Ameliorative Effect and Mechanism of the Purified Anthraquinone-Glycoside Preparation from Rheum Palmatum L. on Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Results
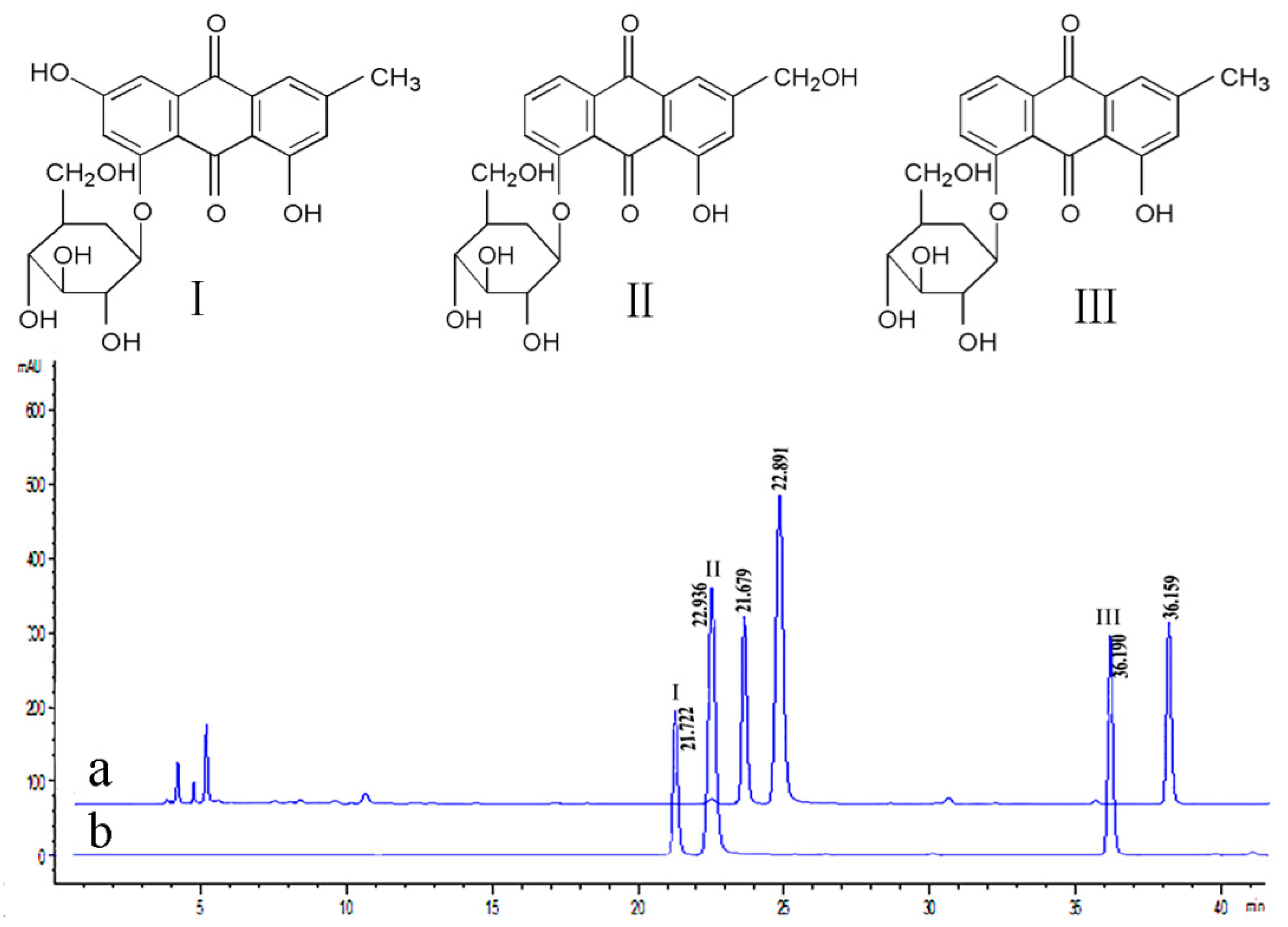
2.1. Anthraquinone-Glycosides Content of PAGR
2.2. Acute Toxicity Study
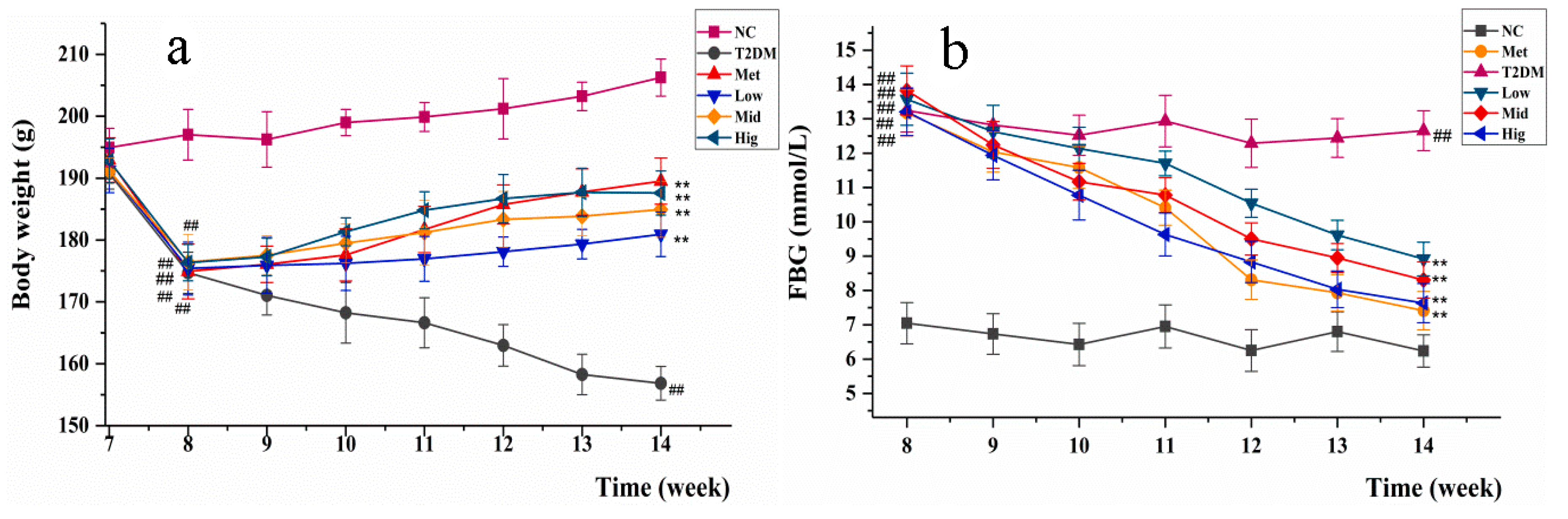
2.3. Changes inBody Weight and Fasting Blood Glucose in Rats
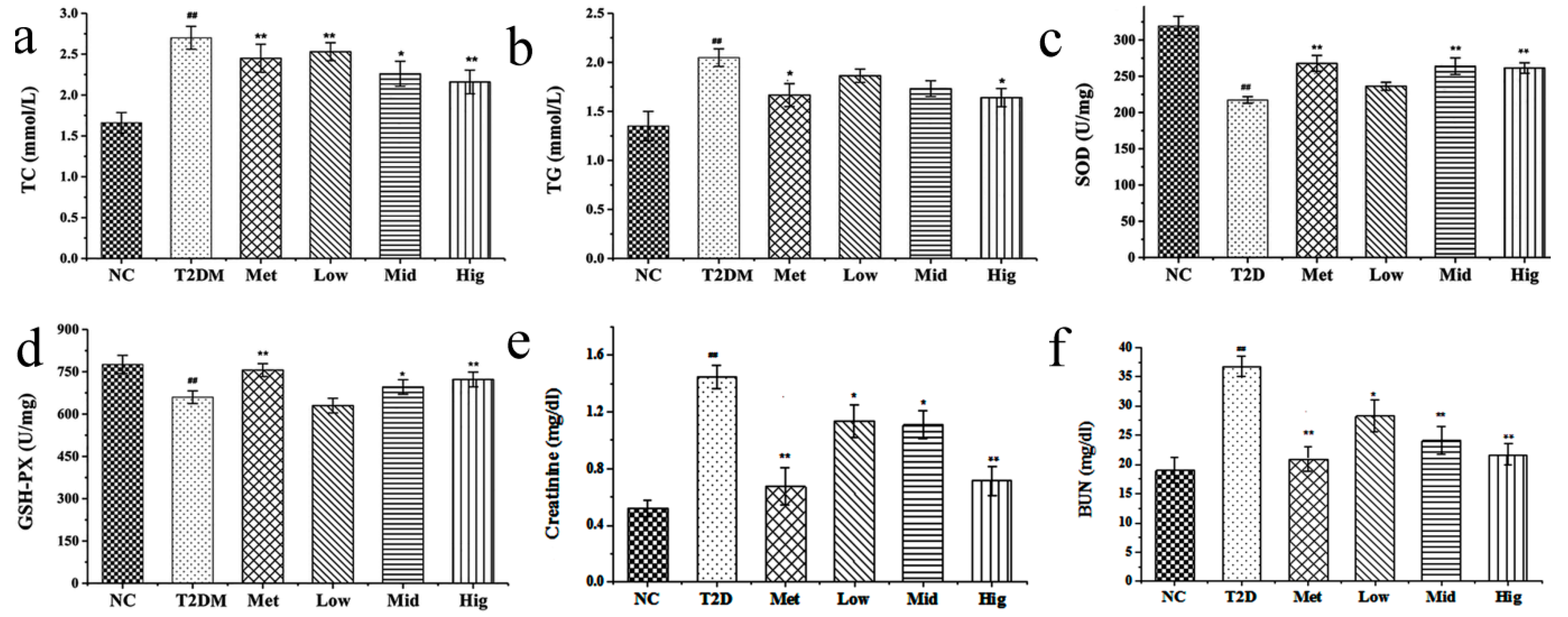
2.4. Effect of PAGR on Lipid Metabolites and Antioxidant Enzyme Activities in Rats
2.5. The Effect of PAGR on Histopathological Changes
2.5.1. Effects of PAGR on Histopathological Changes in Liver
2.5.2. Effects of PAGR on Histopathological Changes in Kidney
2.5.3. Effects of PAGR on Histopathological Changes in Pancreas
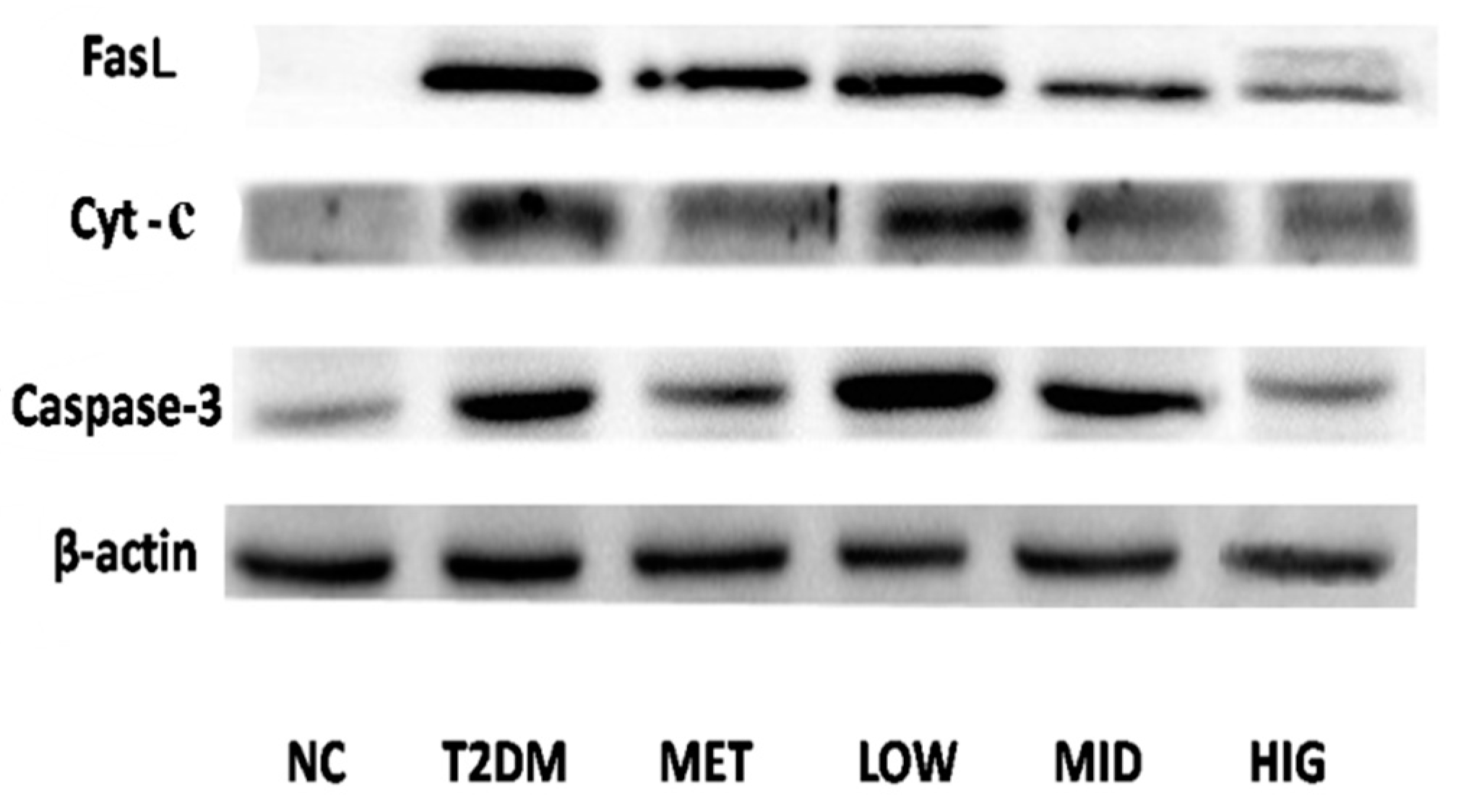
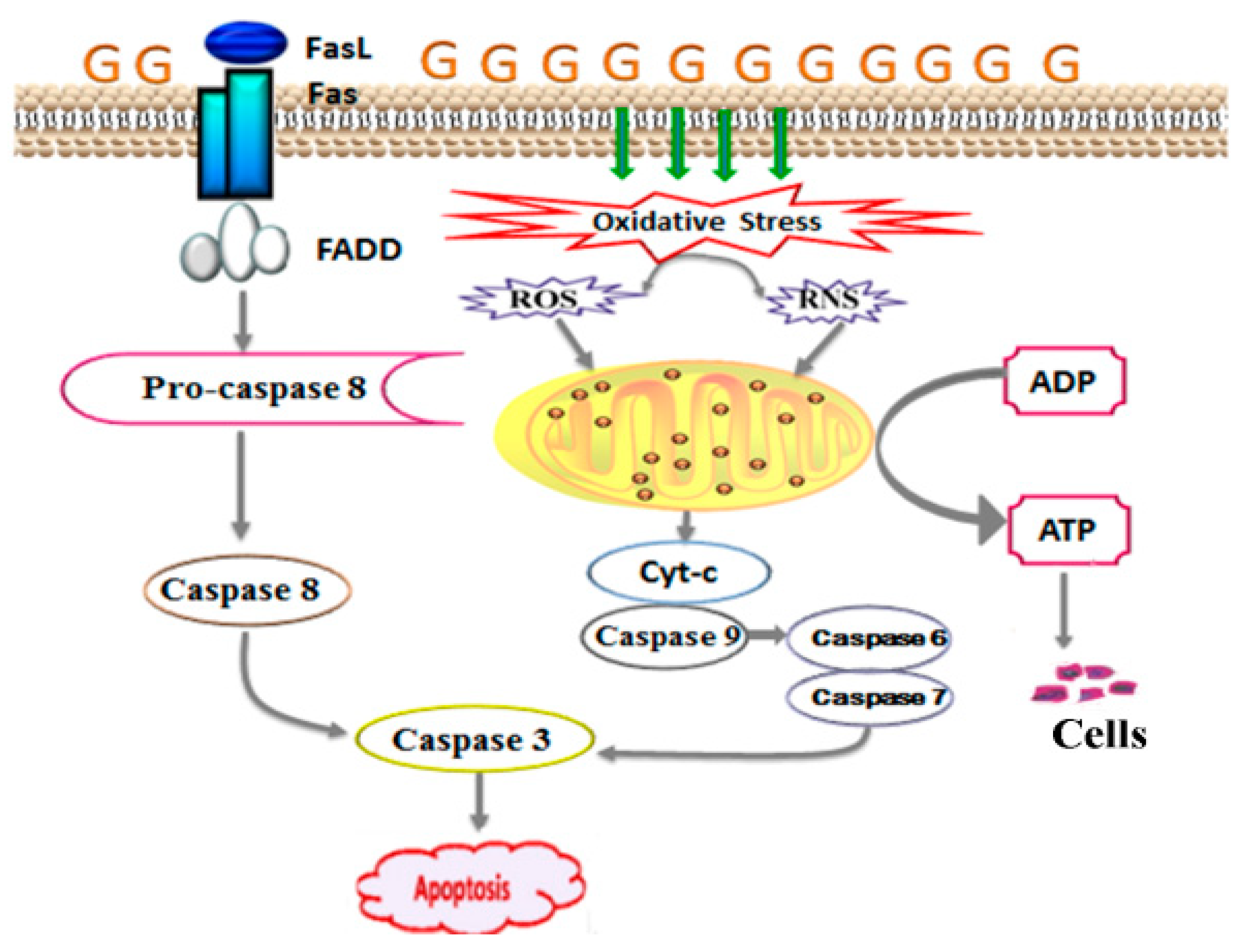
2.6. Effect of PAGR on the Expression of Cytochrome C (Cyt-c), Caspase-3, and FasLin Pancreas
3. Discussions
4. Material and Methods
4.1. Drugs and Chemicals
4.2. The Preparation and Determination of PAGR
4.3. Determination of Three Anthraquinone-Glycosides in PAGR by HPLC
4.3.1. HPLC Chromatographic Conditions
4.3.2. Preparation of Working Solution and Standard Curve
4.3.3. Stability and Precision
4.4. Acute Toxicity Test
4.5. AnimalsTreatment and Sample Collection
4.6. Biochemical Measurements
4.7. Histopathological Examinations
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data availability
Abbreviations
| PAGR | purified anthraquinone-Glycoside from Rheum palmatum L. |
| STZ | streptozotocin |
| T2DM | type 2 diabetes mellitus |
| NC | normal |
| Met | metformin |
| Mid | medium |
| Hig | high |
| FBG | fasting blood glucose |
| TC | total cholesterol |
| TG | triglyceride |
| SOD | superoxide dismutase |
| GSH-PX | glutathione peroxidase |
| Cyt-c | cytochrome C |
| HPLC | High-Performance Liquid Chromatography |
| BUN | blood urea nitrogen |
