Abstract
Red mud is a by-product of alumina production containing lanthanides. Growth of green microalgae on red mud and the intracellular accumulation of lanthanides was tested. The best growing species was Desmodesmus quadricauda (2.71 cell number doublings/day), which accumulated lanthanides to the highest level (27.3 mg/kg/day), if compared with Chlamydomonas reinhardtii and Parachlorella kessleri (2.50, 2.37 cell number doublings and 24.5, 12.5 mg/kg per day, respectively). With increasing concentrations of red mud, the growth rate decreased (2.71, 2.62, 2.43 cell number doublings/day) due to increased shadowing of cells by undissolved red mud particles. The accumulated lanthanide content, however, increased in the most efficient alga Desmodesmus quadricauda within 2 days from zero in red-mud free culture to 12.4, 39.0, 54.5 mg/kg of dry mass at red mud concentrations of 0.03, 0.05 and 0.1%, respectively. Red mud alleviated the metal starvation caused by cultivation in incomplete nutrient medium without added microelements. Moreover, the proportion of lanthanides in algae grown in red mud were about 250, 138, 117% higher than in culture grown in complete nutrient medium at red mud concentrations of 0.03, 0.05, 0.1%. Thus, green algae are prospective vehicles for bio-mining or bio-leaching of lanthanides from red mud.
1. Introduction
The group of rare earth elements (REEs) includes scandium (Sc), yttrium (Y), and a series of 15 other elements from the lanthanide series. Although they differ in atomic number (21 for Sc, 39 for Y and the others from 57 to 71), they exhibit similar physical and chemical properties [1]. In general, lanthanides can be divided into light (from lanthanum to europium) and heavy REEs (from gadolinium to lutetium, including Y and Sc). Due to their unique magnetic and catalytic properties, lanthanides are widely used in almost all electronic and clean energy technologies. As a consequence of the development of modern technologies, the demand and prices for lanthanides are steadily increasing, leading to potential exhaustion of current limited supplies. Lanthanides are therefore critical raw materials because of their high supply risk and above average economic importance in comparison with other raw materials [2].
The risk of reduced availability of resources in China (the main world producer), and possibly of a depletion of other natural resources, raises the need for more efficient and sustainable (bio)mining processes, even from low grade ores, as well as efficient methods for recycling of lanthanides, e.g., from waste material. Although recycling is far from reaching its full potential, it has some advantages such as a lack of radioactive impurities and an economic independence of supply from primary sources.
Another potential, so far very little exploited, source of lanthanides may be found in so called red mud. Red mud is a by-product of the production of alumina (aluminum oxide) from bauxite ore during the standard Bayer process. Red mud is currently being produced at a global rate of 150 million tons annually [3], while a cumulative amount of 4 billion tons of bauxite residue have already been stored worldwide since 2015 [4]. However, less than 2% of the residue produced annually is currently being reused [5], due to difficulties related to high pH, salinity, low solid content, size of fine particles and leaching of metals [6]. Information on the recovery of valuable metals (reviewed in [7]) provides an insight into the full potential of red mud as an economic resource rather than a waste material.
Red mud has been deposited in large dumps for decades, where its chemical and mineralogical composition may change significantly due to interactions with groundwater. As a result, red mud consists of a wide spectrum of minerals, some of which still reflect the source sedimentary rock, bauxite, while others have developed due to chemical treatment under industrial conditions and some mineral phases formed in the deposit ponds.
During the Bayer process, bauxite ore is heated, along with a sodium hydroxide solution, at a temperature of 150 to 200 °C to obtain soluble aluminum (oxy) hydroxides. After separating the aqueous solution of aluminum hydroxide from solid impurities, the remaining solid fraction is called bauxite residue, while the waste sludge is called red mud [8]. The residue of the chemical treatment is still very high in sodium and has a pH > 11. Among the minor components of red mud, lanthanides and scandium are of special interest since, based on recent research, extraction of these components from red mud is a real possibility (e.g., [9]).
Methods for extraction of lanthanides from ores, such as pyrometallurgy and hydrometallurgy have severe negative environmental impacts, as well as being expensive. Currently, industrial extraction of lanthanides from monazite involves either a basic process that uses concentrated sodium hydroxide or an acidic process that uses concentrated sulfuric acid. These processes generate large amounts of hazardous waste containing thorium and uranium [10]. Bio-mining techniques are generally less energy-intensive and less polluting.
Research has therefore recently focused on more environmentally-friendly technologies of metal recovery from secondary resources [11]. Biological methods might offer an alternative to physicochemical recycling techniques, although they are still limited by high costs and low element selectivity [12]. Microbial biotechnologies such as bio-mining, bio-leaching, bio-recovery, bio-sorption, bio-electrochemical recovery, bio-precipitation, bio-flotation, bio-reduction or bio-accumulation that can be used in the recovery of critical and scarce metals are explained in detail and summarized, for example, in [12,13,14,15,16]. Considerable progress has been achieved in the development of efficient biological methods for lanthanide recovery using microorganisms such as bacteria, fungi, cyanobacteria or algae [17,18].
An important aspect of any efficient bio-recovery method is selection of a suitable species. Different microorganisms were investigated for exploitation in scarce metal recovery biotechnology. Filamentous fungi Penicillium tricolor and Aspergillus niger producing citric acid and oxalic acid were used for bio-leaching of lanthanides from red mud [19,20]. In the case of the red alga Galdieria sulphuraria, interactions of phosphoproteins and Ca-binding proteins with lanthanides were identified as factors in the bio-sorption of lanthanides [17]. Park and colleagues reported the expression of lanthanide-binding tags on the surface of Caulobacter crescentus, leading to the adsorption of lanthanides [18]. The only selective bio-accumulation so far was described in the fungus Penidiella sp. T9. This fungus selectively accumulated dysprosium from acidic solutions [21].
Only a few studies of lanthanide recovery by algae or cyanobacteria have been published. With the exception of the red alga Galdieria sulphuraria [17], the live macroalga Gracillaria gracilis was effectively used to recover lanthanides from waste water [22]. Dried or carbonized biomass of the green alga Parachlorella was used for bio-sorption and reversible desorption of lanthanides from aqueous solution [23]. Studies of bio-remediation of red mud were performed with the cyanobacterial species Phormidium and Oscillatoria [24]. Results indicated that these microorganisms were able to reach a high growth rate in the presence of red mud-supplemented nutrient medium. Several studies have shown that lanthanides accumulate in chloroplasts [25,26,27,28]. It was demonstrated that selective deposition of individual lanthanides in chloroplasts or the cytoplasm occurs in the green alga Desmodesmus quadricauda. Nd and Ce were located in the chloroplast while La and Gd were found in the cytoplasm [29]. The advantage of using phototrophic organisms for remediation is the sequestration of CO2 during their growth and assimilation of pollutants present in the waste-water. Moreover, the biomass produced can be further reused for feed, food or fertilizers [30].
