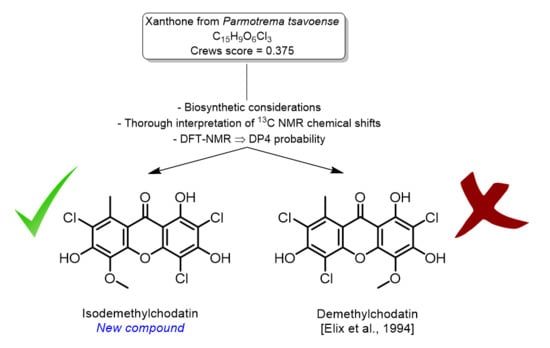
DP4-Assisted Structure Elucidation of Isodemethylchodatin, a New Norlichexanthone Derivative Meager in H-Atoms, from the Lichen Parmotrema tsavoense
Abstract
1. Introduction
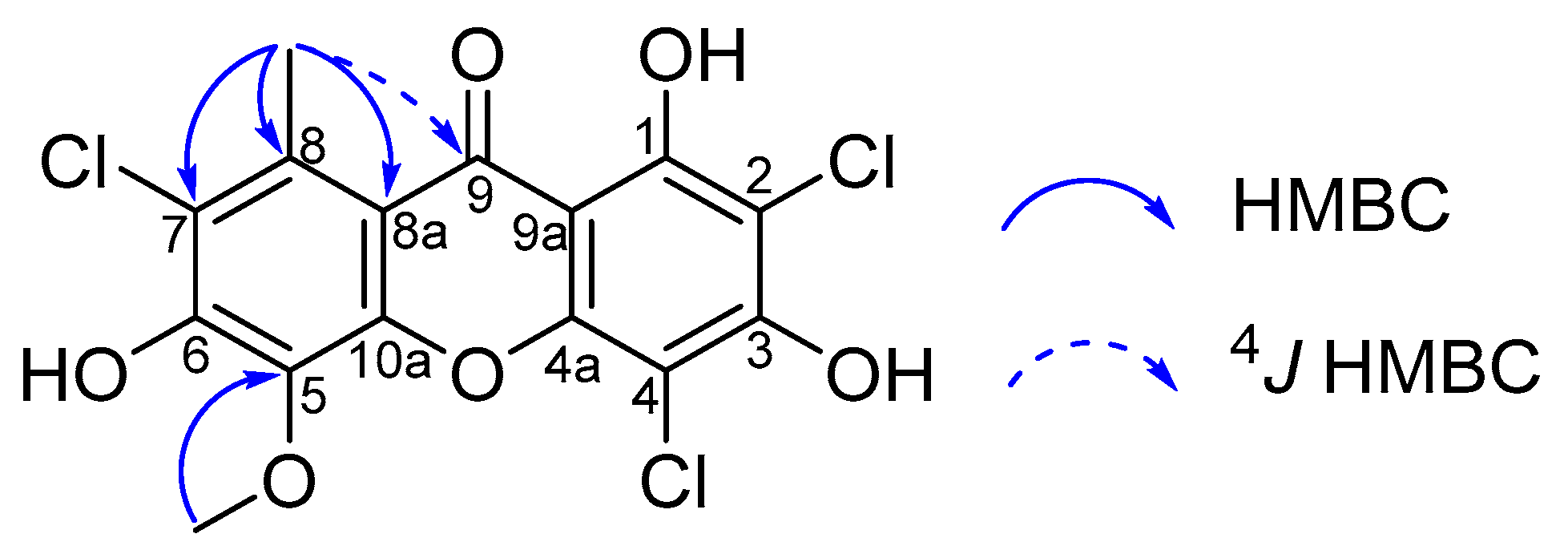
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Lichen Material
3.3. Extraction and Isolation
3.4. Computational Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lesch, B.; Bräse, S. A Short, Atom-Economical Entry to Tetrahydroxanthenones. Angew. Chem. Int. Ed. 2004, 43, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Le Pogam, P.; Boustie, J. Xanthones of lichen source: A 2016 update. Molecules 2016, 21, 294. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: Berlin, Germany, 1996. [Google Scholar]
- Sundholm, E.G. Total syntheses of lichen xanthones: Revision of structures. Tetrahedron 1978, 34, 577–586. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Feige, G.B.; Elix, J.A. The Joint Occurrence of Chloroxanthones in Southern Hemisphere Lecanora Species (Ascomycotina; Lecanoraceae). Bot. Acta 1994, 107, 30–35. [Google Scholar] [CrossRef]
- Ren, F.-C.; Wang, L.-X.; Yu, Q.; Jiang, X.-J.; Wang, F. Lanostane-type triterpenoids from Scilla scilloides and structure revision of drimiopsin D. Nat. Prod. Bioprospecting 2015, 5, 263–270. [Google Scholar] [CrossRef][Green Version]
- Leuckert, C.; Knoph, J.-G. European taxa of saxicolous Lecidella containing chloroxanthones: Identification of patterns using thin layer chromatography. Lichenologist 1992, 24, 383–397. [Google Scholar] [CrossRef]
- Yoshimura, I.; Kinoshita, Y.; Yamamoto, Y.; Huneck, S.; Yamada, Y. Analysis of secondary metabolites from lichen by high performance liquid chromatography with a photodiode array detector. Phytochem. Anal. 1994, 5, 197–205. [Google Scholar] [CrossRef]
- Elix, J.A.; Musidlak, H.W.; Sala, T.; Sargent, M.V. Structure and synthesis of some lichen xanthones. Aust. J. Chem. 1978, 31, 145–155. [Google Scholar] [CrossRef]
- Sundholm, E.G. Syntheses and 13C NMR spectra of some 5-chloro-substituted lichen xanthones. Acta Chem. Scand. B 1979, 33, 475–482. [Google Scholar] [CrossRef]
- Elix, J.A.; Crook, C.E.; Hui, J.; Zhu, Z.N. Synthesis of new lichen xanthones. Aust. J. Chem. 1992, 45, 845–855. [Google Scholar] [CrossRef]
- Elyashberg, M. Identification and structure elucidation by NMR spectroscopy. TrAC Trends Anal. Chem. 2015, 69, 88–97. [Google Scholar] [CrossRef]
- Molinski, T.F.; Morinaka, B.I. Integrated approaches to the configurational assignment of marine natural products. Tetrahedron 2012, 68, 9307–9343. [Google Scholar] [CrossRef]
- White, K.N.; Amagata, T.; Oliver, A.G.; Tenney, K.; Wenzel, P.J.; Crews, P. Structure Revision of Spiroleucettadine, a Sponge Alkaloid with a Bicyclic Core Meager in H-Atoms. J. Org. Chem. 2008, 73, 8719–8722. [Google Scholar] [CrossRef]
- Smith, S.G.; Goodman, J.M. Assigning stereochemistry to single diastereoisomers by GIAO NMR calculation: The DP4 probability. J. Am. Chem. Soc. 2010, 132, 12946–12959. [Google Scholar] [CrossRef]
