Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Analysis
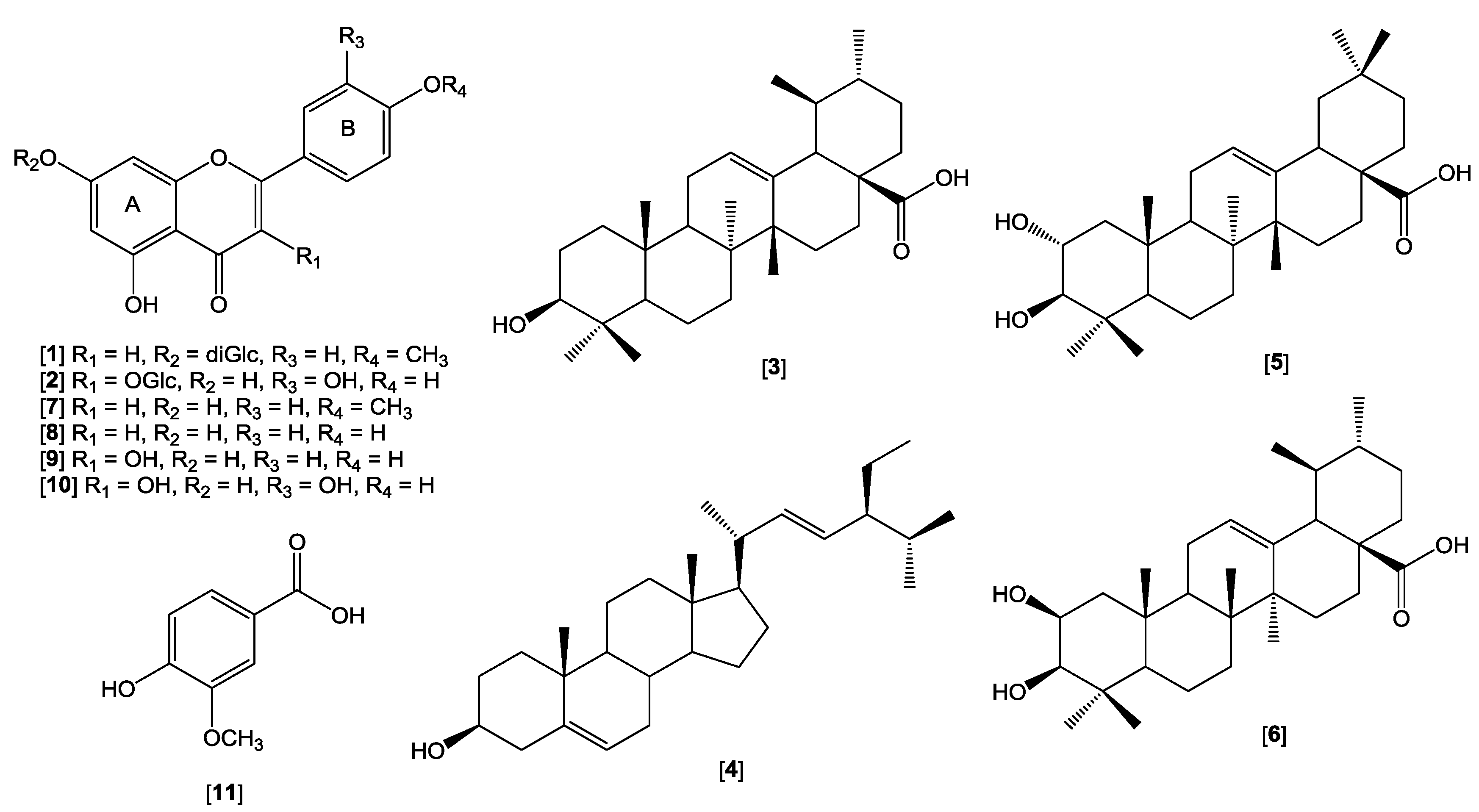
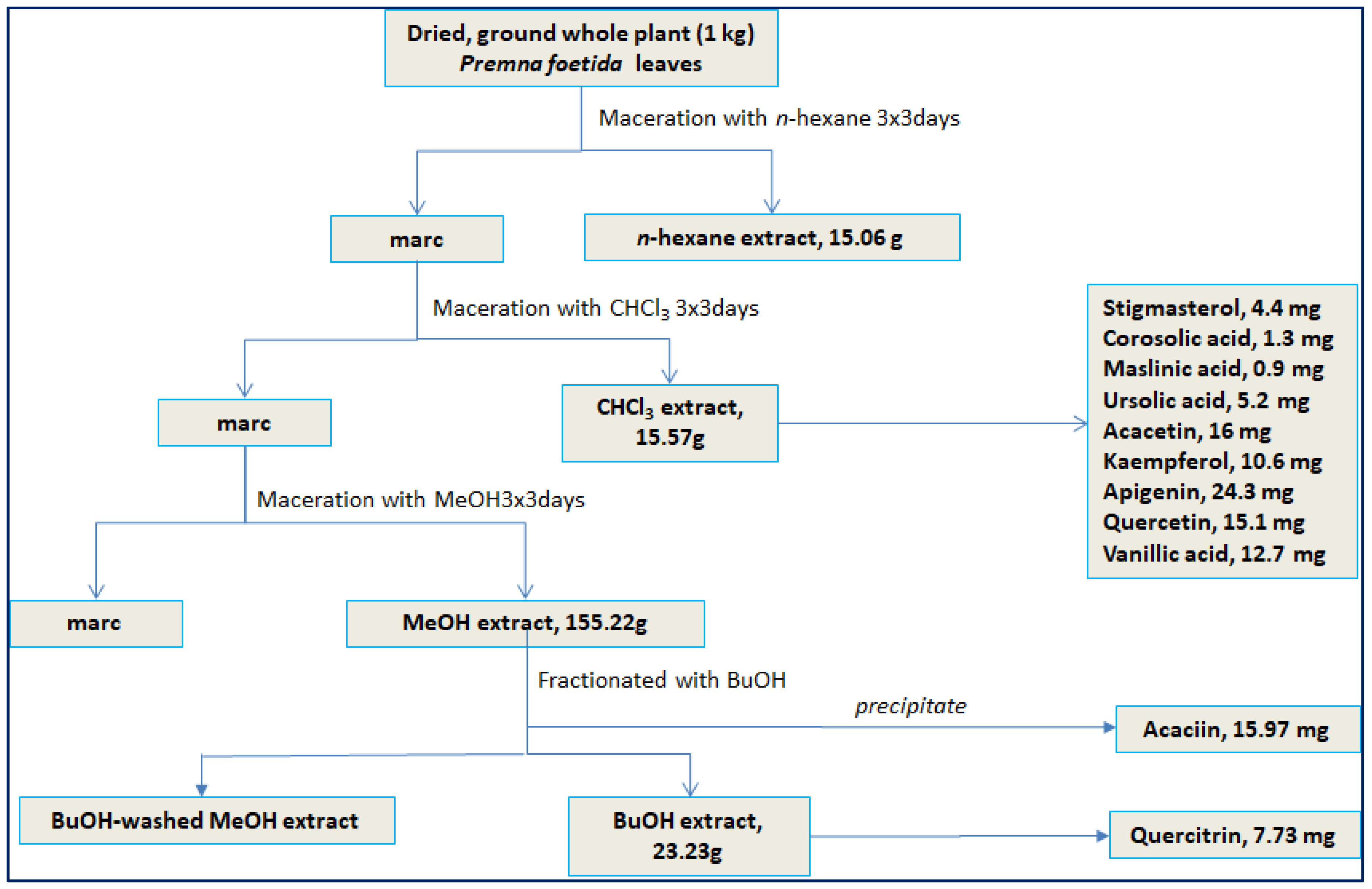
2.2. Isolation and Identification of Compounds
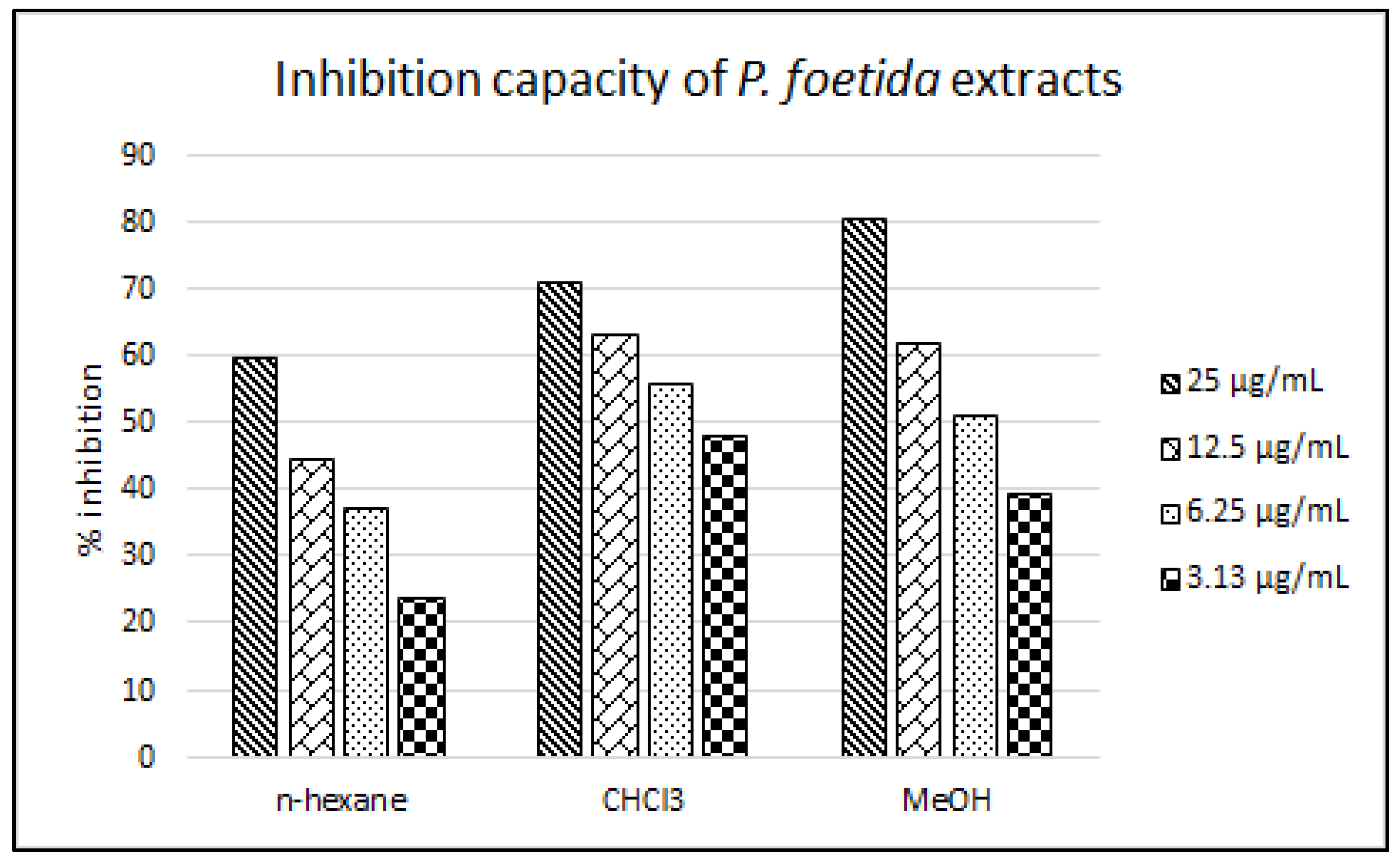
2.3. LDL Antioxidant Activity
2.4. Antiplatelet Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Collection
3.3. Plant Sample Preparation for HPLC Analysis
3.4. HPLC Instrumentation and Analysis
3.5. Isolation Procedure
3.6. Inhibition on Human Low Density Lipoprotein Oxidation Assay
3.6.1. Isolation of LDL
3.6.2. Inhibition of Copper-induced LDL Oxidation
3.6.3. MDA/TBARS Assay
3.6.4. Whole Blood Platelet Aggregation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Singh, R.B. Bioactive foods and nutraceuticals supplementation criteria in cardiovascular protection. Open Nutraceuticals J. 2010, 3, 141–153. [Google Scholar] [CrossRef][Green Version]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Lipoprotein oxidation in cardiovascular disease: Chief culprit or innocent bystander? J. Exp. Med. 2006, 203, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; ShriShriMal, N.; Das, D.K. Phytochemicals from plants to combat cardiovascular disease. Curr. Med. Chem. 2012, 19, 2242–2251. [Google Scholar] [CrossRef]
- Wiart, C. Medicinal Plants of Southeast Asia; Pelanduk Publication Sdn Bhd: Kelana Jaya, Malaysia, 2000; pp. 37–38. [Google Scholar]
- Perry, L.M.; Metzger, J. Medicinal Plants of East and Southeast Asia. Attributed properties and uses; The MIT Press: Cambridge, CA, USA, 1980; pp. 280–282. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants, Vol. III (E-L); Taylor & Francis Group: Boca Raton, FL, USA, 2012; p. 687. [Google Scholar]