| H&E | hematoxylin and eosin |
| SD | standard deviation |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| MAPK | mitogen-activated protein kinase |
| SDS-PAGE | sodium dodecyl sulfate polypropylene gel electrophoresis |
| PVDF | polyvinylidene difluoride |
References
- Sharma, A.K.; Bharti, S.; Goyal, S.; Arora, S.; Nepal, S.; Kishore, K. Upregulation of PPARγ by Aegle marmelos Ameliorates Insulin Resistance and β-cell Dysfunction in High Fat Diet Fed-Streptozotocin Induced Type 2 Diabetic Rats. Phytother. Res. 2011, 25, 1457–1465. [Google Scholar] [CrossRef]
- Orchard, T.J.; Olson, J.C.; Erbey, J.R.; Williams, K.; Forrest, K.Y. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003, 26, 1374–1379. [Google Scholar] [CrossRef]
- Nishihama, K.; Yasuma, T.; Yano, Y.D.; Alessandro-Gabazza, C.N.; Toda, M.; Hinneh, J.A.; Baffour, T.P. Anti-apoptotic activity of human matrix metalloproteinase-2 at tenuates diabetes mellitus. Metabolism 2018, 82, 88–99. [Google Scholar] [CrossRef]
- Manisha, J.O.; Yogesh, A.K. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018, 107, 1119–1127. [Google Scholar]
- Wing, R.R.; Rosen, R.C.; Fava, J.L.; Bahnson, J.; Brancati, F.; Gendrano, I.N.C.; Kitabchi, A.; Schneider, S.H.; Wadden, T.A. Effects of Weight Loss Intervention on Erectile Function in Older Men with Type 2 Diabetes in the Look AHEAD Trial. J. Sex Med. 2010, 7, 156–165. [Google Scholar]
- Olson, J.C.; Erbey, J.R.; Forrest, K.Y.; Williams, K.; Becker, D.J. Glycemia (or, in women, estimated glucose disposal rate) predict lower extremity arterial disease events in type 1 diabetes. Metabolism 2002, 51, 248–254. [Google Scholar] [CrossRef]
- Jakob, G.K.; Patrik, R. β-Cell Dysfunction in Type 2 Diabetes: Draned of Energy? Cell Metabol. 2019, 29, 1–2. [Google Scholar]
