2 | 2144.3 | 2523.4 | 227.6 | 2526.6 | 462.2 | 4561.9 |
| S3 | 623.8 | 4536.4 | 1528.4 | 301.7 | 1017 | 280.3 | 1487.8 |
| S4 | 1325.1 | 1516.1 | 1412.1 | 116.7 | 1906.9 | 402.8 | 3150 |
| S5 | 1659.3 | 2123.3 | 1991.3 | 111.9 | 2141.7 | 383.4 | 4006.4 |
| S6 | 1161 | 1463.7 | 3734.3 | 108.1 | 1867.1 | 390.2 | 3167.6 |
| S7 | 1492.8 | 663.8 | 2991.6 | 85.8 | 1818.7 | 392.5 | 3473.5 |
| S8 | 1470.9 | 823.8 | 1573.2 | 127.1 | 2231.8 | 546.3 | 5104.3 |
| S9 | 1898 | 1876.6 | 2572.7 | 82 | 2629.1 | 595.9 | 4430.8 |
| S10 | 3816.6 | 136.2 | 1316.2 | 110.1 | 2663.3 | 441.9 | 3383.6 |
| S11 | 2353.9 | 970.8 | 1563.7 | 131.9 | 3073.4 | 789.5 | 9224.6 |
| S12 | 1794 | 830 | 2577.2 | 45.7 | 2141.8 | 101.1 | 3845.8 |
| S13 | 2344.5 | 572.4 | 2363.1 | 57.6 | 2039.1 | 499.2 | 5019.1 |
| S14 | 1369.4 | 427.8 | 1961.6 | 41.9 | 2408.9 | 622.1 | 5512.4 |
| S15 | 2177.5 | 1270.2 | 2076.6 | 56.6 | 3133.4 | 791.2 | 8184.9 |
| S16 | 3322.1 | 108.1 | 1240.9 | 73.1 | 1935.1 | 357.8 | 2127.8 |
| S17 | 1081.8 | 322.8 | 2365 | 104.7 | 2363.6 | 500.7 | 5062.6 |
| S18 | 1893.7 | 270.9 | 1719.4 | 78.3 | 2475.6 | 823 | 6072.7 |
| S19 | 380.4 | 4012.7 | 1414.3 | 617.3 | 781.5 | 136.4 | 1789.1 |
| S20 | 300.9 | 3287.7 | 878.9 | 564 | 479.5 | 102.3 | 687.1 |
| S21 | 2175.1 | 1076.8 | 2057.2 | 143.7 | 2826.4 | 94.7 | 6278.5 |
| C.V. (%) 1 | 49.7 | 85.2 | 33.3 | 96.5 | 32.2 | 50.1 | 47.9 |
| No. | Similarity | No. | Similarity | No. | Similarity | No. | Similarity |
|---|---|---|---|---|---|---|---|
| S1 | 0.983 | S7 | 0.970 | S13 | 0.988 | S19 | 0.990 |
| S2 | 0.987 | S8 | 0.988 | S14 | 0.982 | S20 | 0.988 |
| S3 | 0.983 | S9 | 0.989 | S15 | 0.979 | S21 | 0.988 |
| S4 | 0.975 | S10 | 0.982 | S16 | 0.989 | R | 1.000 |
| S5 | 0.983 | S11 | 0.987 | S17 | 0.980 | ||
| S6 | 0.975 | S12 | 0.990 | S18 | 0.964 |
| Instrument | Chromatogram Column | RCF Values | |
|---|---|---|---|
| Agilent 1260 | YMC-Tyiart C18 (250 × 4.6 mm, 5 μm) | fP-hydroxy benzyl alcohol/gastrodin | 2.1090 |
| fparishin E/gastrodin | 0.7589 | ||
| fP-hydroxy benzaldehyde/gastrodin | 2.8194 | ||
| fparishin B/gastrodin | 1.1156 | ||
| fparishin C/gastrodin | 1.6771 | ||
| fparishin A/gastrodin | 0.6316 | ||
| No. | Gastrodin | p-Hydroxy Benzyl Alcohol | Parishin E | p-Hydroxy Benzaldehyde | Parishin B | Parishin C | Parishin A | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | |||