- Burkill, I. A Dictionary of the Economic Products of the Malay Peninsula, Vol. II (I-Z); Ministry of Agriculture and Co-Operatives Malaysia: Kuala Lumpur, Malaysia, 1966; pp. 1311–1839.
- McClatchey, W. The ethnopharmacopoeia of the Rotuma. J. Ethnopharmacol. 1996, 50, 147–156. [Google Scholar] [CrossRef]
- Rajendran, R.; Basha, N.S. Cardioprotective effect of ethanol extract of stem bark and stem wood of Premna serratifolia Lin. (Verbenaceae). Res. J. Pharm. Tech. 2008, 1, 487–491. [Google Scholar]
- Rajendran, R.; Suseela, L.; Meenakshi, S.R.; Saleem, B.N. Cardiac stimulant activity of bark and wood of Premna serratifolia. Bangladesh J. Pharmacol. 2008, 3, 107–113. [Google Scholar]
- Patel, N.G.; Patel, K.V.; Gandhi, T.R.; Patel, K.G.; Suhagia, B.N.; Gevariya, H.B. Evaluation of the cardioprotective effect of Premna mucronata Roxb (Verbenaceae) in the experimental model of myocardial ischemia-reperfusion injury. Int. J. Modern. Pharm. Res. 2012, 1, 20. [Google Scholar]
- Savsani, H.; Shah, H.; Patel, K.; Gandhi, T. Cardioprotective effect of flavonoids rich fraction of Premna mucronata on isoproterenol-induced myocardial infarction in Wistar rats. Int. J. Phytopharmacol. 2014, 5, 95–108. [Google Scholar]
- Garcia-Granados, A.; Martinez, A.; Moliz, J.N.; Parra, A.; Rivas, F. 2a,3b-Dihydroxyoleana-12-en-28-oic acid (maslinic acid). Molecules 1998, 3, M88. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2003, 67, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. In Vitro 2008, 22, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Ryu, S.Y.; Lee, K.R. Phytochemical constituents of Bistorta manshuriensis. Nat. Prod. Sci. 2009, 15, 234–240. [Google Scholar]