- Tania, G.D.; Manuel, S.P.; Fernando, V.A.; Ángel, A.V.; Olga, D.L.; Eduardo, L.G.; Aurora, M.R. Apoptosis in pancreatic β-cells is induced by arsenic and atorvastatin in Wistar rats with diabetes mellitus type 2. J. Trace Elem. Med. Biol. 2018, 46, 146–199. [Google Scholar]
- Tatsuo, T. Apoptosis in pancreaticβ-islet cells in Type 2 diabetes. Bosanian J. Basic Med. 2016, 16, 162–179. [Google Scholar]
- Diana, C.; Minna, W. Executioners of apoptosis in pancreatic β-cells: Not just for cell death. Am. J. Physiol. Endocrinol. Metab. 2010, 298, 735–741. [Google Scholar]
- Guariguata, L. Contribute data to the 6th edition of the IDF Diabetes Atlas. Diabetes Res. Cli. PR. 2013, 100, 280–281. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, P.; Xu, L.; He, C.; Peng, N.; Xiao, P. Evaluation of the content variation of anthraquinone glycosidesin rhubarb by UPLC-PDA. Chem. Cent. J. 2012, 7, 153–160. [Google Scholar]
- Cirillo, C.; Capasso, R. Constipation and Botanical Medicines: An Overview. Phytother. Res. 2015, 29, 1488–1493. [Google Scholar] [CrossRef]
- Ullah, H.; Kim, J.; Rehman, N.U.; Kim, H.J.; Ahn, M.J.; Chung, H.J. A Simple and Sensitive Liquid Chromatography with Tandem Mass Spectrometric Method for the Simultaneous Determination of Anthraquinone Glycosides and Their Aglycones in Rat Plasma: Application to a Pharmacokinetic Study of Rumex acetosa Extract. Pharmaceutics 2018, 10, 100. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Kong, W.; Jin, C.; Zhao, Y.; Qu, Y. Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine 2010, 7, 684–689. [Google Scholar]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Iizuka, A.; Iijima, O.T.; Kondo, K.; Itakura, H.; Yoshie, F.; Miyamoto, H. Evaluation of rhubarb using antioxidative activity as an index of pharmacological usefulness. J. Ethnopharmacolo. 2004, 91, 89–94. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yan, F.F.; Liu, Y.; Zhang, C.; Yu, H.M.; Zhang, Y. Aqueous extract of rhubarb stabilizes vulnerable atherosclerotic plaques due to depression of inflammation and lipid accumulation. Phytother. Res. 2008, 22, 935–942. [Google Scholar] [CrossRef]