| S1 | 5.23 ± 0.16 | 1.77 ± 0.05 | 1.82 ± 0.05 | 5.14 ± 0.01 | 5.35 ± 0.03 | 0.24 ± 0.01 | 0.25 ± 0.06 | 3.54 ± 0.14 | 3.60 ± 0.05 | 0.18 ± 0.00 | 0.19 ± 0.01 | 11.58 ± 0.45 | 11.71 ± 0.49 | 27.68 |
| S2 | 4.51 ± 0.38 | 1.61 ± 0.08 | 1.64 ± 0.06 | 2.27 ± 0.14 | 2.38 ± 0.08 | 0.24 ± 0.01 | 0.25 ± 0.01 | 3.43 ± 0.21 | 3.47 ± 0.01 | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.18 ± 0.00 | 0.00 ± 0.00 | 12.38 |
| S3 | 2.44 ± 0.28 | 1.07 ± 0.08 | 1.09 ± 0.10 | 4.82 ± 0.25 | 5.00 ± 0.04 | 0.26 ± 0.01 | 0.26 ± 0.02 | 2.84 ± 0.10 | 2.88 ± 0.04 | 0.16 ± 0.02 | 0.16 ± 0.02 | 8.33 ± 0.31 | 8.32 ± 0.11 | 19.91 |
| S4 | 1.35 ± 0.02 | 3.36 ± 0.12 | 3.39 ± 0.08 | 2.62 ± 0.06 | 2.62 ± 0.23 | 0.23 ± 0.01 | 0.24 ± 0.04 | 1.45 ± 0.04 | 1.46 ± 0.09 | 0.16 ± 0.00 | 0.16 ± 0.10 | 4.23 ± 0.12 | 4.10 ± 0.31 | 13.41 |
| S5 | 3.41 ± 0.38 | 1.55 ± 0.10 | 1.58 ± 0.07 | 2.31 ± 0.22 | 2.36 ± 0.10 | 0.12 ± 0.02 | 0.13 ± 0.01 | 2.49 ± 0.12 | 2.51 ± 0.11 | 0.20 ± 0.00 | 0.20 ± 0.01 | 8.94 ± 0.63 | 8.92 ± 0.29 | 19.03 |
| S6 | 1.91 ± 0.12 | 1.03 ± 0.07 | 1.06 ± 0.10 | 7.05 ± 0.13 | 7.26 ± 0.23 | 0.23 ± 0.02 | 0.24 ± 0.05 | 2.69 ± 0.03 | 2.73 ± 0.17 | 0.17 ± 0.00 | 0.17 ± 0.02 | 7.64 ± 0.31 | 7.63 ± 0.61 | 20.72 |
| S7 | 3.12 ± 0.01 | 0.51 ± 0.00 | 0.531 ± 0.00 | 6.02 ± 0.14 | 6.20 ± 0.09 | 0.21 ± 0.01 | 0.21 ± 0.00 | 2.71 ± 0.02 | 2.73 ± 0.01 | 0.15 ± 0.00 | 0.16 ± 0.00 | 8.87 ± 0.02 | 8.86 ± 0.08 | 21.58 |
| S8 | 3.06 ± 0.10 | 0.62 ± 0.01 | 0.64 ± 0.01 | 3.00 ± 0.18 | 3.13 ± 0.17 | 2.59 ± 0.02 | 2.60 ± 0.05 | 3.25 ± 0.15 | 3.27 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.04 | 12.77 ± 0.58 | 12.75 ± 0.54 | 25.44 |
| S9 | 2.85 ± 0.37 | 1.22 ± 0.18 | 1.24 ± 0.20 | 4.54 ± 0.03 | 4.69 ± 0.06 | 0.28 ± 0.01 | 0.28 ± 0.01 | 3.61 ± 0.06 | 3.63 ± 0.15 | 0.17 ± 0.01 | 0.16 ± 0.05 | 10.78 ± 0.15 | 10.77 ± 0.08 | 23.44 |
| S10 | 5.89 ± 0.22 | 0.10 ± 0.01 | 0.10 ± 0.01 | 3.37 ± 0.24 | 3.52 ± 0.23 | 0.11 ± 0.01 | 0.11 ± 0.06 | 3.84 ± 0.13 | 3.91 ± 0.05 | 0.15 ± 0.02 | 0.16 ± 0.00 | 7.90 ± 0.67 | 7.91 ± 0.63 | 21.36 |
| S11 | 4.74 ± 0.37 | 0.69 ± 0.08 | 0.71 ± 0.08 | 3.40 ± 0.22 | 3.55 ± 0.22 | 0.26 ± 0.02 | 0.26 ± 0.04 | 5.191 ± 0.09 | 5.23 ± 0.02 | 0.18 ± 0.01 | 0.19 ± 0.00 | 26.70 ± 0.46 | 26.93 ± 0.54 | 41.15 |