The aim of this study was to examine the ability of selected species of green microalgae to grow in the presence of red mud and to accumulate lanthanides from this lanthanide-rich material. In order to examine bio-absorption capacity and physiological effects of lanthanides, the three species Desmodesmus quadricauda, Chlamydomonas reinhardtii, and Parachlorella kessleri were cultivated in the presence of different concentrations of red mud. As a comprehensive determination of the content of lanthanides accumulated in algal biomass, inductively coupled plasma mass spectrometry was used. The simultaneous verification of accumulation and the localization of lanthanides were examined using fluorescence microscopy. The work describes the potential of green algae for bio-mining of lanthanides from red mud or bio-leaching.
2. Results
2.1. Composition of Lanthanides and Other Metals in Red Mud
To consider the extensive waste red mud deposits as a potential source for bio-mining lanthanides, the composition of these elements in different locations and depths of the mud disposal site had to be analyzed. For experiments, samples were collected at a depth of approximately 1–1.2 m measured from the red mud surface. At this depth, the state of the red mud was gelatinous and wet. From the list of lanthanides analyzed (Table 1), cerium, lanthanum and neodymium were found to be proportionally the most abundant at 36.5, 17.2, and 14.7% respectively, i.e., representing 68.4% of the total amount of lanthanides.

Table 1.
Data on the quality and homogeneity of lanthanides in the red mud from Almásfüzítő, Hungary.
High proportions of the heavy REEs scandium and yttrium, 8.2 and 10.1% respectively, were also found. The proportion of other lanthanides were relatively low and ranged from 3.2 to 0.2% (Table 1).
Analyses of other elements (Table 2) demonstrated their presence at very high levels, about three orders of magnitude higher (g/kg) than lanthanides (mg/kg). The red color of the mud derives from compounds of the most frequent element, iron (53% of the total content of metal elements, Table 2). Most of these elements, particularly microelements, could be used as inorganic compounds for the growth of algal cultures (see the composition section in Methods).

Table 2.
Concentration range of the main constituents of red mud.
2.2. Selection of a Suitable Model Organism for Cultivation with Red Mud
To investigate the bio-mining of lanthanides from red mud by algae, initial experiments were carried out to select the appropriate algal species for their ability to grow in the presence of red mud and accumulate lanthanides from it. The three species of green algae Desmodesmus quadricauda, Chlamydomonas reinhardtii and Parachlorella kessleri were selected.
A stock 10% suspension of red mud in water (w/v) was used for all experiments. Aliquots of this stock suspension were added to corresponding algal nutrient media (see Methods) to final concentrations of 0.03, 0.05 and 0.1% (w/v). The control culture was grown in the absence of red mud in a nutrient medium (Figure 1). The final cell number was estimated after 48 h of growth at 30 °C, and an incident light intensity of 500 μmol/m2/s. All experimental cultures started growth at a cell concentration of 8 × 105/mL.
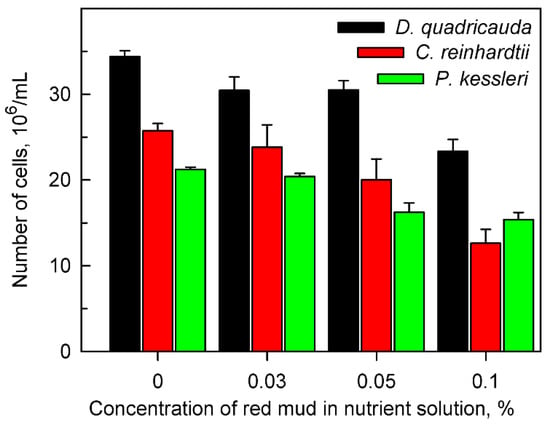
Figure 1.
Cell number of Desmodesmus quadricauda, Chlamydomonas reinhardtii and Parachlorella kessleri after 48 h of growth in the absence (0%) or presence of different concentrations (0.03, 0.05, 0.1%) of red mud in nutrient medium suitable for the given species. All the cultures were diluted to the same initial number of cells (8 × 105/mL) at the beginning of each experiment.
Particles of red mud suspended in nutrient medium were only partially solubilized and with increasing amounts of added suspension, the insolubilized particle content increased. Shadowing of cells by insoluble particles of red mud caused a decrease in the mean light intensity (light intensity experienced by cells, for determination see Material and Methods). The measured mean light intensities in cultures grown at concentrations of 0, 0.03, 0.05 and 0.1% red mud were 500, 400, 200 and 100 μmol/m2/s, respectively. The decrease in mean light intensity with increasing levels of red mud caused slower growth of cell cultures for all species tested (Figure 1, Table 3). Nevertheless, for any concentration of red mud, D. quadricauda grew better than the other two algal species (Figure 1, Table 3).

Table 3.
The growth rate (µ) of Desmodesmus quadricauda, Chlamydomonas reinhardtii and Parachlorella kessleri at different concentrations of red mud expressed as doubling of number of cells per day.
The cells of all algal species tested were able to accumulate lanthanides intracellularly from the red mud suspension (Figure 2). The concentration of bio-absorbed lanthanides increased with increasing concentrations of red mud in the nutrient medium. In spite of the fact that cells in the presence of high concentrations of red mud (0.1%) grew much slower than those at lower concentrations, their intracellular content of lanthanides was much higher (Figure 2). The alga D. quadricauda accumulated more lanthanides in comparison with C. reinhardtii and P. kessleri (Figure 2).
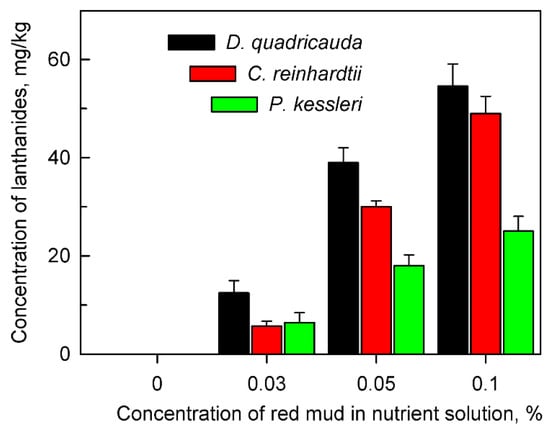
Figure 2.
Total amount of lanthanides accumulated in cells of Desmodesmus quadricauda, Chlamydomonas reinhardtii and Parachlorella kessleri after 48 h of growth in the absence (0%) or presence of different concentrations (0.03, 0.05, 0.1%) of red mud in nutrient medium suitable for the given species. No lanthanides were found in cells grown in the absence of red mud.
To find the most appropriate species for more detailed experiments, the proportional bio-absorption of three most frequent lanthanides, cerium, lanthanum and neodymium from red mud (36.5, 17.2 and 14.7% of total lanthanides, respectively, see Table 1) was followed in all species, at concentrations of red mud (0.1% and 0.03%), the level of which was assumed to be sufficient for this purpose.
It was confirmed that the highest levels of all three lanthanides, at both lowest and highest concentrations of red mud were found in Desmodesmus quadricauda (Figure 3). Higher levels of lanthanides accumulated with higher concentrations of red mud. Lanthanides accumulated in about the same proportion as they were present in an extracellular suspension of red mud (compare data in Table 1 and in Figure 3).
