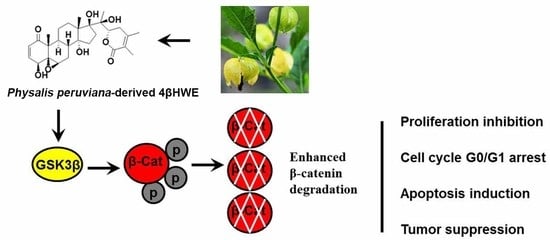
Physalis peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo
Abstract
1. Introduction
2. Results
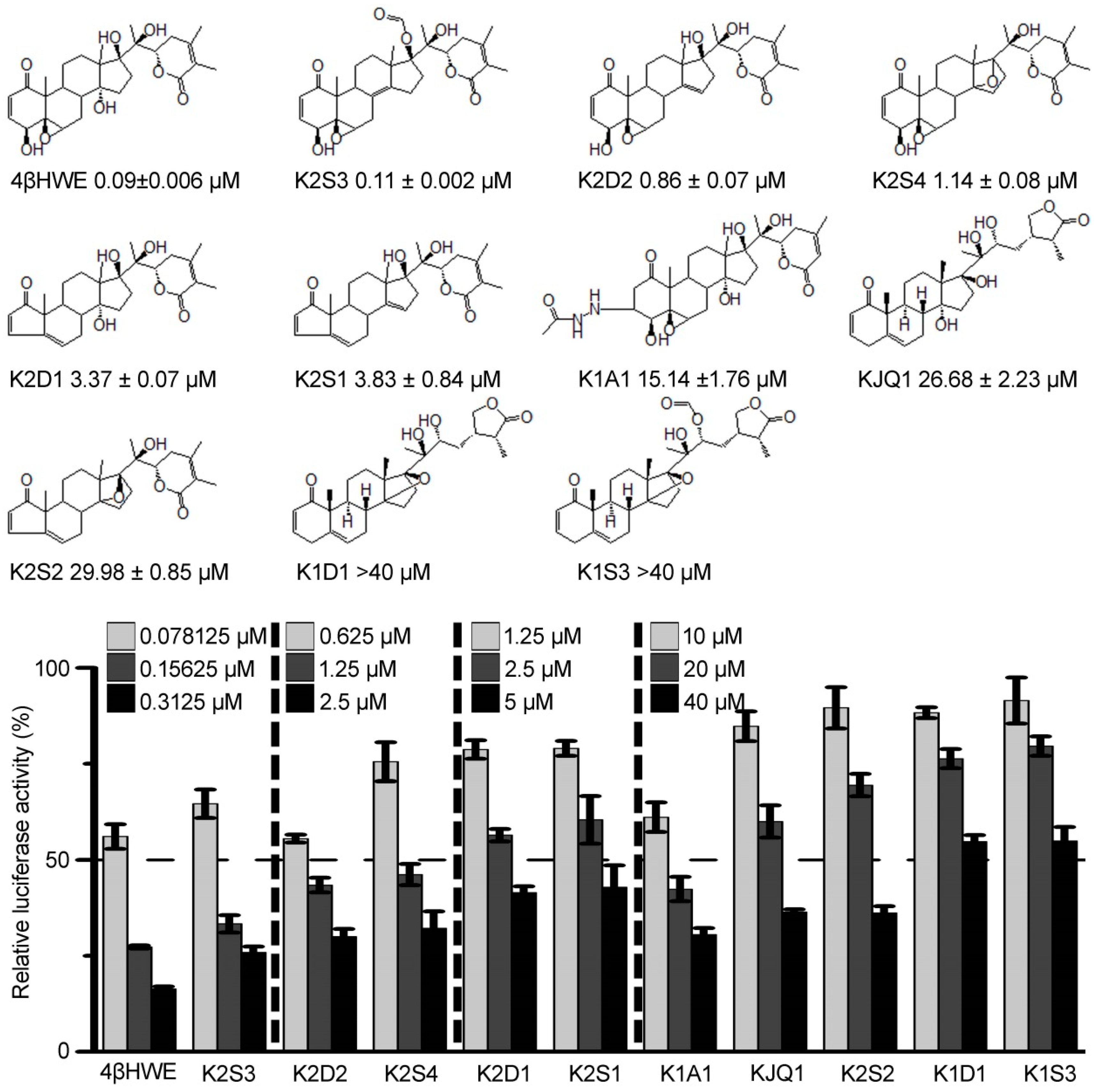