| S12 | 7.10 ± 0.27 | 0.65 ± 0.04 | 0.66 ± 0.01 | 4.88 ± 0.23 | 5.04 ± 0.11 | 0.08 ± 0.12 | 0.08 ± 0.02 | 3.03 ± 0.16 | 3.18 ± 0.01 | 0.15 ± 0.02 | 0.15 ± 0.00 | 9.43 ± 0.54 | 9.80 ± 0.10 | 25.32 |
| S13 | 4.03 ± 0.03 | 0.37 ± 0.01 | 0.39 ± 0.01 | 3.69 ± 0.11 | 3.86 ± 0.11 | 0.16 ± 0.01 | 0.17 ± 0.02 | 2.37 ± 0.06 | 2.41 ± 0.06 | 0.15 ± 0.00 | 0.15 ± 0.00 | 10.27 ± 0.24 | 10.33 ± 0.24 | 21.04 |
| S14 | 2.59 ± 0.03 | 0.19 ± 0.16 | 0.20 ± 0.00 | 2.97 ± 0.08 | 3.10 ± 0.07 | 0.20 ± 0.00 | 0.21 ± 0.02 | 2.88 ± 0.06 | 2.90 ± 0.05 | 0.15 ± 0.01 | 0.15 ± 0.00 | 11.61 ± 0.37 | 11.58 ± 0.33 | 20.59 |
| S15 | 4.29 ± 0.15 | 0.92 ± 0.04 | 0.94 ± 0.04 | 3.76 ± 0.16 | 3.90 ± 0.16 | 0.31 ± 0.02 | 0.31 ± 0.07 | 4.27 ± 0.17 | 4.30 ± 0.10 | 0.13 ± 0.00 | 0.14 ± 0.00 | 19.30 ± 0.83 | 19.42 ± 0.84 | 32.98 |
| S16 | 6.48 ± 0.21 | 0.08 ± 0.00 | 0.08 ± 0.00 | 2.35 ± 0.11 | 2.41 ± 0.13 | 0.11 ± 0.00 | 0.11 ± 0.05 | 2.75 ± 0.11 | 2.78 ± 0.04 | 0.15 ± 0.01 | 0.15 ± 0.01 | 5.24 ± 0.15 | 5.16 ± 0.20 | 17.15 |
| S17 | 5.09 ± 0.39 | 0.13 ± 0.22 | 0.13 ± 0.26 | 4.20 ± 0.05 | 4.39 ± 0.05 | 0.24 ± 0.00 | 0.25 ± 0.07 | 1.54 ± 0.17 | 1.56 ± 0.08 | 0.15 ± 0.01 | 0.14 ± 0.01 | 11.65 ± 0.22 | 11.77 ± 0.23 | 23.01 |
| S18 | 3.71 ± 0.05 | 0.20 ± 0.01 | 0.20 ± 0.01 | 4.22 ± 0.09 | 4.41 ± 0.13 | 0.20 ± 0.00 | 0.20 ± 0.04 | 3.47 ± 0.08 | 3.52 ± 0.03 | 0.15 ± 0.00 | 0.16 ± 0.01 | 14.60 ± 0.21 | 14.76 ± 0.24 | 26.54 |
| S19 | 0.42 ± 0.01 | 1.23 ± 0.02 | 1.26 ± 0.02 | 1.10 ± 0.01 | 1.14 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.00 | 0.46 ± 0.01 | 0.47 ± 0.01 | 0.38 ± 0.01 | 0.39 ± 0.04 | 1.99 ± 0.06 | 1.98 ± 0.05 | 5.76 |
| S20 | 0.36 ± 0.02 | 1.01 ± 0.01 | 1.04 ± 0.01 | 0.64 ± 0.00 | 0.65 ± 0.00 | 0.12 ± 0.00 | 0.11 ± 0.00 | 0.28 ± 0.00 | 0.29 ± 0.00 | 0.30 ± 0.01 | 0.30 ± 0.03 | 0.83 ± 0.09 | 0.82 ± 0.00 | 3.52 |
| S21 | 1.50 ± 0.14 | 0.33 ± 0.00 | 0.34 ± 0.00 | 1.70 ± 0.40 | 1.71 ± 0.04 | 0.68 ± 0.02 | 0.69 ± 0.01 | 1.75 ± 0.02 | 1.78 ± 0.01 | 0.17 ± 0.00 | 0.16 ± 0.01 | 6.62 ± 0.03 | 6.61 ± 0.03 | 12.75 |
| Mean | 3.53 | 0.91 | 3.65 | 0.34 | 2.79 | 0.18 | 9.53 | 20.70 | ||||||
| No. | p-Hydroxy Benzyl Alcohol | Parishin E | p-Hydroxy Benzaldehyde | Parishin B | Parishin C | Parishin A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Error | RSD | Relative Error | RSD | Relative Error | RSD | Relative Error | RSD | Relative Error | RSD | Relative Error | RSD | |