- El-Domiaty, M.M.; Wink, M.; Abdel-Aal, M.; Abou-Hashem, M.M.; Abd-Alla, R.H. Antihepatotoxic activity and chemical constituents of Buddleja asiatica Lour. Z Naturforsch C 2009, 64c, 11–19. [Google Scholar] [CrossRef]
- Martins, D.; Carrion, L.L.; Ramos, D.F.; Salomé, K.S.; da Silva, P.E.A.; Barison, A.; Nunez, V. Triterpenes and the antimycobacterial activity of Duroia macrophylla Huber (Rubiaceae). Biomed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.; Bah, M.; Ibarra-Alvarado, C.; Rivero-Cruz, J.F.; Rojas-Molina, A.; Rojas-Molina, J.I.; Cabrera-Luna, J.A. Aortic relaxant activity of Crataegus gracilior Phipps and identification of some of its chemical constituents. Molecules 2014, 19, 20962–20974. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Dianita, R.; Jantan, I. Ethnomedicinal uses, phytochemistry, and pharmacological aspects of the genus Premna: A review. Pharm. Biol. 2017, 55, 1715–1739. [Google Scholar] [CrossRef]
- Steinberg, D. Studies on the mechanism of action of probucol. Am. J. Cardiol. 1986, 57, 16H–21H. [Google Scholar] [CrossRef]
- Kuzuya, M.; Kuzuya, F. Probucol as an antioxidant and antiatherogenic drug. Free Radic Biol. Med. 1993, 14, 67–77. [Google Scholar] [CrossRef]
- Franceschini, G.; Sirtori, M.; Vaccariano, V.; Gianfranceschi, G.; Rezzonico, L.; Chiesa, G.; Sirtori, C.R. Mechanism of HDL reduction after probucol. Arteriosclerosis 1989, 9, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.A.; Tangirala, R.K.; Fruebis, J.; Steinberg, D.; Witztum, J.L.; Palinski, W. Effect of probucol on LDL oxidation and atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 1998, 39, 1079–1090. [Google Scholar]