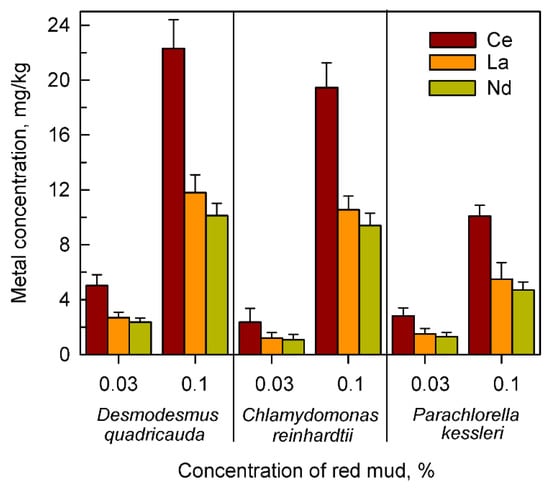
Figure 3.
The content of cerium, lanthanum and neodymium in cells of Desmodesmus quadricauda, Chlamydomonas reinhardtii and Parachlorella kessleri grown in the presence of different concentrations (0.03, 0.1%) of red mud in complete nutrient medium suitable for the given species.
2.3. Cultivation of Desmodesmus quadricauda with Red Mud in Incomplete Nutrient Medium
Analyses of red mud for the content of different metal elements (Table 1 and Table 2) provided evidence for the presence of many metals that are also present in algal nutrient media (see Materials and Methods). From the comparative list of red mud elements and the elements used in nutrient medium, it is apparent that elements B, Mn, Co, Ni, Cu, Zn are essential microelements for algal growth (compare Table 2 and Table 4, bottom part).

Table 4.
Composition of nutrient media for all experimental species.
To substitute these metals in a nutrient medium with those present in red mud could decrease the cost and simplify the preparation of these media, particularly in scale-up photo-bioreactors for biotechnological applications, as well as to improve the bio-leaching of red mud waste.
The alga D. quadricauda was selected for these experiments because, from algal species tested, it had the best growth and the highest bio-sorption of lanthanides. Algal cultures grown either in complete medium containing microelements (+m) or in incomplete medium lacking added microelements (−m) (for the composition of the media see Material and Methods), were treated with red mud at concentrations of 0.03, 0.05 and 0.1% (w/v). Control cultures were grown in the same medium in the absence of red mud (0%) (Figure 4, Figure 5 and Figure 6). The incident light intensity (Ii) used for all red mud-treated cultures was about 500 µE (μmol/m2/s), but the corresponding mean light intensity (Im) (see Materials and Methods for measurement and calculation) decreased with increasing concentrations of red mud due to shadowing effect of undissolved particles. Thus, Im was about 400, 200 and 100 µE for concentrations of 0.03, 0.05 and 0.1%, respectively. To enable a comparison with growth of control cultures, the cultures were grown separately at the same mean light intensities as for algae grown in the corresponding red mud concentrations (Figure 4).
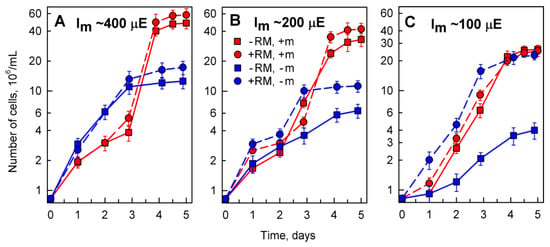
Figure 4.
Changes in number of Desmodesmus quadricauda cells grown in the absence (-RM) or presence (+RM) of different red mud concentrations in different nutrient media: 0.03% (panel A), 0.05% (panel B), 0.1% (panel C). The experimental variants were grown either in complete (+m) or in incomplete nutrient media (−m) lacking added microelements. Mean light intensity (Im) decreased due to increasing shadowing by solid particles with increasing concentration of red mud: 400 µE (panel A), 200 µE (panel B), 100 µE (panel C). Mean light intensity of the control cultures (−RM) was adjusted so it matched the mean light intensities of the corresponding red mud treated culture.
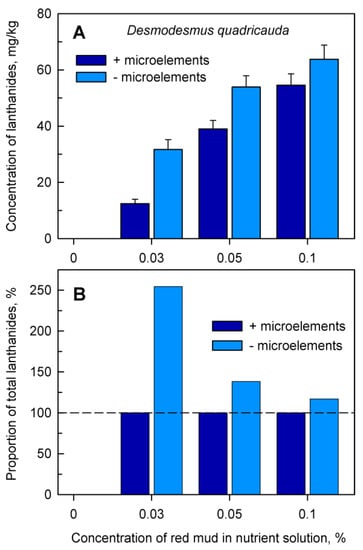
Figure 5.
Total amount of accumulated lanthanides in Desmodesmus quadricauda cells (Panel A) after 48 h of growth in the absence (0%) or in the presence of different concentrations (0.03, 0.05, 0.1%) of red mud, either in complete nutrient medium (+microelements) or in medium without added microelements (−microelements). Panel B: Proportion of total lanthanides in cells grown in nutrient medium without added microelements normalized to the content of lanthanides in cells grown in complete nutrient medium (set to 100%, highlighted by dashed horizontal line).
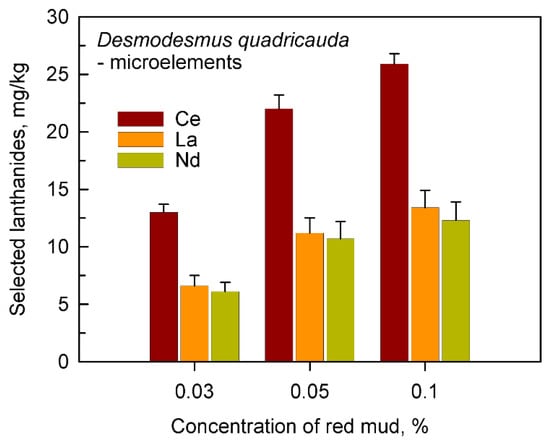
Figure 6.
The content of cerium, lanthanum and neodymium in cells of Desmodesmus quadricauda grown in incomplete nutrient medium lacking added microelements in the presence of 0.03, 0.05 and 0.1% (w/v) of red mud.
Growth of algal cultures was followed as changes in cell number over a 5 day period. During this period, the cultures underwent lag and exponential phases of growth and entered into the stationary phase of growth. The stationary phase was reached already after 3 days in culture grown in incomplete medium (−m) (Figure 4, blue lines) and after 4 days of growth in complete nutrient medium (+m) (Figure 4, red lines).
The findings illustrated in Figure 4 revealed that the cells not only could grow in red mud in incomplete nutrient medium without added microelements but they grew better than in control red mud-free medium (Figure 4A,B). This effect was most pronounced at low mean light intensity at high concentrations of red mud (Figure 4C) suggesting that the presence of red mud is advantageous for the full supply of elements missing from the nutrient medium. Such cultures grew to the same cell concentrations (Figure 4C, blue circles) as the cultures grown in complete nutrient medium, either in the absence (Figure 4C, red squares) or at a high concentration of red mud (Figure 4C, red circles).
Analyses of the accumulation of total lanthanides in cells provided evidence that at any concentration of red mud, cultures grown in nutrient medium lacking added microelements accumulated significantly higher levels of lanthanides (Figure 5, light blue columns) than those grown in complete nutrient medium. (Figure 5, dark blue columns). When the lanthanide content in the cells grown in complete medium in the presence of microelements was set as 100% (Figure 5B, dark blue columns), the proportion of lanthanides decreased from 250% to 117% of the control value with increasing concentrations of red mud (Figure 5B, light blue columns).