| S1 | 2.38% | 1.70% | 4.04% | 2.92% | 2.39% | 1.71% | 1.76% | 1.25% | 1.47% | 1.05% | 1.15% | 0.82% |
| S2 | 1.78% | 1.27% | 4.56% | 3.30% | 1.72% | 1.23% | 1.10% | 0.78% | 1.55% | 1.11% | 0.00% | 0.00% |
| S3 | 2.38% | 1.70% | 3.72% | 2.68% | 1.94% | 1.39% | 1.37% | 0.97% | 1.56% | 1.11% | 0.02% | 0.10% |
| S4 | 0.72% | 0.51% | 0.10% | 0.07% | 1.11% | 0.79% | 0.60% | 0.43% | 0.94% | 0.67% | 0.18% | 2.13% |
| S5 | 1.52% | 1.08% | 2.13% | 1.52% | 2.14% | 1.53% | 0.90% | 0.64% | 1.11% | 0.79% | 0.03% | 0.18% |
| S6 | 2.50% | 1.79% | 2.95% | 2.12% | 2.01% | 1.44% | 1.38% | 0.98% | 0.66% | 0.47% | 0.01% | 0.07% |
| S7 | 3.39% | 2.43% | 2.84% | 2.03% | 1.70% | 1.21% | 0.97% | 0.69% | 3.09% | 2.22% | 0.02% | 0.12% |
| S8 | 2.38% | 1.70% | 4.27% | 3.08% | 0.32% | 0.23% | 0.37% | 0.26% | 3.81% | 2.74% | 0.02% | 0.09% |
| S9 | 1.65% | 1.17% | 3.18% | 2.29% | 1.17% | 0.83% | 0.60% | 0.43% | 0.74% | 0.52% | 0.02% | 0.10% |
| S10 | 1.29% | 0.92% | 4.24% | 3.06% | 2.81% | 2.01% | 1.59% | 1.13% | 3.05% | 2.19% | 0.21% | 0.15% |
| S11 | 2.57% | 1.84% | 4.20% | 3.03% | 1.38% | 0.98% | 0.74% | 0.53% | 4.84% | 3.51% | 0.88% | 0.62% |
| S12 | 0.77% | 0.54% | 3.22% | 2.32% | 3.59% | 2.59% | 4.72% | 3.42% | 0.31% | 0.22% | 3.81% | 2.75% |
| S13 | 4.77% | 3.45% | 4.54% | 3.29% | 2.41% | 1.73% | 1.48% | 1.05% | 0.90% | 0.64% | 0.61% | 0.43% |
| S14 | 4.79% | 3.47% | 4.31% | 3.12% | 1.15% | 0.82% | 0.39% | 0.27% | 0.66% | 0.47% | 0.27% | 0.19% |
| S15 | 1.95% | 1.39% | 3.72% | 2.68% | 1.10% | 0.78% | 0.59% | 0.42% | 4.61% | 3.34% | 0.61% | 0.44% |
| S16 | 4.71% | 3.41% | 2.10% | 1.50% | 2.69% | 1.93% | 1.15% | 0.82% | 0.39% | 0.28% | 1.44% | 1.02% |
| S17 | 0.76% | 0.54% | 4.31% | 3.12% | 2.23% | 1.60% | 1.61% | 1.15% | 3.77% | 2.72% | 0.99% | 0.70% |
| S18 | 0.35% | 0.25% | 4.31% | 3.11% | 2.23% | 1.59% | 1.43% | 1.02% | 4.95% | 3.59% | 1.14% | 0.81% |
| S19 | 2.54% | 1.82% | 3.76% | 2.71% | 2.50% | 1.79% | 2.33% | 1.67% | 0.94% | 0.67% | 0.38% | 0.27% |
| S20 | 3.25% | 2.33% | 1.43% | 1.02% | 3.59% | 2.59% | 3.34% | 2.40% | 0.07% | 0.05% | 1.13% | 0.80% |
| S21 | 2.53% | 1.81% | 0.70% | 0.50% | 1.75% | 1.24% | 1.70% | 1.21% | 2.10% | 1.50% | 0.20% | 0.14% |