- Roland, A.; Patterson, R.A.; Leake, D.S. Measurement of copper-binding sites on low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 594–602. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S. Low-density lipoprotein oxidation, antioxidants, and atherosclerosis: A clinical biochemistry perspective. Clin. Chem. 1996, 42, 498–506. [Google Scholar]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food. Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef]
- Vaya, J.; Mahmood, S.; Goldblum, A.; Aviram, M.; Volkova, N.; Shaalan, A.; Musa, R.; Tamir, S. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry 2003, 62, 89–99. [Google Scholar] [CrossRef]
- Yao, Y.; Li, S.; Guo, C.; Zhang, C.; Wu, D.; Ren, Y.; Yu, H.S.; Sun, M.Z.; Chen, H. Function-structural study of anti-LDL-oxidation effects of flavonoid phytochemicals. J. Med. Plant Res. 2012, 6, 5895–5904. [Google Scholar]
- Siess, W. Molecular mechanisms of platelet activation. Physiol. Rev. 1989, 69, 58–178. [Google Scholar] [CrossRef]
- Sangkuhl, K.; Shuldiner, A.R.; Klein, T.E.; Altman, R.B. Platelet aggregation pathway. Pharmacogenet Genomics 2011, 21, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Karlíčková, J.; Říha, M.; Filipský, T.; Macáková, K.; Hrdina, R.; Mladěnka, P. Antiplatelet effects of flavonoids mediated by inhibition of arachidonic acid-based pathway. Planta Med. 2016, 82, 76–83. [Google Scholar] [CrossRef]
- Liu, B.M.; Zhang, J.; Bai, C.L.; Wang, X.; Qiu, X.Z.; Wang, X.L.; Liu, B. Spectroscopic study on flavonoid-drug interactions: Competitive binding for human serum albumin between three flavonoid compounds and ticagrelor, a new antiplatelet drug. J. Lumin. 2015, 168, 69–76. [Google Scholar] [CrossRef]