2.1. 4βHWE is A Potent Inhibitor of the Wnt/β-Catenin Pathway
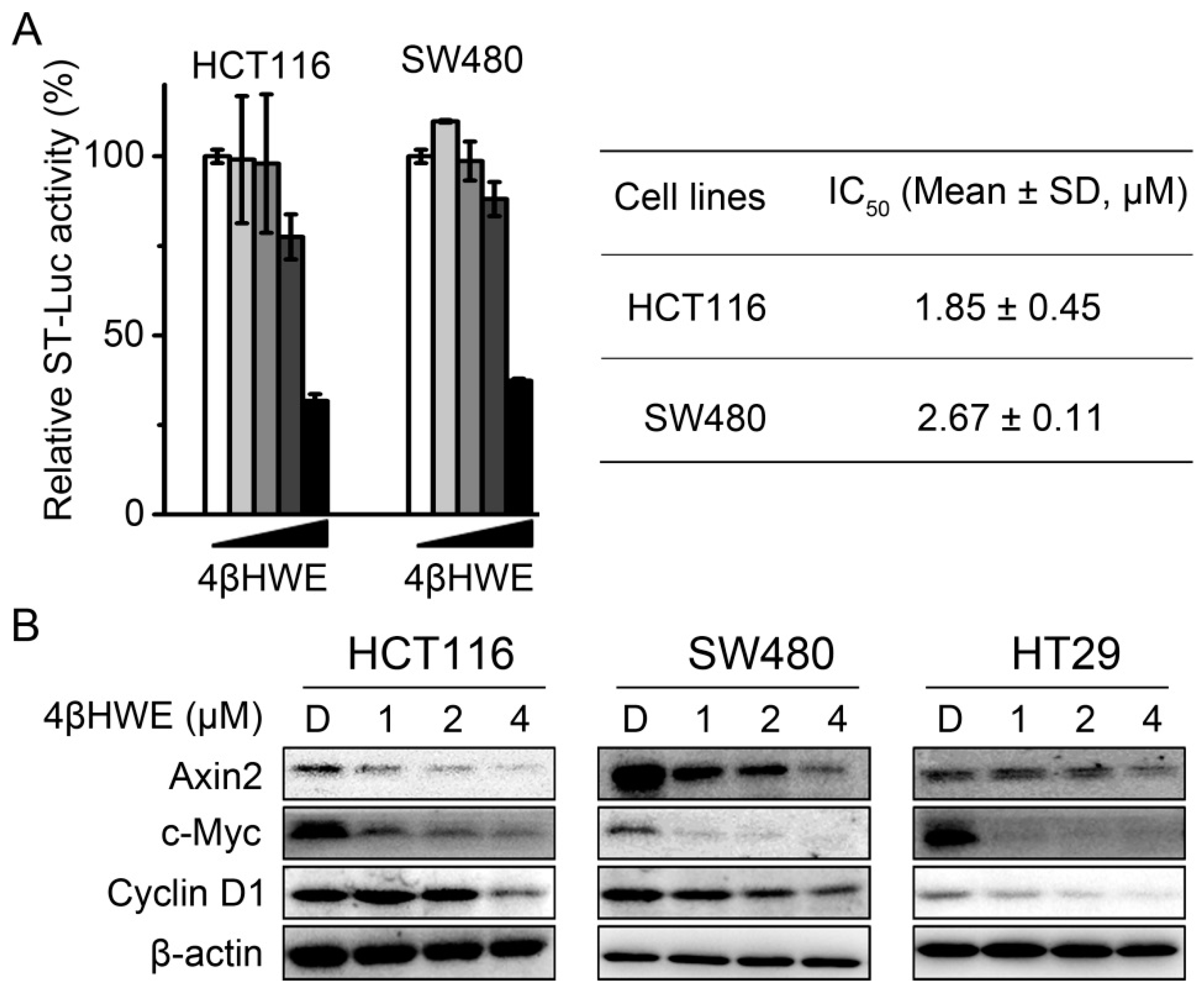
2.2. 4βHWE Interferes with the Wnt Signaling in Colorectal Cancer Cells