- Latcha, S.; Lubetzky, M.; Weinstein, A.M. Severe hyperosmolarity and hypernatremia in an adipsic young woman. Clin. Nephrol. 2011, 76, 407–411. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kanawat, D.S.; Mishr, A.; Dhakad, P.K.; Sharma, P.; Srivastava, V.; Joshi, S.; Joshi, M.; Raikwar, S.K.; Kurmi, M.K.; et al. Dual therapy of vildagliptin and telmisartan on diabetic nephropathy in experimentally induced type 2 diabetes mellitus rats. J. Renin Angiotensin Aldosterone Syst. 2013, 15, 410–418. [Google Scholar]
- Li, J.P.; Yuan, Y.; Zhang, W.Y.; Jiang, Z.; Hu, T.J.; Feng, Y.T. Effect of Radix isatidis polysaccharide on alleviating insulin resistance in type 2 diabetes mellitus cells and rats. J. Pharm. Pharmacol. 2018, 71, 220–229. [Google Scholar] [CrossRef]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A. Non-alcoholic fatty liver disease in type 2 diabetes: Pathogenesis and treatment options. Curr. Vasc. Pharmacol. 2012, 10, 162–172. [Google Scholar] [CrossRef]
- Petersen, K.F.; Shulman, G.I. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity (Silver Spring) 2006, 14 (Suppl. 1), 34S–40S. [Google Scholar]
- Rerolle, J.P.; Hertig, A.; Nguyen, G.; SraerEric, J.D.; Rondeau, E.P. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000, 58, 1841–1850. [Google Scholar] [CrossRef][Green Version]
- Graus, N.F.; Marinho, T.S.; Barbosa, S.S.; Aguila, M.B.; Mandarim, L.; Souza, M.V. Differential effects of angiotensin receptor blockers on pancreatic islet remodelling and glucose homeostasis in diet-induced obese mice. Mol. Cell Endocrinol. 2017, 439, 54–64. [Google Scholar]
- Radenković, M.; Stojanović, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharm. Toxicolo. Methods. 2016, 78, 13–31. [Google Scholar]