- Rodríguez, I.; Genta-Jouve, G.; Alfonso, C.; Calabro, K.; Alonso, E.; Sánchez, J.A.; Alfonso, A.; Thomas, O.P.; Botana, L.M. Gambierone, a Ladder-Shaped Polyether from the Dinoflagellate Gambierdiscus belizeanus. Org. Lett. 2015, 17, 2392–2395. [Google Scholar] [CrossRef]
- Zang, Y.; Genta-Jouve, G.; Zheng, Y.; Zhang, Q.; Chen, C.; Zhou, Q.; Wang, J.; Zhu, H.; Zhang, Y. Griseofamines A and B: Two Indole-Tetramic Acid Alkaloids with 6/5/6/5 and 6/5/7/5 Ring Systems from Penicillium Griseofulvum. Org. Lett. 2018, 20, 2046–2050. [Google Scholar] [CrossRef]
- Roulland, E.; Solanki, H.; Calabro, K.; Zubia, M.; Genta-Jouve, G.; Thomas, O.P. Stereochemical Study of Puna’auic Acid, an Allenic Fatty Acid from the Eastern Indo-Pacific Cyanobacterium Pseudanabaena sp. Org. Lett. 2018, 20, 2311–2314. [Google Scholar] [CrossRef]
- Duong, T.-H.; Chavasiri, W.; Boustie, J. New meta-depsidones and diphenyl ethers from the lichen Parmotrema tsavoense (Krog & Swinscow) Krog & Swinscow, Parmeliaceae. Tetrahedron 2015, 71, 9684–9691. [Google Scholar]
- Duong, T.H.; Huynh, B.L.C.; Chavasiri, W.; Chollet-Krugler, M.; Nguyen, T.H.T.; Hansen, P.E.; Le Pogam, P.; Thüs, H.; Boustie, J.; Nguyen, K.P.P. New erythritol derivatives from the fertile form of Roccella montagnei. Phytochemistry 2017, 137, 156–164. [Google Scholar] [CrossRef]
- Duong, T.H.; Ha, X.-P.; Chavasiri, W.; Beniddir, M.A.; Genta-Jouve, G.; Boustie, J.; Chollet-Krugler, M.; Ferron, S.; Nguyen, H.-H.; Yamin, B.M.; et al. Sanctis A-C: Three Racemic Procyanidin Analogues from the Lichen Parmotrema sancti-angelii. Eur. J. Org. Chem. 2018, 2247–2253. [Google Scholar] [CrossRef]
- Duong, T.-H.; Beniddir, M.; Genta-Jouve, G.; Aree, T.; Chollet-Krugler, M.; Boustie, J.; Ferron, S.; Sauvager, A.; Nguyen, H.-H.; Nguyen, K.P.P.; et al. Tsavoenones A-C: Unprecedented Polyketides with a 1, 7-dioxadispiro [4.0. 4.4] tetradecane core from the Lichen Parmotrema tsavoense. Org. Biomol. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Elix, J.A.; Robertson, F.; Wardlaw, J.H.; Willis, A.C. Isolation and Structure Determination of Demethylchodatin—A New Lichen Xanthone. Aust. J. Chem. 1994, 47, 2291–2295. [Google Scholar] [CrossRef]
- Frahm, A.W.; Chaudhuri, R.K. 13C-NMR spectroscopy of substituted xanthones—II: 13C-NMR spectral study of polyhydroxy xanthones. Tetrahedron 1979, 35, 2035–2038. [Google Scholar] [CrossRef]
- Pomilio, A.B.; Tettamanzi, M.C.; Romanelli, G.P.; Autino, J.C.; Vitale, A.A. NMR Study of Substituted 1-Bromo-2-aryloxyethanes and Monosubstituted Xanthones. Magn. Reson. Chem. 1996, 34, 165–171. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Silva, A.M.; Cavaleiro, J.A.; Silva, F.M.; Fernanda, M.; Borges, M.; Pinto, M.M. 1H- and 13C-NMR Spectroscopy of mono-, di-, tri-and tetrasubstituted xanthones. Magn. Reson. Chem. 1998, 36, 305–309. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Koorbanally, C.; Crouch, N.R.; Sandor, P. Xanthones from Drimiopsis maculata. J. Nat. Prod. 2004, 67, 1726–1728. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.P.; Thumser, A.E.; Langat, M.K.; Crouch, N.R.; Mulholland, D.A. COX-2 inhibitory activity of homoisoflavanones and xanthones from the bulbs of the Southern African Ledebouria socialis and Ledebouria ovatifolia (Hyacinthaceae: Hyacinthoideae). Phytochemistry 2013, 95, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.-B.; Yin, H.; Zhang, X.-W.; Zhou, W.; Liu, T. Three New Xanthones from the Fungus Penicillium sp. NH-7-1. Helv. Chim. Acta 2015, 98, 699–703. [Google Scholar] [CrossRef]
- He, Q.; Yin, H.; Jiang, J.; Bai, Y.; Chen, N.; Liu, S.; Zhuang, Y.; Liu, T. Fermentative Production of Phenolic Glucosides by Escherichia coli with an Engineered Glucosyltransferase from Rhodiola sachalinensis. J. Agric. Food Chem. 2017, 65, 4691–4697. [Google Scholar] [CrossRef]
- Sundholm, E.G. 13C-NMR Spectra of Lichen Xanthones. Temperature Dependent of Collapse of Long-range Couplings to Hydrogen-bonded Hydroxyl Protons. Acta Chem. Scand. B 1978, 32, 177–181. [Google Scholar] [CrossRef]
- Stothers, J.B. Carbon-13 NMR Spectroscopy: Organic Chemistry, A Series of Monographs; Academic Press: New York, NY, USA, 1972; Volume 24, p. 197. [Google Scholar]
- Gil, S.; Palanca, P.; Sanz, V.; Tortajada, A. Synthesis of 1, 2, 3, 8-Tetraoxygenated Xanthones. J. Nat. Prod. 1990, 53, 1198–1211. [Google Scholar] [CrossRef]
- Silveira, E.R.; Falcão, M.J.; Menezes Jr, A.; Kingston, D.G.; Glass, T.E. Pentaoxygenated xanthones from Bredemeyera floribunda. Phytochemistry 1995, 39, 1433–1436. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Awale, S.; Kadota, S.; Morita, H.; Tezuka, Y. Heptaoxygenated xanthones as anti-austerity agents from Securidaca longepedunculata. Bioorg. Med. Chem. 2013, 21, 7663–7668. [Google Scholar] [CrossRef]
- Klaiklay, S.; Sukpondma, Y.; Rukachaisirikul, V.; Phongpaichit, S. Friedolanostanes and xanthones from the twigs of Garcinia hombroniana. Phytochemistry 2013, 85, 161–166. [Google Scholar] [CrossRef]
- Tanahashi, T.; Takenaka, Y.; Ikuta, Y.; Tani, K.; Nagakura, N.; Hamada, N. Xanthones from the cultured lichen mycobionts of Pyrenula japonica and Pyrenula pseudobufonia. Phytochemistry 1999, 52, 401–405. [Google Scholar] [CrossRef]
- Takenaka, Y.; Tanahashi, T.; Nagakura, N.; Hamada, N. Production of xanthones with free radical scavenging properties, emodin and sclerotiorin by the cultured lichen mycobionts of Pyrenula japonica. Z. Naturforschung C 2000, 55, 910–914. [Google Scholar] [CrossRef]
- Řezanka, T.; Jáchymová, J.; Dembitsky, V.M. Prenylated xanthone glucosides from Ural’s lichen Umbilicaria proboscidea. Phytochemistry 2003, 62, 607–612. [Google Scholar] [CrossRef]
- Řezanka, T.; Dembitsky, V.M. Identification of acylated xanthone glycosides by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry in positive and negative modes from the lichen Umbilicaria proboscidea. J. Chromatogr. A 2003, 995, 109–118. [Google Scholar] [CrossRef]