The same was also confirmed in more detailed analyses for the most frequently found individual lanthanides Ce, La, and Nd (Figure 6). The absolute level of Ce, La and Nd in cultures grown in the absence of added microelements and in the presence of any concentration of red mud was higher than in cells grown in complete nutrient medium containing microelements. This is well illustrated when comparing cells grown in complete medium in the presence of microelements from Figure 3 or in the absence of added microelements (Figure 6).
2.4. Localization of Lanthanides in Algal Cells
To verify that lanthanides from red mud found in algal biomass by ICP-MS had accumulated intracellularly and were not simply adsorbed to cell walls, the cells of three experimental algal species were stained with the fluorescent dye Fluo-4 (see Materials and Methods).
The increased fluorescence signal is a consequence of lanthanide cations binding to the Fluo-4 dye. Staining showed frequent small bright bodies, presumably containing lanthanides in cells grown in the presence of 0.1% red mud (Figure 7D–F). These bodies were not found in untreated cells (Figure 7A–C). The lanthanides were localized both in chloroplasts and the cytoplasm (see arrows in Figure 7D–F).
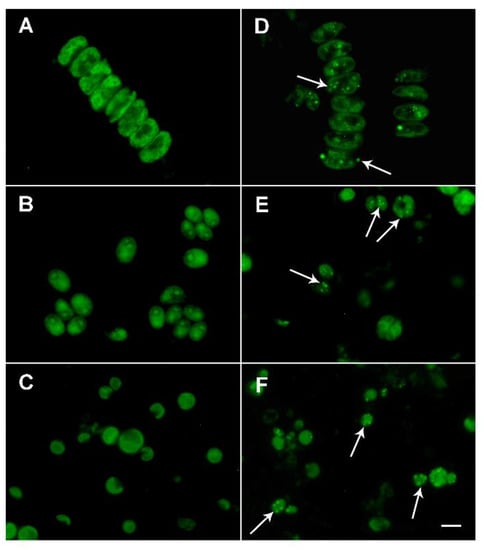
Figure 7.
Fluorescence microphotographs after Fluo-4 staining of Desmodesmus quadricauda (A,D), Chlamydomonas reinhardtii (B,E) and Parachlorella kessleri (C,F) grown in the absence (A–C) or in the presence (D–F) of 0.1% red mud. Intracellular localization of lanthanides inside the red mud-treated cell are seen as small bright-green bodies, some of them indicated by arrows. The scale bar is 10 µm.
3. Discussion
3.1. Red Mud as a Source of Valuable Metals
Production of red mud has increased, providing an almost inexhaustible resource, but due to its toxicity, it is a burden on the environment. Among red mud contaminants, arsenic (As) is of considerable concern [31]. The concentration of As in our samples collected 1–1.2 m deep in the Almásfüzítő landfill (100 mg/kg) exceeded the standards for Protection of Aquatic Life, where As levels in water are regarded as being toxic at concentrations ≥50 µg/L and sediments are toxic at As levels ≥17 mg/kg [32]. On the other hand, the summed content of lanthanides in red mud was about 170 mg per kg of red mud, from which about 80% form the most industrially important elements, cerium, neodymium and lanthanum (Table 1), implying it could serve as a possible source of lanthanides. Besides lanthanides, red mud could also be a valuable source of other metals, such as iron [33] or microelements, which, in the case of algae, are required for their growth. Thus, red mud appears to be a potential raw material for the recovery of valuable lanthanides and a broad range of minor trace elements and, especially in the case of lanthanides, it seems more appropriate to speak about re-mining rather than re-cycling.
3.2. Bio-Mining of Red Mud by Green Algae
Our study showed that at least three species of green algae, D. quadricauda, C. reinhardtii and P. kessleri, not only grow but also divide their cells in the harsh red mud environment (Figure 1). All the selected species have been extensively used as model organisms in basic physiological research of algae, cell cycle regulation, molecular biology as well as in biotechnological applications [34,35,36,37] and are well established as rapidly growing and tolerant to possible toxins. Moreover, they were able to efficiently accumulate lanthanides (and other essential metals) from the red mud (Figure 2). Despite different growth rates, all the strains showed a reduction in growth rates in higher red mud concentrations (Figure 1 and Figure 4; Table 3). This could be caused either by red mud toxicity or by light limiting conditions in highly concentrated suspensions of red mud (Figure 8). When the growth conditions were normalized to light intensity (Figure 4), the presence of red mud did not have a toxic but rather a beneficial effect on algal growth. This suggests the reduced growth rates are dictated rather by light conditions and not by red mud toxicity.
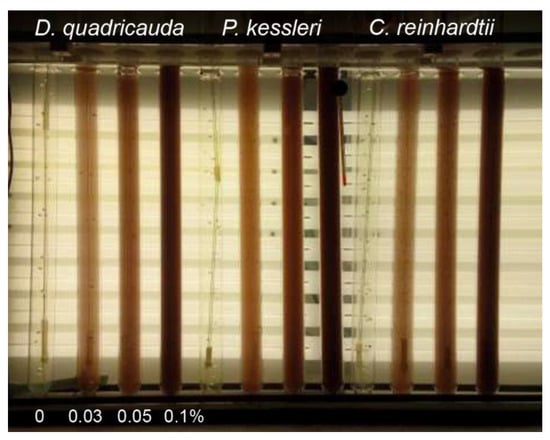
Figure 8.
Photobioreactor used for experiments. The photobioreactor consisted of glass cylinders placed in thermostatic water bath illuminated from one side by a panel of dimmable fluorescent lamps. The experimental species cultures were grown either in absence (0%) (control) or in different red mud concentrations (0.03, 0.05, 0.1%).
The intracellular content of both total (Figure 2) and individual lanthanides (Figure 3) increased with increasing concentrations of red mud. This was valid for all three algal species tested even though efficiencies of lanthanide absorption increased from P. kessleri to C. reinhardtii and then D. quadricauda (see Figure 1, Figure 2 and Figure 3). The three algae accumulated the highest concentration of cerium, followed by lanthanum and neodymium thus reflecting the proportion of lanthanides in red mud (Table 1). At least five-times more lanthanides were accumulated if cultured with 0.1% than with 0.03% red mud (Table 1, Figure 3). This pattern was the same for all taxa, indicating that the mechanism of lanthanide uptake was not species-specific and that the increase in lanthanide accumulation could be driven by higher concentrations of red mud or by a beneficial effect of some red mud component(s) (see below). It has been established that light lanthanides (La, Ce, Pr, Nd) are taken up by organisms preferentially because they are present in the environment at the highest concentrations. This was proven in experiments examining lanthanide concentrations from biota of natural systems, where concentrations within an organism varied with the same pattern (light lanthanides > heavy lanthanides) as observed in surrounding environments [38,39]. Our results clearly prove lanthanide bio-mining capacity of aquatic microalgae but additional investigation is needed to understand the detailed mechanisms behind the bioaccumulation of metals from red mud-containing environments. The differences observed could be caused by many factors such as species-specific growth rates, differing growth optima (red mud concentrations and corresponding differences in illumination), and differences in affinities for lanthanide [40,41]. Previously, the removal of lanthanides from sewage water was demonstrated using algae such as Chlorella vulgaris, Isochrysis galbana or Euglena gracilis [42,43,44]. Another organism with proven ability to concentrate and recover critical rare elements from contaminated water is Gracilaria gracilis [44]. In many studies, Desmodesmus spp. was used as a bioremediation agent to remove inorganic and organic substances from polluted water [45,46].