- Zhu, C.F.; Peng, H.B.; Liu, G.Q.; Zhang, F.; Li, Y. Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetes. Nutrition 2010, 26, 1014–1020. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Lydiasrat, C.L.; Kaul, P.R. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Pazdro, R.; Burgess, J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2017, 131, 276–286. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef]
- Unger, R.H.; Zhou, Y.T. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001, 50, S118–S121. [Google Scholar] [CrossRef]
- Khavandi, M.; Duarte, F.; Ginsberg, H.N.; Reyes, S.G. Treatment of Dyslipidemias to Prevent Cardiovascular Disease in Patients with Type 2 Diabetes. Curr. Cardiol. Rep. 2017, 19, 7–16. [Google Scholar] [CrossRef]
- Ming, Z.; Zhou, J.M.; Liu, Y.; Sun, X.Z.; Luo, X.P.; Han, C.Y. Risk of type 2 diabetes mellitus associated with plasma lipid levels: The rural Chinese cohort study. Diabetes Res. Clin. Pract. 2018, 35, 150–157. [Google Scholar]
- Styskal, J.; Van, R.H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef]
- Bravi, M.C.; Armiento, A.; Laurenti, O.; Cassone, F.M.; De, L.O. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2006, 55, 691–695. [Google Scholar] [CrossRef]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H. Reactive oxygen species promote TNF alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef]
- Emamaullee, J.A.; Shapiro, A.M. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes 2006, 55, 1907–1914. [Google Scholar] [CrossRef]
- Yang, R.Y.; Zhang, Z.F.; Pei, X.R.; Han, X.L.; Wang, J.B.; Wang, L.L. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, Y.Q.; Kang, Y.L.; Lu, H.; Niu, H.G.; Liu, W. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell Physiol. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Mohamad, N.; Buang, F.; Lazim, A.M.; Ahmad, N.; Martin, C.; Mohd Amin, M.C.I. Characterization and biocompatibility evaluation of bacterial cellulose-based wound dressing hydrogel: Effect of electron beam irradiation doses and concentration of acrylic acid. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2553–2564. [Google Scholar]