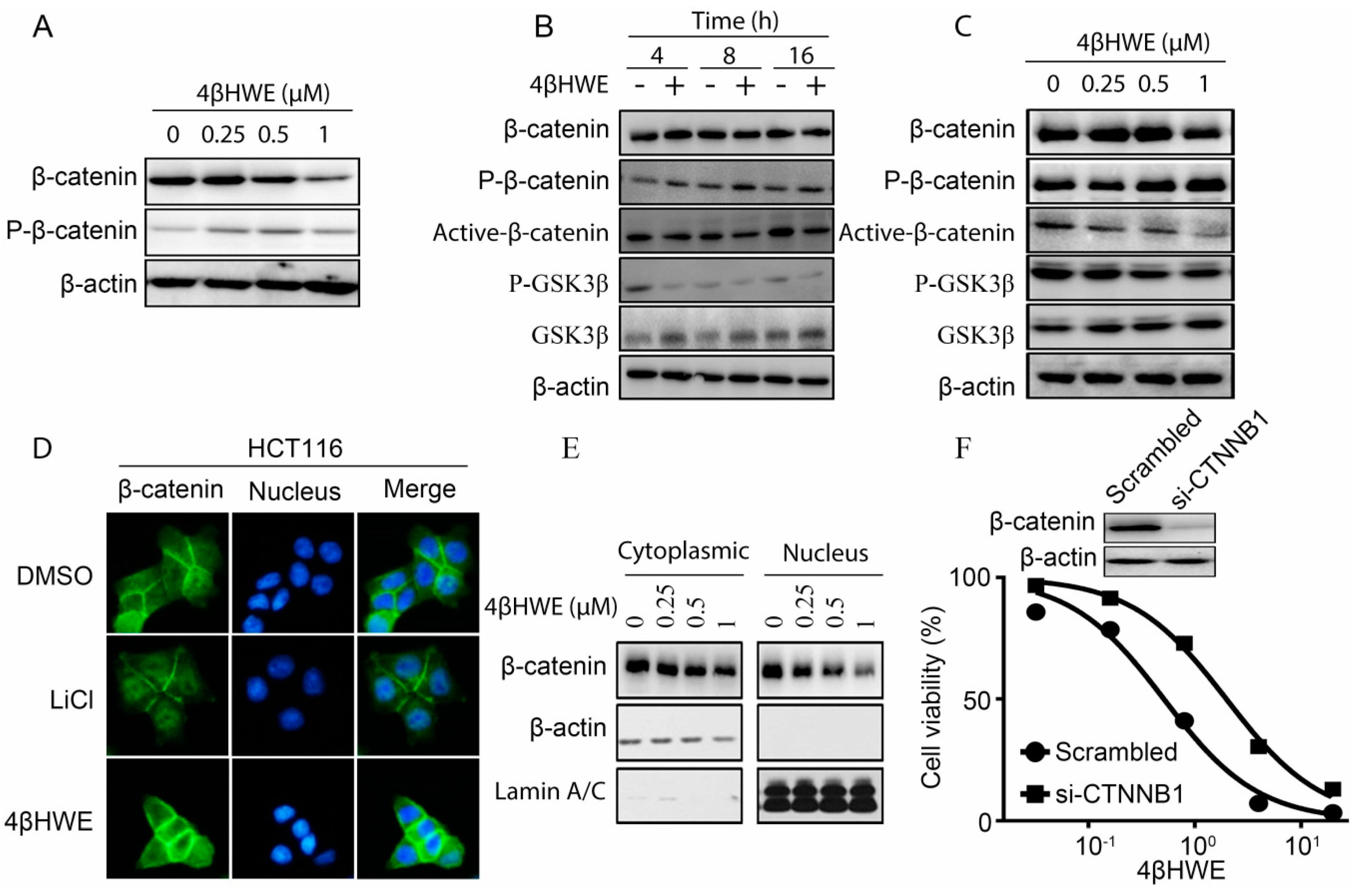
2.3. 4βHWE Inhibits the Stability and Nuclear Translocation of β-Catenin in CRC
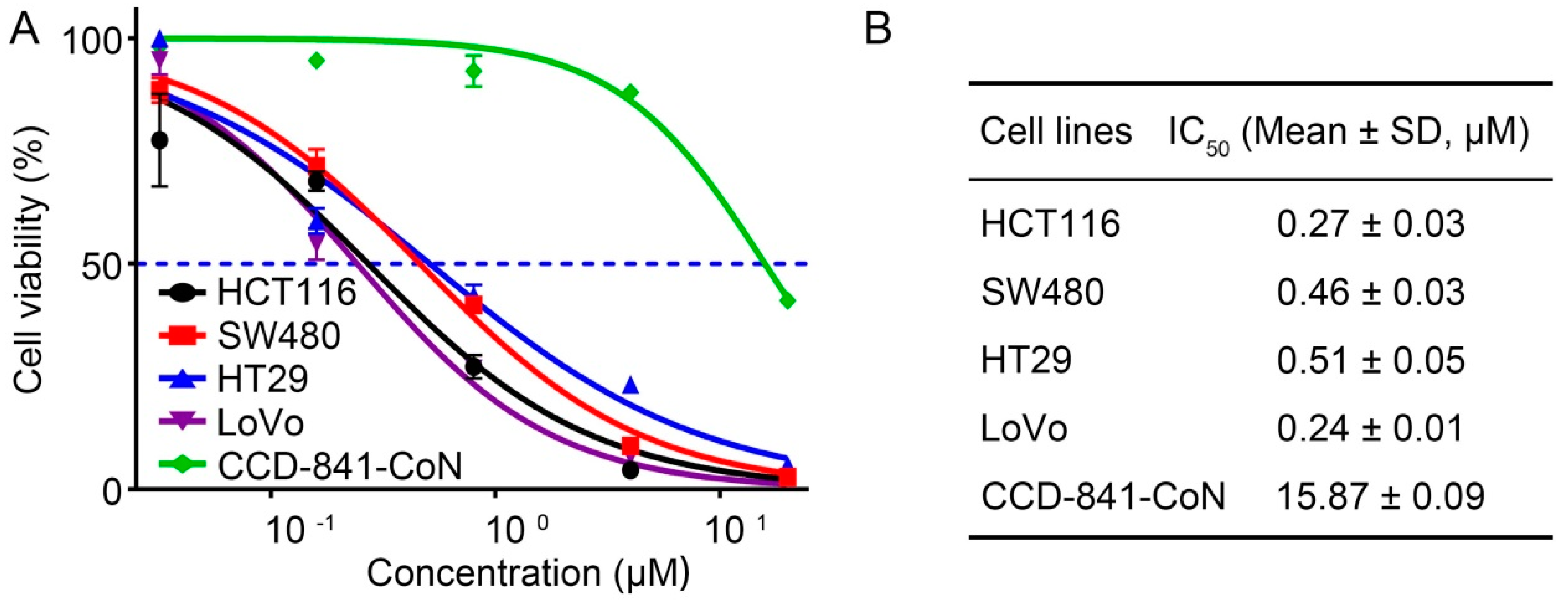