The role of photosynthetic algae in the bioremediation of elements from red mud has not been studied in detail. However, several cyanobacteria and fungi have been tested for bio-leaching of bauxite residue [19,24]. Cyanobacteria were found to be less efficient in the re-vegetation of red mud field deposits than fungi because they suffered more under alkaline conditions and a total lack of energy sources. But if the pH was reduced they formed a crust-like structure on the red mud surface [24]. In contrast, fungi were less vulnerable to harsh conditions of red mud, and could even excrete metabolites (such as organic acids, amino acids, and proteins) and form complexes with metal ions. The fungus RM-10 (originally isolated from red mud), was used to extract metals from red mud because of its ability to drastically reduce the pH of the medium supplemented with high concentrations of bauxite residue [19].
For possible future scale-up culturing of algae for bio-mining of lanthanides from red mud, it is important to make sure that the lanthanides are really bio-absorbed into intracellular structures not just adsorbed onto the cell surface. Thus in our study, cells prepared for analysis of the intracellular content of lanthanides (by ICP-MS) were repeatedly washed to remove compounds or metals adsorbed onto the cell surface. Because it cannot be totally excluded that some lanthanides could remain attached to cell walls, even after intensive washing, direct fluorescence microscopic observation was carried out to verify the intracellular accumulation of lanthanides. The Fluo-4 dye is commonly used as a calcium indicator [47] but lanthanides have a higher affinity for the dye molecules, so it can be used to visualize lanthanides. Lanthanides in the cells were concentrated in bodies of different sizes and locations (Figure 7). Some were inside chloroplasts and others in the cytoplasm. We were not able to distinguish which lanthanides were localized in the individual cell structures. However, a previously published study on the localization of individual lanthanides in D. quadricauda showed that neodymium and cerium were localized specifically in the chloroplast, while lanthanum and gadolinium were in the cytoplasm [29]. Cerium, neodymium and lanthanum were the most predominant lanthanides found in red mud (Table 1; 35.3, 17.6 and 16.4%, respectively), so the observed bodies should comprise at least one of them. However, it is not clear whether the observed bodies were specific for only one lanthanide or a mixture and more detailed experiments with different single lanthanides (as in [29]) are needed. Lanthanide localization in different cellular compartments (chloroplast and cytoplasm) could play an important role in increasing photosynthetic rate by activation of Rubisco occurring in the stromal compartment of chloroplasts [48], while in the cytoplasm, particularly lanthanum could have a function in stabilization of the cytoskeleton and be involved in some intracellular signaling pathways [49]. Separation of lanthanides from other metals is technologically challenging due to their similar chemical properties. Thus, an understanding of specific compartmentalization of lanthanides in algal cells might be useful for their biotechnological separation by cell fractionation.
3.3. Beneficial Effects of Red Mud on Algal Growth
Red mud contains many different elements including some routinely added to algal nutrient media as microelements. Moreover, it also contains other metals necessary for algal growth, such as calcium, magnesium, and iron. The addition of the elements to the growth medium in pure form increases the price of algal cultivation, especially in a scaled up system. Thus, it was of interest to determine whether the red mud could supplement such micronutrients when they are absent from the nutrient medium. Indeed, D. quadricauda grew in the red mud even in the absence of added microelements. With increasing red mud concentration there was a noticeably better growth in the presence of red mud over the control cultures. The growth improvement was best evidenced at the highest red mud concentrations and low mean light intensity (Figure 4C). This could be caused by two factors or their combination. Firstly, more metals would be present at high red mud concentrations and they might accumulate more in the cells. Secondly, at low light intensity, the growth of algae is limited and any advantage (e.g., presence of micronutrients) compared to controls will be most evident. Cultures grown in the presence of red mud but the absence of added microelements accumulated more total (Figure 5) and individual lanthanides (Ce, La, Nd) (Figure 6) than cultures grown in complete nutrient medium with added microelements (Figure 5 and Figure 6). This suggests that nutrient limitation promoted accumulation of lanthanides. High levels of accumulation demonstrated in algal cells was previously observed and explained through their ability to form metabolites chelated with compounds [50]. Cultures might also have profited from the presence of red mud compounds, stimulated by bio-absorption of essential metal ions required for microbial metabolic activity and thus achieve higher growth rates than expected [19,40,41].
Given the higher accumulation of lanthanides under conditions of nutrient limitation, their presence might be another possible explanation for growth improvement. Complete nutrient medium contains optimal concentrations of all compounds necessary for growth and reproduction of algae [51]. The only metals not present in nutrient medium, or in the control cells, are the lanthanides. Numerous papers have reported that lanthanides, can stimulate growth and development of plants [52] despite the fact that they are not essential elements. Lanthanides have been demonstrated to exhibit diverse physiological effects on plants and animals [53] due to their involvement in different metabolic pathways, photosynthesis, membrane stability, and stress resistance [54]. They can interact with a great number of biological macromolecules to form stable complexes. Consequently, they were found to act as a substitute for Ca2+ ions in some biological functions [55,56]. Although almost all experiments were carried out on higher plants, the substitution of missing calcium by lanthanides was also demonstrated in algae [57]. The feasibility of this hypothesis and identification of affected metabolic processes remains a challenge for future research.
Thus, green algae could be used for bio-mining of lanthanides from red mud as well as for red mud recycling. Algal intracellular accumulation capacity increased with red mud concentration perhaps due to beneficial effects of lanthanides. Interestingly, red mud was able to replace the micronutrients normally added to algal growth medium, thus, decreasing the price of algal cultivation, especially in large scale. Moreover, under micronutrient limitation conditions, lanthanide accumulation further improved. This study provides an initial examination of the bio-mining capacity of green algae. At this point, there are at least two possible downstream applications of algal biomass enriched in lanthanides. The biomass could be directly used as fertilizer [30]. Alternatively, the lanthanides could be isolated from the enriched biomass either chemically or after biomass fractionation, taking into account the information on lanthanide localization to different cell compartments.
4. Materials and Methods
4.1. Microalgal Strains
For these experiments, three model organisms were used: Desmodesmus quadricauda (Turpin) Brébisson (previously named as Scenedesmus quadricauda, strain Greifswald/15) and Parachlorella kessleri, strain 255, were obtained from the Culture Collection of Autotrophic Organisms, Institute of Botany (CCALA, Czech Acad. Sci., Třeboň, Czech Republic), Chlamydomonas reinhardtii wild-type strain CC-1690 was obtained from Chlamydomonas Resource Center at (University of Minnesota, St. Paul, MN, USA). All the cultures were obtained sterile and were maintained under sterile conditions both for routine sub-culturing and for the experiments. Cultures of D. quadricauda and P. kessleri were cultivated in liquid mineral medium as described before (Table 4, upper part) [58,59]. Cultures of C. reinhardtii were cultured in modified HS medium (Table 4, middle part) [60]. The cultures were maintained by sub-culturing to appropriate nutrient medium solidified by agar approximately every three weeks and grown at room temperature on a light shelf illuminated by an incident light intensity of 100 µmol/m2/s.