- Routray, I.; Ali, S. Boron inhibits apoptosis in hyperapoptosis condition: Acts by stabilizing the mitochondrial membrane and inhibiting matrix. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 144–152. [Google Scholar]
- Nolsøe, R.L.; Hamid, Y.H.; Pociot, F.; Paulsen, S.; Andersen, K.M.; Borch-Johnsen, K. Association of a microsatellite in FASL to type II diabetes and of the FAS-670G4A genotype to insulin resistance. Genes Immun. 2016, 7, 316–321. [Google Scholar] [CrossRef]
- Loweth, A.C.; Williams, G.T.; James, R.F.L.; Scarpello, J.H.B.; Morgan, N.G. Human islets of Langerhans express fas ligand and undergo apoptosis in response to interleukin-1b and fas ligation. Diabetes 1998, 47, 727–732. [Google Scholar] [CrossRef]
- Tian, C.; Chang, H.; La, X.; Li, J.A.; Ma, L. Wushenziye Formula Inhibits Pancreatic β Cell Apoptosis in Type 2 Diabetes Mellitus via MEK-ERK-Caspase-3 Signaling Pathway. Evid. Based Complement Alternat Med. 2018, 25, 259–298. [Google Scholar] [CrossRef]
- Maedler, K.; Fontana, A.; Ris, F.; Sergeev, P.; Toso, C.; Oberholzer, J. FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc. Natl. Acad. Sci. USA 2002, 99, 8236–8241. [Google Scholar] [CrossRef]
- Hong, S.C.; So, H.T.; Sonia, C.; Wen, P.; Joash, B.; Lee, T. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J. Adv. Res. 2017, 8, 30106–30116. [Google Scholar]
- Arvindekar, A.; More, T.; Payghan, P.V.; Laddha, K.; Ghoshal, N.; Arvindekar, A. Evaluation of anti-diabetic and alpha glucosidase inhibitory action of anthraquinones from Rheum emodi. Food Funct. 2015, 6, 2693–2700. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
 , hepatocyte;
, hepatocyte;  , inflammatory cell;
, inflammatory cell;  , lipid droplet.