2.4. 4βHWE Selectively Suppresses the Proliferation of CRC
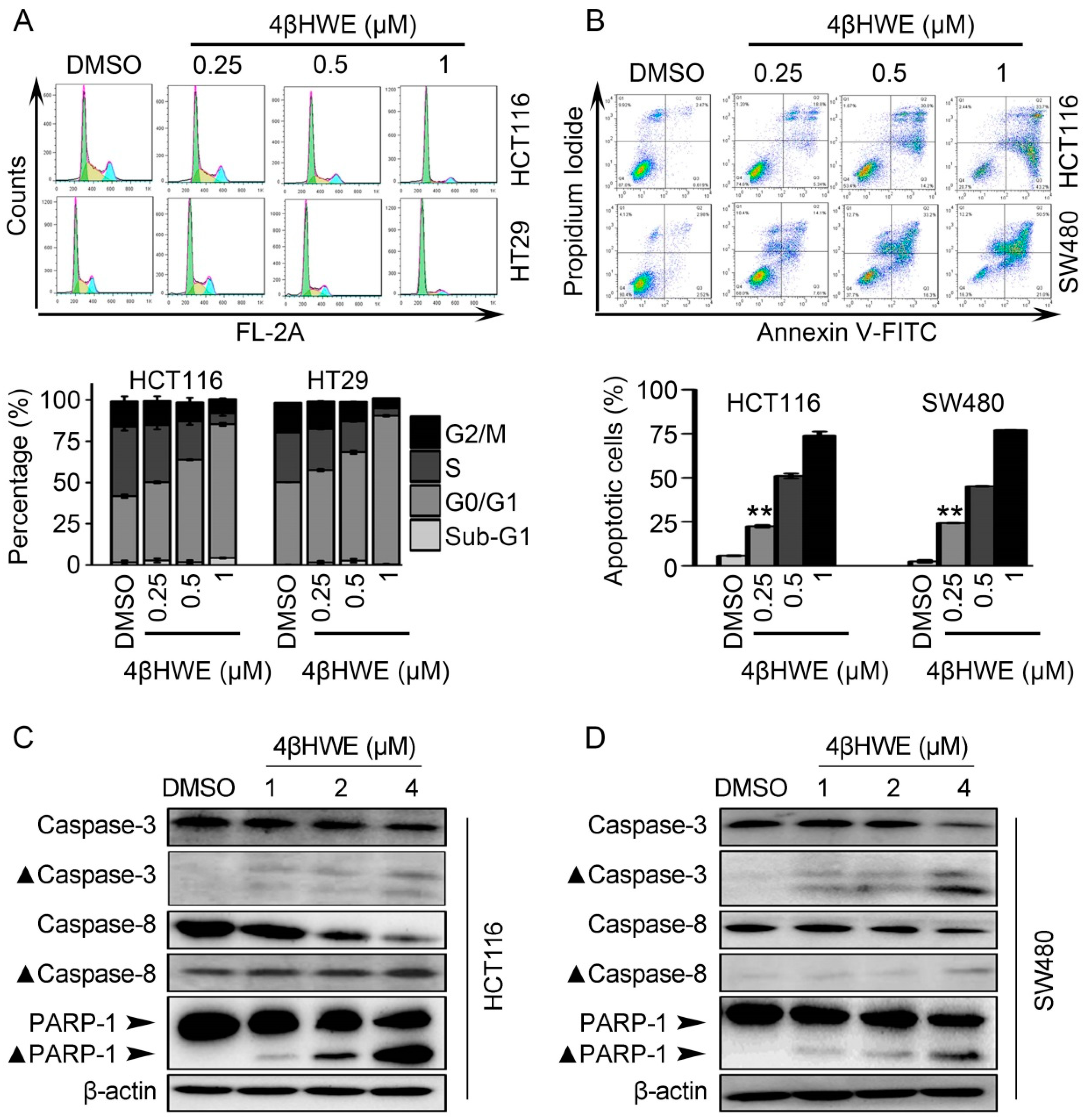
2.5. 4βHWE causes G0/G1 Cell Cycle Arrest and Induces Apoptosis in CRC
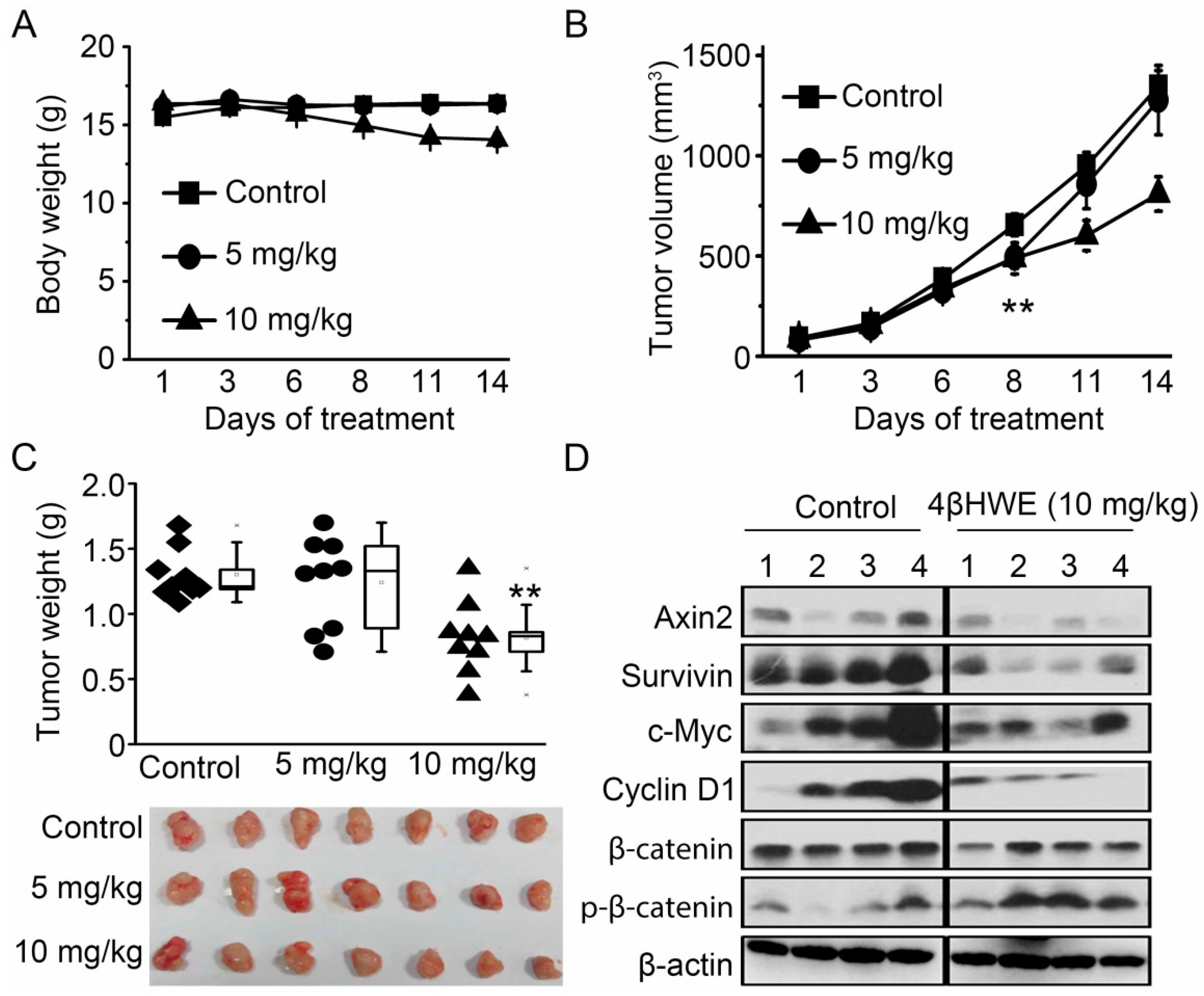
2.6. 4βHWE Suppresses In Vivo Tumor Growth Through Downregulation of Wnt Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Transfection and Luciferase Reporter Assay
4.3. Western Blotting Assay
4.4. Nuclear and Cytoplasmic Fractionation
4.5. Small Interfering RNAs
- Scrambled control: 5’-UUCUCCGAACGUGUCACGU-3’;
- CTNNB1: 5’-AGCUGAUAUUGAUGGACAG-3’.