4.2. Laboratory Experimental Photobioreactor
A set of glass cylinders (inner diameter 36 mm, height 500 mm, volume 300 mL) were placed in a thermostatic bath (30 °C) and continuously illuminated from one side by a panel of dimmable fluorescent lamps (DULUX L55 W/950 Daylight, OSRAM, Milano, Italy) allowing adjustment of the incident light intensity from 16 to 780 µmol/m2/s. The cylinders were ‘‘aerated’’ using a mixture of air and CO2 (2%, v/v) at a flow rate of 15 L/h. The pH of cultures was maintained in the range 6.5–7.5 by the addition of 1 M NaOH. The experiments were carried out in a batch culture regime.
4.3. Measurement of Light Intensity
For routine light measurement, a quantum/radiometer-photometer (LI-COR, Lincoln, NE, USA) was used. For mean light intensity measurements in the cylinders, an ULM-500 light meter (WALZ, Effeltrich, Germany) equipped with microspherical quantum sensor US-SQS/L was used. To obtain a measure of light energy absorbed by a layer of cell suspension grown at different incident light intensities and different optical densities (concentrations of cells), the mean light intensity (Im) was calculated according to the Lambert–Beer formula: Im = (Ii It)/ln(Ii/It), where Ii is the incident light intensity measured by the microspherical quantum sensor US-SQS/L positioned in geometrical center of the cultivation cylinder filled with clear nutrient medium and It is the transmitted light intensity measured at the same position in cultivation cylinder filled either with algal suspension only (control) or by algal suspension supplemented by corresponding amount of red mud suspension (red mud treated cultures).
4.4. Preparation of Experimental Cultures
Cultures were inoculated from plates and cultivated for approximately three days at 30 °C and at a continuous incident light intensity of 500 μmol/m2/s. The number of cells in the pre-grown cultures was counted in a Bürker chamber (Meopta, Přerov, Czech Republic) and the cultures for the experiment were diluted to the same initial cell concentration (8 × 105 cells/mL) at the beginning of the experiment and then cultivated in batch culture without any dilution until the end of the experiment (2–5 days). For the experiments, the microalgae were cultivated at a constant temperature (30 °C) and under continuous light of incident light intensity 500 μmol/m2/s.
The composition of incomplete medium lacking added microelements, used for some experiments with D. quadricauda was the standard nutrient medium (Table 4, upper part) without addition of microelements (Table 4, bottom part). To prepare cultures limited for microelements, cells of D. quadricauda from the pre-grown culture were pelleted and washed twice with medium lacking added microelements (Table 4, upper part) before resuspension to a known cell concentration (8 × 105 cells/mL) in the same medium. Cultures were cultivated in batch culture for 3 days. The pH of the red mud-supplemented nutrient medium (measured by OrionTM Versa Star ProTM pH meter, ThermoFisher Scientific, Waltham, MA, USA) was in the range from 6.5 to 7.5 at the beginning of cultivation, providing consistent conditions for growth and development of microalgae.
4.5. Determination of Cell Number
The number of algal cells was followed by counting cells under transmitted light in a Bürker counting chamber using a BX51 microscope (Olympus, Tokyo, Japan). Values were expressed as the number of cells/mL.
4.6. Estimation of Growth Rates of Cultures
The growth rate µ was expressed as a doubling of cell number per day. The growth parameters were calculated according to the formula: Nt = N0 2µ t, from which µ = (log2 (Nt/N0))/t. Parameters N0 and Nt are cell numbers in cultures at the beginning and end of experiment of time interval t; µ is the growth rate expressed in doubling/day.
4.7. Preparation of Samples for ICP-MS
Cultures were centrifuged (ROTINA 380R, HETTICH, Tuttlingen, Germany) at 4750 rpm for 5 min, washed three times in distilled water and the pellet was frozen at −70 °C and freeze-dried. Samples of algal biomass (0.1 g) were digested with 3 mL of 67% HNO3 and 0.5 mL of 30% H2O2 in a PTFE microwave oven (MLS1200 MEGA, Gemini bv, Apeldoorn, Netherland) at 250–600 W for 20 min. After evaporation of excess acid, the resulting solution was transferred to a volumetric flask supplemented with 0.67% HNO3 [57].
4.8. Determination of Element Content (ICP-MS)
For the determination of lanthanide content in the stock solution and in algal biomass, the ICP-MS analytical method was used. ICP-MS measurements were performed using an Elan DRC-e (Perkin Elmer, Concord, ON, Canada) equipped with a concentric PTFE nebuliser, a cyclonic spray chamber, a high-efficiency quartz torch and a dynamic reaction cell (DRC) for the elimination of spectral interference. Distilled and demineralized water (Millipore, Bedford, MA, USA) was used to prepare all solutions. Samples were passed through a 0.45 μm nylon syringe filter and diluted 1:10 using water. Values were expressed as milligram per kilogram (mg/kg) of dry weight [57].
4.9. Fluorescence Microscopy
Fluo-4 fluorescent dye was used to display intensification of the fluorescence signal as a consequence of binding to lanthanide cations. A dense algal suspension (1 mL) from control cultures, and from cultures treated with red mud were centrifuged at 5000× g for 3 min. The cell pellets were washed with 1 mL of 0.1% sodium dodecyl sulfate, mixed well and centrifuged again at 5000× g for 3 min. This washing step was repeated until all possible red mud contaminants were removed (at least three times). The samples were further washed with 1 mL of PBS (phosphate buffered saline), spun at 5000× g for 3 min, and the pellet was re-suspended in 1 mL of PBS. In order to stain algal cells, 100 μL of algal suspension, 150 μL of zirconium beads (diameter 0.7 mm), and 100 μL of 2.5 μM Fluo-4 (Molecular Probes, Eugene, OR, USA) freshly diluted in PBS were mixed in 2-mL Eppendorf tubes and vortexed (Vortex Genie2, Scientific Industries, Bohemia, NY, USA) at 4 °C for 1 min at full speed. To complete the staining process, the samples were incubated at room temperature for 2 h while covered with aluminum foil. The samples were then centrifuged at 5000× g for 3 min and re-suspended in 100 μL of PBS. To observe the location of the lanthanides, 15 μL of cell suspension was put onto a glass slide and mixed with SlowFade Gold Antifade Reagent (Molecular Probes), preventing the fluorescent dye from fading. For microscopy, a BX51 fluorescence microscope (Olympus) equipped with U-MWIBA2 filter block (excitation 460–490 nm/emission 510–550 nm) was used. Photomicrographs were taken using a DP72 digital camera [29].
