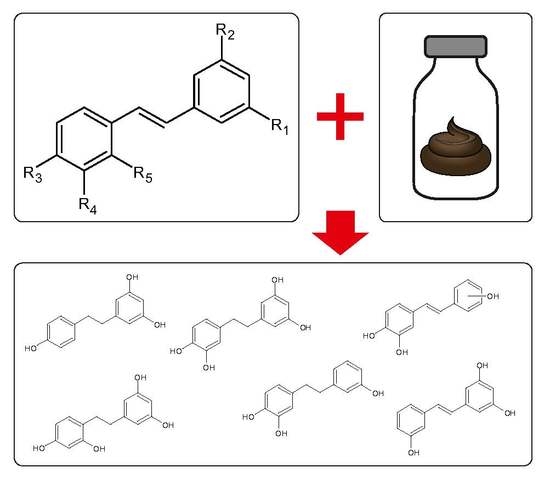
Metabolism of Stilbenoids by Human Faecal Microbiota
Abstract
1. Introduction
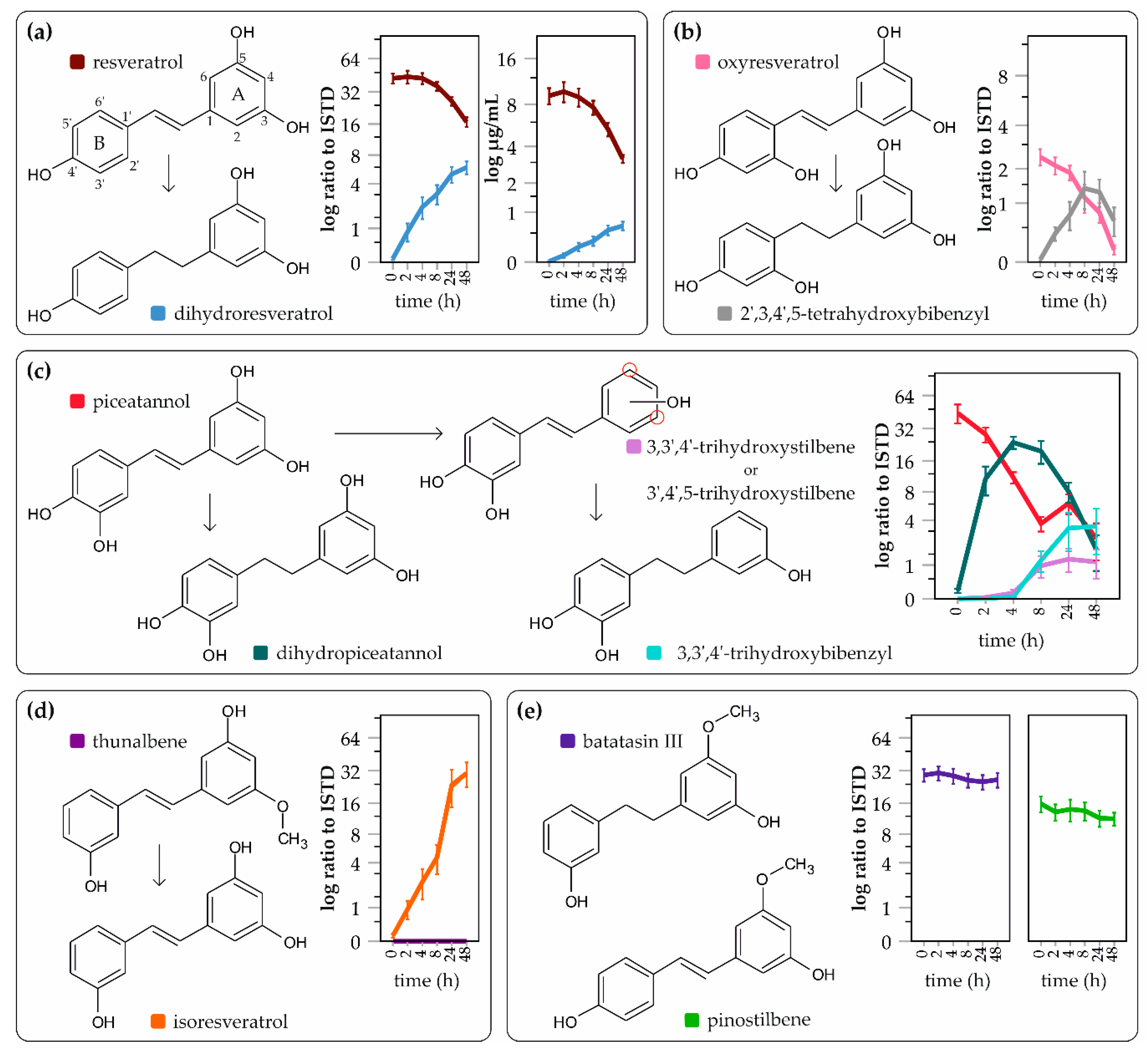
2. Results
3. Discussion
4. Materials and Methods
4.1. In vitro Faecal Fermentation System
4.1.1. Fermentation Medium
4.1.2. Sodium Phosphate Buffer and Reducing Solution
4.1.3. Stilbenoid Preparation
4.1.4. Faecal Samples and Ethics Statement
4.1.5. In Vitro Incubations
4.2. LC/MS analyses
4.2.1. Standards
4.2.2. Sample Purification
4.2.3. LC/MS Analysis of Metabolites
4.2.4. Statistical Evaluation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
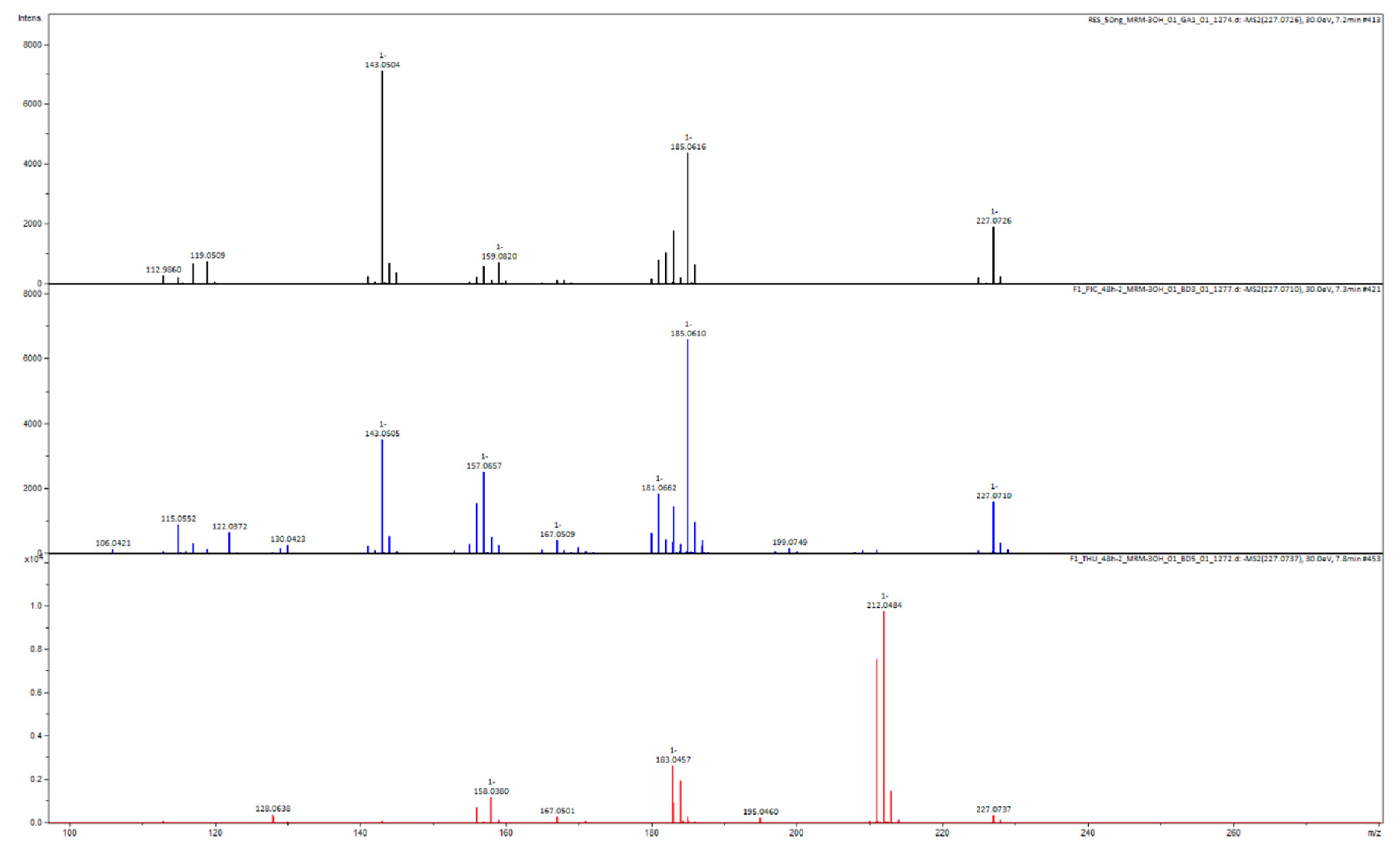
Appendix B

Appendix C
| Name | Molecular Formula | Neutral Molecule Exact Mass | Measured [M − H]− Exact Mass | Comparison with Standard |
|---|---|---|---|---|
| monitored: | ||||
| thunalbene | C15H14O3 | 242.0943 | 241.0865 | YES |
| pinostilbene | C15H14O3 | 242.0943 | 241.0865 | YES |
| piceatannol | C14H12O4 | 244.0736 | 243.0657 | YES |
| oxyresveratrol | C14H12O4 | 244.0736 | 243.0657 | YES |
| batatasin III | C15H16O3 | 244.1099 | 243.1021 | YES |
| resveratrol | C14H12O3 | 228.0786 | 227.0708 | YES |
| lunularin | C14H14O2 | 214.0994 | 213.0916 | YES |
| detected: | ||||
| dihydroresveratrol | C14H14O3 | 230.0943 | 229.0865 | YES |
| 2,3′,4,5′-tetrahydroxybibenzyl | C14H14O4 | 246.0892 | 245.0814 | NO |
| dihydropiceatannol | C14H14O4 | 246.0892 | 245.0814 | NO |
| trihydroxystilbene | C14H12O3 | 228.0786 | 227.0708 | NO |
| 3,3′,4-trihydroxybibenzyl | C14H14O3 | 230.0943 | 229.0865 | NO |
| isoresveratrol | C14H12O3 | 228.0786 | 227.0708 | NO |
References
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Leláková, V.; Šmejkal, K.; Jakubczyk, K.; Veselý, O.; Landa, P.; Václavík, J.; Bobá’, P.; Pížová, H.; Temml, V.; Steinacher, T.; et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019, 285, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Kairisalo, M.; Bonomo, A.; Hyrskyluoto, A.; Mudò, G.; Belluardo, N.; Korhonen, L.; Lindholm, D. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci. Lett. 2011, 488, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Buryanovskyy, L.; Fu, Y.; Boyd, M.; Ma, Y.; Hsieh, T.C.; Wu, J.M.; Zhang, Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry 2004, 43, 11417–11426. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wilk, S.A.; Wang, A.; Zhou, L.; Wang, R.H.; Ogawa, W.; Deng, C.; Dong, L.Q.; Liu, F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 2010, 285, 36387–36394. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.A.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jäger, W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure-activity relationship. Bioorganic Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.N.; Potdar, S.; Tapias, V.; Parmar, M.; Mizuno, C.S.; Rimando, A.; Cavanaugh, J.E. Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J. Nutr. Biochem. 2018, 54, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, C.; Xiang, W.; Chen, H.; Qin, X.; Huang, X. Advances in the study of oxyresveratrol. Int. J. Pharmacol. 2014, 10, 44–54. [Google Scholar] [CrossRef]
- Kantartzis, K.; Fritsche, L.; Bombrich, M.; Machann, J.; Schick, F.; Staiger, H.; Kunz, I.; Schoop, R.; Lehn-Stefan, A.; Heni, M.; et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes. Metab. 2018, 20, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Di, Y.; Jiang, Z.; Xu, J. Resveratrol improves efficacy of oral amoxicillin against childhood fast breathing pneumonia in a randomized placebo-controlled double blind clinical trial. Microb. Pathog. 2018, 114, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2018, 45, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; Van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295. [Google Scholar] [CrossRef]