- Beretz, A.; Cazenave, J.P.; Anton, R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: Structure-activity relationships. Agents Actions 1982, 12, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Tindall, M.J.; Lovegrove, J.A.; Gibbins, J.M. Investigating flavonoids as molecular templates for the design of small molecule inhibitors of cell signaling. J. Food Sci. 2013, 78, N1921–N1928. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Spencer, J.P.E.; Lovegrove, J.A.; Gibbins, J.A. Flavonoid inhibitory pharmacodynamics on platelet function in physiological environment. Food Funct. 2013, 4, 1803–1810. [Google Scholar] [CrossRef]
- Tomkin, G.H.; Owens, D. LDL as cause of atherosclerosis. Open Atheroscler. Thromb. J. 2012, 5, 13–21. [Google Scholar] [CrossRef]
- Gibbins, J.M. Platelet adhesion signaling and the regulation of thrombus formation. J. Cell. Sci. 2004, 117, 3415–3425. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2-(R1), Step 4 Version; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Saputri, F.C.; Jantan, I. Effects of selected medicinal plants on human low-density lipoprotein oxidation, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and human platelet aggregation. J. Med. Plants Res. 2011, 5, 6182–6191. [Google Scholar]
Sample Availability: Not available. |

| Conc. (µg/mL) | % Recovery * | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| RT * (min) | Response ** | RT * (min) | Response ** | ||
| 31.25 | 109.93 ± 6.01 | 30.04 ± 0.34 | 16700.7 ± 1.2 | 29.83 ± 0.09 | 16,611.3 ± 2.3 |
| 62.5 | 101.00 ± 2.03 | 29.79 ± 0.18 | 156,978.9 ± 2.3 | 29.69 ± 0.20 | 1568,90.3 ± 2.5 |
| 125 | 103.14 ± 1.86 | 29.94 ± 0.11 | 844,282.4 ± 0.8 | 29.89 ± 0.10 | 842,740.7 ± 0.4 |
| Fractions/Compounds | Final Conc. (µg/mL) | Inhibition (%) | IC50, µg/mL (µM) |
|---|---|---|---|
| n-hexane fraction | 25 | 59.80 ± 0.02 | 14.71 ± 0.11 |
| 12.5 | 44.78 ± 1.38 | ||
| 6.25 | 37.26 ± 1.35 | ||