| Correlation coefficient | 0.999 ** | 0.999 ** | 0.999 ** | 1.000 ** | 0.998 ** | 0.999 ** | ||||||
| p values | 0.940 | 0.802 | 0.978 | 0.923 | 0.960 | 0.986 | ||||||
| No. | Sample | Producing Areas | No. | Sample | Producing Areas |
|---|---|---|---|---|---|
| S1 | G. elata tubers | Zhaotong, Yunnan, China | S12 | G. elata tubers | Enshi, Hubei, China |
| S2 | G. elata tubers | Zhaotong, Yunnan, China | S13 | G. elata tubers | Yichang, Hubei, China |
| S3 | G. elata tubers | Zhaotong, Yunnan, China | S14 | G. elata tubers | Hanzhong, Shanxi, China |
| S4 | G. elata tubers | Zhaotong, Yunnan, China | S15 | G. elata tubers | Qinling, Shanxi, China |
| S5 | G. elata tubers | Zhaotong, Yunnan, China | S16 | G. elata tubers | Qinchuan, Sichuang, China |
| S6 | G. elata tubers | Zhaotong, Yunnan, China | S17 | G. elata tubers | Longnan, Gansu, China |
| S7 | G. elata tubers | Lijiang, Yunnan, China | S18 | G. elata tubers | Anhui, China |
| S8 | G. elata tubers | Bijie, Guizhou, China | S19 | G. elata tubers | Moju, South Korea |
| S9 | G. elata tubers | Zhengyuan, Guizhou, China | S20 | G. elata tubers | Chun chuan, South Korea |
| S10 | G. elata tubers | Qiandongnan, Guizhou, China | S21 | G. elata tuber slices | Yingyang, South Korea |
| S11 | G. elata tubers | Bijie, Guizhou, China |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; Zhang, Z.; Hu, Y.; Cui, X.; Xiong, Y. Quality Evaluation of Gastrodia Elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker. Molecules 2019, 24, 1521. https://doi.org/10.3390/molecules24081521
Li Y, Zhang Y, Zhang Z, Hu Y, Cui X, Xiong Y. Quality Evaluation of Gastrodia Elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker. Molecules. 2019; 24(8):1521. https://doi.org/10.3390/molecules24081521
Chicago/Turabian StyleLi, Yehong, Yiming Zhang, Zejun Zhang, Yupiao Hu, Xiuming Cui, and Yin Xiong. 2019. "Quality Evaluation of Gastrodia Elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker" Molecules 24, no. 8: 1521. https://doi.org/10.3390/molecules24081521
APA StyleLi, Y., Zhang, Y., Zhang, Z., Hu, Y., Cui, X., & Xiong, Y. (2019). Quality Evaluation of Gastrodia Elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker. Molecules, 24(8), 1521. https://doi.org/10.3390/molecules24081521