, lipid droplet.
 , hepatocyte;
, hepatocyte;  , inflammatory cell;
, inflammatory cell;  , lipid droplet.
, lipid droplet.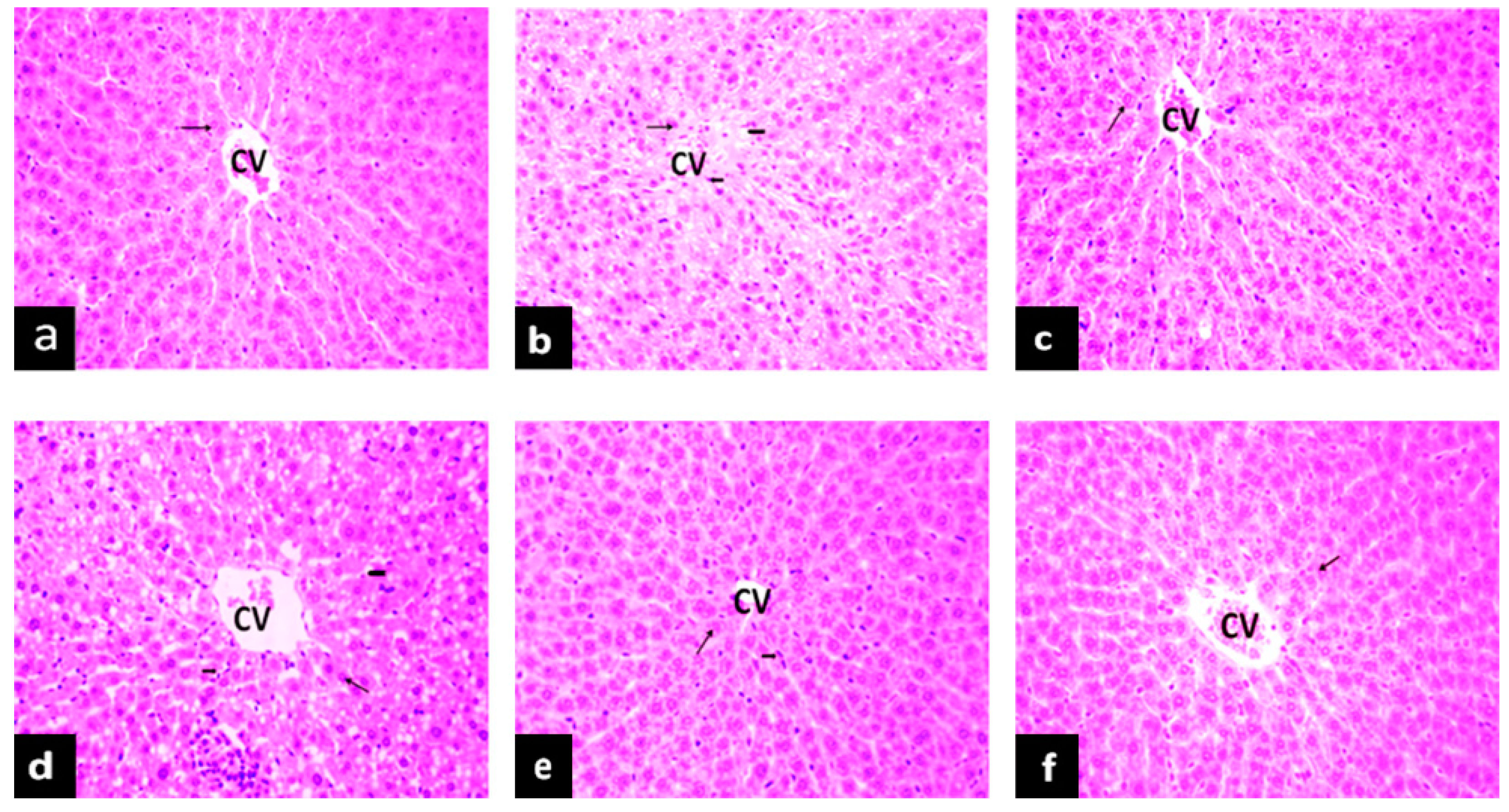
 , glomerulus;
, glomerulus;  , base membrane;
, base membrane;  , mesangial.
, mesangial.
 , glomerulus;
, glomerulus;  , base membrane;
, base membrane;  , mesangial.