- Tsang, S.W.; Guan, Y.F.; Wang, J.; Bian, Z.X.; Zhang, H.J. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: Implication for treatment of acute pancreatitis. Sci. Rep. 2016, 6, 22859. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-S.; Ku, C.F.; Guan, Y.-F.; Xiao, H.-T.; Shi, X.-K.; Wang, H.-Q.; Bian, Z.-X.; Tsang, S.W.; Zhang, H.-J. Dihydro-Resveratrol Ameliorates Lung Injury in Rats with Cerulein-Induced Acute Pancreatitis. Phytother. Res. 2016, 30, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.M.; Heinze, T.M.; Schnackenberg, L.K.; Mullis, L.B.; Elkins, S.A.; Elkins, C.A.; Steele, R.S.; Sutherland, J.B. Interaction of dietary resveratrol with animal-associated bacteria. FEMS Microbiol. Lett. 2009, 297, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hang, T.; Wu, C.; Liu, W. Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 829, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, J.D.; Jarosova, V.; Vesely, O.; Mekadim, C.; Mrazek, J.; Marsik, P.; Killer, J.; Smejkal, K.; Kloucek, P.; Havlik, J. Effect of selected stilbenoids on human fecal microbiota. Molecules 2019, 24, 744. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of Stilbenoids by Human Faecal Microbiota. Molecules 2019, 24, 1155. https://doi.org/10.3390/molecules24061155
Jarosova V, Vesely O, Marsik P, Jaimes JD, Smejkal K, Kloucek P, Havlik J. Metabolism of Stilbenoids by Human Faecal Microbiota. Molecules. 2019; 24(6):1155. https://doi.org/10.3390/molecules24061155
Chicago/Turabian StyleJarosova, Veronika, Ondrej Vesely, Petr Marsik, Jose Diogenes Jaimes, Karel Smejkal, Pavel Kloucek, and Jaroslav Havlik. 2019. "Metabolism of Stilbenoids by Human Faecal Microbiota" Molecules 24, no. 6: 1155. https://doi.org/10.3390/molecules24061155
APA StyleJarosova, V., Vesely, O., Marsik, P., Jaimes, J. D., Smejkal, K., Kloucek, P., & Havlik, J. (2019). Metabolism of Stilbenoids by Human Faecal Microbiota. Molecules, 24(6), 1155. https://doi.org/10.3390/molecules24061155