4.10. Red Mud
4.10.1. Red Mud Sampling
The red mud used for this study was provided by Envirotis Holding zrt. (Budapest, Hungary) (www.envirotis.hu), a company concerned with reclamation of red mud deposits. Red Mud originated in Depo #7 of the red mud disposal site, located in Almásfüzítő, Hungary, situated on the banks of the river Danube. The coordinates of the sampling site are (N 59°21′33′′; W 26°47′18′′), close to the sonic core drilling #25. The samples were collected from approx. 1–1.2 m depth measured from the red mud surface. At this depth the texture of red mud resembles jelly and wet soil, as opposed to the top surface of the deposit being carbonated due to CO2 uptake from the atmosphere.
4.10.2. Red Mud Treatment
To determine the effect of red mud on the selected species of microalgae and to verify their ability to take up lanthanides, red mud-supplemented nutrient medium was used for cultivation. The red mud stock solution was prepared by mixing 10% (w/v) red mud with de-ionized water and sterilized by autoclaving. The red mud solution was added to the appropriate nutrient medium to a final concentration (w/v) of 0.03%, 0.05% and 0.1%, forming a suspension.
Author Contributions
Conceptualization, M.V.; Data curation, D.M., K.B. and V.Z.; Investigation, M.Č., V.N. and V.L.; Methodology, M.R. and T.M.T.; Supervision, M.V.; Visualization, V.Z.; Writing—original draft, M.Č. and V.N.; Writing—review & editing, M.Č., D.M., M.R., T.M.T., V.N., V.L., K.B., V.Z. and M.V.
Funding
This work was supported by the program Interreg V-A: ATCZ172, the National Program of Sustainability I. ID: LO1416, and by Institutional Research Concept no. AV0Z61388971.
Acknowledgments
We are grateful to Balász Kovács, Envirotis Holding, Budapest, Hungary for co-operation and supply of red mud samples for our experiments. We thank Piotr Pawlica for his help during experiments. We acknowledge J. D. Brooker for critical reading and language editing of the text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, Z.Z.; Wang, Z.L.; Li, J.; Li, Y.; Zhang, Z.G.; Zhang, P. Distribution of rare earth elements in sewage-irrigated soil profiles in Tianjin, China. J. Rare Earths 2012, 30, 609–613. [Google Scholar]
- European Commission. Study on the Review of the List of Critical Raw Materials. Critical Raw Materials Factsheets. Catalogue Number ET-04-15-307-ENN. 2017. Available online: https://publications.europa.eu/en/publication-detail/-/publication/7345e3e8-98fc-11e7-b92d-01aa75ed71a1/language-en (accessed on 15 May 2018).
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metallurgy 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Wang, W.W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Separ. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Ujaczki, E.; Feigl, V.; Molnar, M.; Cusack, P.; Curtin, T.; Courtney, R.; O’Donoghue, L.; Davris, P.; Hugi, C.; Evangelou, M.W.; et al. Re-using bauxite residues: Benefits beyond (critical raw) material recovery. J. Chem. Technol. Biotechnol. 2018, 93, 2498–2510. [Google Scholar]
- Cusack, P.B.; Courtney, R.; Healy, M.G.; O’Donoghue, L.M.T.; Ujaczki, E. An evaluation of the general composition and critical raw material content of bauxite residue in a storage area over a twelve-year period. J. Clean. Prod. 2019, 208, 393–401. [Google Scholar] [CrossRef]
- Liu, Y.J.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar]
- Abreu, R.D.; Morais, C.A. Purification of rare earth elements from monazite sulphuric acid leach liquor and the production of high-purity ceric oxide. Miner. Eng. 2010, 23, 536–540. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, K.; Kutschke, S.; Matys, S.; Raff, J.; Hlawacek, G.; Lederer, F.L. Bio-recycling of metals: Recycling of technical products using biological applications. Biotechnol. Adv. 2018, 36, 1048–1062. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Mohan, S.V.; Lens, P.N.L. Biological and bioelectrochemical recovery of critical and scarce metals. Trends Biotechnol. 2016, 34, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining–biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y. Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ. J. Sci. Technol. 2016, 50, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lian, B.; Mo, B.B.; Liu, C.Q. Bioleaching of heavy metals from red mud using Aspergillus niger. Hydrometallurgy 2013, 136, 71–77. [Google Scholar] [CrossRef]
- Horiike, T.; Yamashita, M. A New Fungal Isolate, Penidiella sp. Strain T9, Accumulates the Rare Earth Element Dysprosium. Appl. Environ. Microbiol. 2015, 81, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and recovery of critical rare elements from contaminated waters by living Gracilaria gracilis. J. Hazard. Mater. 2018, 344, 531–538. [Google Scholar] [CrossRef]
- Ponou, T.; Wang, L.P.; Dodbiba, G.; Okaya, K.; Fujita, T.; Mitsuhashi, K.; Atarashi, T.; Satoh, G.; Noda, M. Recovery of rare earth elements from aqueous solution obtained from Vietnamese clay minerals using dried and carbonized parachlorella. J. Environ. Chem. 2014, 2, 1070–1081. [Google Scholar] [CrossRef]
- Dubey, K.; Dubey, K.P. A study of the effect of red mud amendments on the growth of cyanobacterial species. Bioremed. J. 2011, 15, 133–139. [Google Scholar] [CrossRef]
- Kang, L.; Shen, Z.; Jin, C. Neodymium cations Nd3+ were transported to the interior of Euglena gracilis 277. Chin. Sci. Bull. 2000, 45, 585–592. [Google Scholar] [CrossRef]
- Shen, H.; Ren, Q.G.; Mi, Y.; Shi, X.F.; Yao, H.Y.; Jin, C.Z.; Huang, Y.Y.; He, W.; Zhang, J.; Liu, B. Investigation of metal ion accumulation in Euglena gracilis by fluorescence methods. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 189, 506–510. [Google Scholar] [CrossRef]
- Guo, A.; Wang, J.; Li, X.; Zhu, J.; Reinert, T.; Heitmann, J.; Spemann, D.; Vogt, J.; Flagmeyer, R.H.; Butz, T. Study of metal bioaccumulation by nuclear microscope analysis of algae fossils and living algae cells. Nucl. Instrum. Meth. Phys. Res. Sect. B 2000, 161–163. [Google Scholar]
- Shen, C.D.; Xu, J.R.; Yu, J.F. Effect of the rare earth element of Eu on the growth and chlorophyll content of Chlorella vulgaris. Freshw. Fish. 2003, 33, 23–26. [Google Scholar]
- Řezanka, T.; Kaineder, K.; Mezricky, D.; Řezanka, M.; Bišová, K.; Zachleder, V.; Vítová, M. The effect of lanthanides on photosynthesis, growth, and chlorophyll profile of the green alga Desmodesmus quadricauda. Photosynth. Res. 2016, 130, 335–340. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Vivekanand, V.; Pareek, N. Photoautotrophic microorganisms and bioremediation of industrial effluents: Current status and future prospects. 3 Biotech 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Olszewska, J.P.; Meharg, A.A.; Heal, K.V.; Carey, M.; Gunn, I.D.M.; Searle, K.R.; Winfield, I.J.; Spears, B.M. Assessing the legacy of red mud pollution in a shallow freshwater lake: Arsenic accumulation and speciation in macrophytes. Environ. Sci. Technol. 2016, 50, 9044–9052. [Google Scholar] [CrossRef]
- EEC Council Directive 76/464/EEC on Pollution Caused by Certain Dangerous Substances Discharged into the Aquatic Environment of the Community (Dangerous Substances Directive)–List II Substances. Off. J. Eur. Communities 1976, L129, 23–29.