- Dieu, A.; Millot, M.; Champavier, Y.; Mambu, L.; Chaleix, V.; Sol, V.; Gloaguen, V. Uncommon chlorinated xanthone and other antibacterial compounds from the lichen Cladonia incrassata. Planta Med. 2014, 80, 931–935. [Google Scholar] [CrossRef]
- Gómez-Serranillos, M.P.; Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A. Parmeliaceae family: Phytochemistry, pharmacological potential and phylogenetic features. RSC Adv. 2014, 4, 59017–59047. [Google Scholar] [CrossRef]
- Eliasaro, S.; Adler, M.T. Two new species and new reports in the Parmeliaceae sensu stricto (Lichenized Ascomycotina) from Brazil. Mycotaxon 1997, 63, 49–56. [Google Scholar]
- Micheletti, A.C.; Beatriz, A.; de Lima, D.P.; Honda, N.K.; Pessoa, C.d.Ó.; de Moraes, M.O.; Lotufo, L.V.; Magalhães, H.I.F.; Carvalho, N.C.P. Constituintes quimicos de Parmotrema lichexanthonicum Eliasaro & Adler-Isolamento, Modificacoes estruturais e avaliacao das atividades antibiotica e citotoxica. Quím. Nova 2009, 32, 12–20. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Becke’s three parameter hybrid method using the LYP correlation functional. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Frisch, M.J.; Trucks, H.B.; Schlegel, G.W.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism: I. A gauge-invariant LCAO method for NMR chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
Sample Availability: Samples of compound 1 are not available. |

| Position | δC | δH |
|---|---|---|
| 1 | 153.7 1 | |
| 2 | 102.0 | |
| 3 | 153.7 1 | |
| 4 | 96.9 | |
| 4a | 147.4 | |
| 5 | 131.4 | |
| 6 | 154.1 1 | |
| 7 | 126.9 | |
| 8 | 135.9 | |
| 8a | 103.1 | |
| 9 | 179.6 | |
| 9a | 102.0 | |
| 10a | 153.7 1 | |
| 5-OCH3 | 60.5 | 3.76, s |
| 8-CH3 | 19.2 | 2.82, s |
| 1-OH | 13.98, br s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, T.-H.; Beniddir, M.A.; Boustie, J.; Nguyen, K.-P.-P.; Chavasiri, W.; Bernadat, G.; Le Pogam, P. DP4-Assisted Structure Elucidation of Isodemethylchodatin, a New Norlichexanthone Derivative Meager in H-Atoms, from the Lichen Parmotrema tsavoense. Molecules 2019, 24, 1527. https://doi.org/10.3390/molecules24081527
Duong T-H, Beniddir MA, Boustie J, Nguyen K-P-P, Chavasiri W, Bernadat G, Le Pogam P. DP4-Assisted Structure Elucidation of Isodemethylchodatin, a New Norlichexanthone Derivative Meager in H-Atoms, from the Lichen Parmotrema tsavoense. Molecules. 2019; 24(8):1527. https://doi.org/10.3390/molecules24081527
Chicago/Turabian StyleDuong, Thuc-Huy, Mehdi A. Beniddir, Joël Boustie, Kim-Phi-Phung Nguyen, Warinthorn Chavasiri, Guillaume Bernadat, and Pierre Le Pogam. 2019. "DP4-Assisted Structure Elucidation of Isodemethylchodatin, a New Norlichexanthone Derivative Meager in H-Atoms, from the Lichen Parmotrema tsavoense" Molecules 24, no. 8: 1527. https://doi.org/10.3390/molecules24081527
APA StyleDuong, T.-H., Beniddir, M. A., Boustie, J., Nguyen, K.-P.-P., Chavasiri, W., Bernadat, G., & Le Pogam, P. (2019). DP4-Assisted Structure Elucidation of Isodemethylchodatin, a New Norlichexanthone Derivative Meager in H-Atoms, from the Lichen Parmotrema tsavoense. Molecules, 24(8), 1527. https://doi.org/10.3390/molecules24081527