| 3.13 | 24.07 ± 0.75 | ||
| CHCl3 fraction | 25 | 71.35 ± 0.31 | 3.95 ± 0.65 |
| 12.5 | 63.16 ± 0.46 | ||
| 6.25 | 55.89 ± 1.85 | ||
| 3.13 | 48.09 ± 2.56 | ||
| MeOH fraction | 25 | 80.55 ± 1.38 | 5.77 ± 0.08 |
| 12.5 | 62.16 ± 0.25 | ||
| 6.25 | 51.05 ± 0.60 | ||
| 3.13 | 39.49 ± 0.17 | ||
| Linarin/Acaciin (1) | 25 | 77.67 ± 0.32 | 7.41 ± 0.55 |
| 12.5 | 66.16 ± 1.01 | (12.51 ± 0.93) | |
| 6.25 | 56.97 ± 0.26 | ||
| 3.13 | 49.20 ± 2.01 | ||
| Quercitrin (2) | 25 | 70.84 ± 0.60 | 4.29 ± 0.13 |
| 12.5 | 67.49 ± 0.28 | (9.94 ± 0.29) | |
| 6.25 | 53.14 ± 1.43 | ||
| 3.13 | 44.19 ± 0.38 | ||
| Ursolic acid (3) | 25 | 54.95 ± 0.04 | 33.68 ± 1.28 |
| 12.5 | 47.84 ± 1.56 | (73.85 ± 2.80) | |
| 6.25 | 31.26 ± 1.99 | ||
| 3.13 | 13.66 ± 1.94 | ||
| Stigmasterol (4) | 25 | 56.95 ± 0.58 | 32.95 ± 0.92 |
| 12.5 | 49.72 ± 0.14 | (79.60 ± 2.23) | |
| 6.25 | 36.51 ± 0.23 | ||
| 3.13 | 22.55 ± 0.35 | ||
| Acacetin (7) | 25 | 73.61 ± 1.77 | 4.84 ± 0.30 |
| 12.5 | 62.33 ± 2.03 | (17.05 ± 1.06) | |
| 6.25 | 53.49 ± 2.33 | ||
| 3.13 | 44.61 ± 0.62 | ||
| Apigenin (8) | 25 | 77.77 ± 0.30 | 4.08 ± 0.04 |
| 12.5 | 65.89 ± 0.70 | (15.11 ± 0.16) | |
| 6.25 | 4.44 ± 0.30 | ||
| 3.13 | 47.95 ±0.17 | ||
| Kaempferol (9) | 25 | 83.55 ± 1.90 | 2.29 ± 0.13 |
| 12.5 | 77.30 ± 0.34 | (8.02 ± 9.44) | |
| 6.25 | 66.23 ± 0.94 | ||
| 3.13 | 53.74 ± 1.17 | ||
| Quercetin (10) | 25 | 100.00 ± 0.00 | 1.28 ± 0.04 |
| 12.5 | 90.45 ± 0.55 | (4.25 ± 0.12) | |
| 6.25 | 78.27 ± 0.04 | ||
| 3.13 | 64.31 ± 0.26 | ||
| Vanillic acid (11) | 25 | 63.74 ± 1.26 | 10.08 ± 0.22 |
| 12.5 | 50.94 ± 0.04 | (59.99 ± 1.28) | |
| 6.25 | 42.66 ± 1.98 | ||
| 3.13 | 33.18 ± 0.59 | ||
| Probucol (positive control) | 2.5 | 71.94 ± 1.55 | 0.21 ± 0.01 |