, mesangial.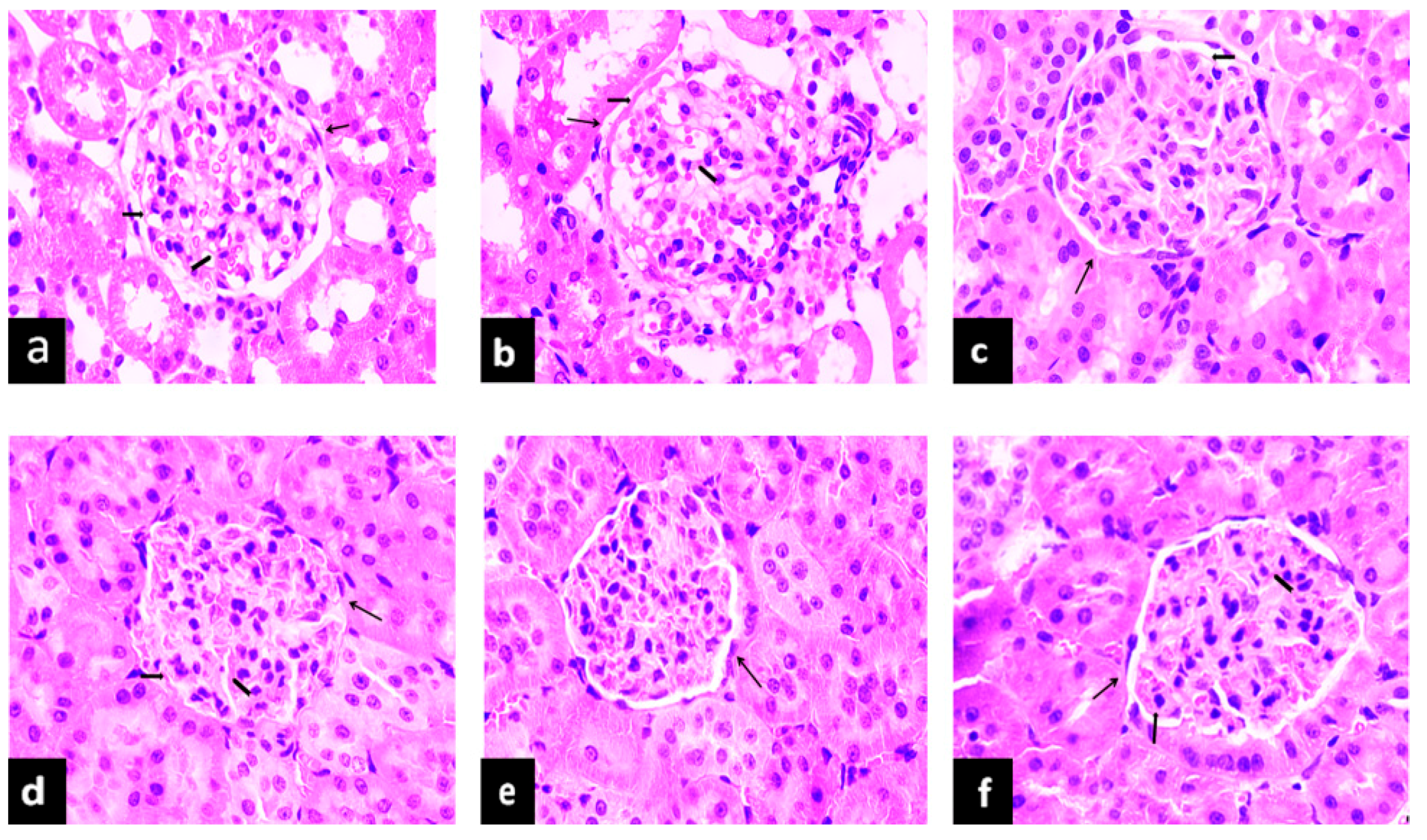
 , islet;
, islet;  , islet cell;
, islet cell;  , lipid droplet.
, lipid droplet.
 , islet;
, islet; 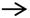 , islet cell;
, islet cell;  , lipid droplet.
, lipid droplet.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.-R.; Cui, H.-X.; Fang, J.-L.; Yuan, K.; Guo, Y. Ameliorative Effect and Mechanism of the Purified Anthraquinone-Glycoside Preparation from Rheum Palmatum L. on Type 2 Diabetes Mellitus. Molecules 2019, 24, 1454. https://doi.org/10.3390/molecules24081454
Cheng F-R, Cui H-X, Fang J-L, Yuan K, Guo Y. Ameliorative Effect and Mechanism of the Purified Anthraquinone-Glycoside Preparation from Rheum Palmatum L. on Type 2 Diabetes Mellitus. Molecules. 2019; 24(8):1454. https://doi.org/10.3390/molecules24081454
Chicago/Turabian StyleCheng, Fang-Rong, Hong-Xin Cui, Ji-Li Fang, Ke Yuan, and Ying Guo. 2019. "Ameliorative Effect and Mechanism of the Purified Anthraquinone-Glycoside Preparation from Rheum Palmatum L. on Type 2 Diabetes Mellitus" Molecules 24, no. 8: 1454. https://doi.org/10.3390/molecules24081454
APA StyleCheng, F.-R., Cui, H.-X., Fang, J.-L., Yuan, K., & Guo, Y. (2019). Ameliorative Effect and Mechanism of the Purified Anthraquinone-Glycoside Preparation from Rheum Palmatum L. on Type 2 Diabetes Mellitus. Molecules, 24(8), 1454. https://doi.org/10.3390/molecules24081454