- Laguna, C.; Gonzalez, F.; Garcia-Balboa, C.; Ballester, A.; Blazquez, M.L.; Munoz, J.A. Bioreduction of iron compounds as a possible clean environmental alternative for metal recovery. Miner. Eng. 2011, 24, 10–18. [Google Scholar] [CrossRef]
- Schroda, M.; Hemme, D.; Muhlhaus, T. The Chlamydomonas heat stress response. Plant. J. 2015, 82, 466–480. [Google Scholar] [CrossRef]
- Zachleder, V.; Bišová, K.; Vítová, M. The cell cycle of microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland; Heidelberg, Germnay; New York, NY, USA; Dordrecht, the Netherlands; London, UK, 2016; pp. 3–46. [Google Scholar]
- Umen, J.G. Sizing up the cell cycle: Systems and quantitative approaches in Chlamydomonas. Curr. Opin. Plant Biol. 2018, 46, 96–103. [Google Scholar] [CrossRef]
- Vitova, M.; Bisova, K.; Kawano, S.; Zachleder, V. Accumulation of energy reserves in algae: From cell cycles to biotechnological applications. Biotechnol. Adv. 2015, 33, 1204–1218. [Google Scholar] [CrossRef]
- Gonzalez, V.; Vignati, D.A.L.; Pons, M.N.; Montarges-Pelletier, E.; Bojic, C.; Giamberini, L. Lanthanide ecotoxicity: First attempt to measure environmental risk for aquatic organisms. Environ. Pollut. 2015, 199, 139–147. [Google Scholar] [CrossRef]
- Yang, G.; Wilkinson, K.J. Biouptake of a rare earth metal (Nd) by Chlamydomonas reinhardtii—Bioavailability of small organic complexes and role of hardness ions. Environ. Pollut. 2018, 243, 263–269. [Google Scholar] [CrossRef]
- Mishra, V.K.; Upadhyay, A.R.; Pathak, V.; Tripathi, B.D. Phytoremediation of mercury and arsenic from tropical opencast coalmine effluent through naturally occurring aquatic macrophytes. Water Air Soil Pollut. 2008, 192, 303–314. [Google Scholar] [CrossRef]
- Goecke, F.; Aránguiz-Acuña, A.; Palacios, M.; Muñoz-Muga, P.; Rucki, M.; Vítová, M. Latitudinal distribution of lanthanides contained in macroalgae in Chile: An inductively coupled plasma-mass spectrometric (ICP-MS) determination. J. Appl. Phycol. 2017, 29, 2117–2128. [Google Scholar] [CrossRef]
- Qu, K.M.; Yuan, Y.; Xin, F. Enhancement of 3 rare earth elements to Isochrysis galbana J. Fish. Sci. Chin. 1998, 5, 42–47. [Google Scholar]
- Wang, X.; Sun, H.; Xu, Z.; Dai, L.; Li, Z.; Chen, Y. The effects and bioconcentration of REE La and its EDTA complex on the growth of algae Chlorella vulgaris Beijerinck. J. Nanjing Univ. 1996, 32, 460–475. (In Chinese) [Google Scholar]
- Ishii, N.; Tagami, K.; Uchida, S. Removal of rare earth elements by algal flagellate Euglena gracilis. J. Alloys Compd. 2006, 408–412, 417–420. [Google Scholar] [CrossRef]
- Martinez, M.E.; Sanchez, S.; Jimenez, J.M.; El Yousfi, F.; Munoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Biores. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Kim, G.Y.; Yun, Y.M.; Shin, H.S.; Kim, H.S.; Han, J.I. Scenedesmus-based treatment of nitrogen and phosphorus from effluent of anaerobic digester and bio-oil production. Biores. Technol. 2015, 196, 235–240. [Google Scholar] [CrossRef]
- Gee, K.R.; Brown, K.A.; Chen, W.N.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and physiological characterization of Fluo-4 Ca2+-indicator dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Liu, C.; Hong, F.-S.; Wu, K.; Ma, H.-B.; Zhang, X.-G.; Hong, C.-J.; Wu, C.; Gao, F.-Q.; Yang, F.; Zheng, L.; et al. Effect of Nd3+ ion on carboxylation activity of ribulose-1,5-bisphosphate carboxylase/oxygenase of spinach. Biochem. Biophys. Res. Commun. 2006, 342, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hasenstein, K.H. La3+ uptake and its effect on the cytoskeleton in root protoplasts of Zea mays L. Planta 2005, 220, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Richter, H.; Sparovek, G.; Schnug, E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: A review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Li, X.; Přibyl, P.; Bišová, K.; Kawano, S.; Cepák, V.; Zachleder, V.; Čížková, M.; Brányiková, I.; Vítová, M. The microalga Parachlorella kessleri—A novel highly-efficient lipid producer. Biotechnol. Bioeng. 2013, 110, 97–107. [Google Scholar] [CrossRef]
- Kastori, R.; Maksimovic, I.; Zeremski-Skoric, T.; Putnik-Delic, M. Rare earth elements: Yttrium and higher plants. Zbornik Matice Srpske za Prirodne Nauke 2010, 87–98. [Google Scholar] [CrossRef]
- Wang, X.P.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Distribution of rare earth elements among chloroplast components of hyperaccumulator Dicranopteris dichotoma. Anal. Bioanal. Chem. 2003, 376, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Abu Bakar, N.K.; Abu Bakar, A.F.; Ashraf, M.A. Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: A review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789. [Google Scholar] [CrossRef] [PubMed]
- Squier, T.C.; Bigelow, D.J.; Fernandezbelda, F.J.; Demeis, L.; Inesi, G. Calcium and lanthanide binding in the sarcoplasmic-reticulum atpase. J. Biol. Chem. 1990, 265, 13713–13720. [Google Scholar]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Rare earth elements in biological systems. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Eyring, L., Eds.; Elsevier: North Holland, The Netherland, 1990; pp. 423–452. [Google Scholar]
- Goecke, F.; Jerez, C.; Zachleder, V.; Figueroa, F.L.; Bišová, K.; Řezanka, T.; Vítová, M. Use of lanthanides to alleviate the effects of metal ion-deficiency in Desmodesmus quadricauda (Sphaeropleales, Chlorophyta). Front. Microbiol. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Vítová, M.; Bišová, K.; Hlavová, M.; Zachleder, V.; Rucki, M.; Čížková, M. Glutathione peroxidase activity in the selenium-treated alga Scenedesmus quadricauda. Aquat. Toxicol. 2011, 102, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Teixeira, J.; Dragone, G.; Vicente, A.A.; Kawano, S.; Bišová, K.; Přibyl, P.; Zachleder, V.; Vítová, M. Relationship between starch and lipid accumulation induced by nutrient depletion and replenishment in the microalga Parachlorella kessleri. Bioresour. Technol. 2013, 144, 268–274. [Google Scholar] [CrossRef]
- Sueoka, N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1960, 46, 83–91. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).