| 1.25 | 65.50 ± 0.71 | (0.40 ± 0.02) | |
| 0.63 | 60.49 ± 0.64 | ||
| 0.31 | 56.15 ± 0.16 | ||
| 0.16 | 45.52 ± 0.37 |
| Compounds | Conc. (µg/mL) | AA (0.5 µM) | ADP (10 µM) | ||
|---|---|---|---|---|---|
| % Inhib. | IC50 (µM) | % Inhib. | IC50 (µM) | ||
| Apigenin (8) | 100 | 100.0 ± 0.0 | 52.3 ± 1.3 | 99.4 ± 1.4 | 127.4 ± 2.3 |
| 50 | 84.9 ± 1.2 | 63.2 ± 0.9 | |||
| 25 | 63.8 ± 0.7 | 33.0 ± 0.6 | |||
| 12.5 | 46.9 ± 1.4 | 9.3 ± 0.2 | |||
| Kaempferol (9) | 100 | 74.4 ± 0.6 | 163.6 ± 5.0 | 65.2 ± 1.0 | 181.9 ± 4.6 |
| 50 | 48.1 ± 1.9 | 51.5 ± 0.6 | |||
| 25 | 32.6 ± 0.5 | 31.7 ± 0.4 | |||
| 12.5 | 12.3 ± 0.1 | 11.1 ± 0.1 | |||
| Quercetin (10) | 100 | 85.1 ± 0.6 | 124.9 ± 3.4 | 74.6 ± 1.1 | 173.2 ± 7.3 |
| 50 | 55.0 ± 2.2 | 47.4 ± 0.7 | |||
| 25 | 37.3 ± 0.6 | 24.8 ± 0.5 | |||
| 12.5 | 14.1 ± 0.1 | 7.0 ± 0.2 | |||
| Aspirin (ASA) | 25 | 100.0 ± 0.0 | 26.0 ± 1.3 | 44.8 ± 1.8 | ND * |
| 12.5 | 85.2 ± 4.5 | ||||
| 6.25 | 58.2 ± 2.3 | ||||
| 3.13 | 36.5 ± 0.4 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dianita, R.; Jantan, I. Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds. Molecules 2019, 24, 1469. https://doi.org/10.3390/molecules24081469
Dianita R, Jantan I. Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds. Molecules. 2019; 24(8):1469. https://doi.org/10.3390/molecules24081469
Chicago/Turabian StyleDianita, Roza, and Ibrahim Jantan. 2019. "Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds" Molecules 24, no. 8: 1469. https://doi.org/10.3390/molecules24081469
APA StyleDianita, R., & Jantan, I. (2019). Inhibition of Human Platelet Aggregation and Low-Density Lipoprotein Oxidation by Premna foetida Extract and Its Major Compounds. Molecules, 24(8), 1469. https://doi.org/10.3390/molecules24081469