4.6. Immunofluorescence Staining
4.7. MTS Assay
4.8. Cell Cycle Analysis
4.9. Cell Apoptosis Analysis
4.10. HCT116 Colorectal Xenografts
Author Contributions
Funding
Conflicts of Interest
References
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Nusse, R. Wnt signaling in disease and in development. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef]
- Moon, R.T.; Kohn, A.D.; De Ferrari, G.V.; Kaykas, A. WNT and beta-catenin signalling: Diseases and therapies. Nat. Rev. Genet. 2004, 5, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Oving, I.M.; Clevers, H.C. Molecular causes of colon cancer. Eur. J. Clin. Investig. 2002, 32, 448–457. [Google Scholar] [CrossRef]
- Tarapore, R.S.; Siddiqui, I.A.; Mukhtar, H. Modulation of Wnt/beta-catenin signaling pathway by bioactive food components. Carcinogenesis 2012, 33, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T.; Chen, C.H.; Lin, D.L.; Wang, S.S.; Lin, C.C. Antihepatoma activity of Physalis angulata and P. peruviana extracts and their effects on apoptosis in human Hep G2 cells. Life Sci. 2004, 74, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T.; Lin, D.L.; Huang, S.N.; Wang, S.S.; Lin, C.C. Physalis peruviana extract induces apoptosis in human Hep G2 cells through CD95/CD95L system and the mitochondrial signaling transduction pathway. Cancer Lett. 2004, 215, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Ishii, H.; Kobayashi, S.; Iwao, T. Isolation of 4 beta-hydroxywithanolide E, a new withanolide from Physalis peruviana L. Chem. Pharm. Bull. 1976, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Kirson, I.; Abraham, A.; Sethl, P.D.; Subramanlan, S.S.; Glotter, E. 4β-Hydroxywithanolide E, a new steroid with a 17α-oriented side-chain. Phytochemistry 1976, 15, 340–342. [Google Scholar] [CrossRef]
- Takimoto, T.; Kanbayashi, Y.; Toyoda, T.; Adachi, Y.; Furuta, C.; Suzuki, K.; Miwa, T.; Bannai, M. 4beta-Hydroxywithanolide E isolated from Physalis pruinosa calyx decreases inflammatory responses by inhibiting the NF-kappaB signaling in diabetic mouse adipose tissue. Int. J. Obes. 2014, 38, 1432–1439. [Google Scholar] [CrossRef]
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef]
- Ray, A.B.; Gupta, M. Withasteroids, a growing group of naturally occurring steroidal lactones. Fortschr. Chem. Org. Nat. 1994, 63, 1–106. [Google Scholar]
- Machin, R.P.; Veleiro, A.S.; Nicotra, V.E.; Oberti, J.C.; Padrón, J.M. Antiproliferative activity of withanolides against human breast cancer cell lines. J. Nat. Prod. 2010, 73, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.Y.; Chiu, C.C.; Chang, F.R.; Chen, J.Y.; Hwang, C.C.; Hseu, Y.C.; Yang, H.L.; Lee, A.Y.; Tsai, M.T.; Guo, Z.L.; et al. 4beta-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest. BMC Cancer 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Haung, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar] [CrossRef] [PubMed]
- You, B.J.; Wu, Y.C.; Lee, C.L.; Lee, H.Z. Non-homologous end joining pathway is the major route of protection against 4beta-hydroxywithanolide E-induced DNA damage in MCF-7 cells. Food. Chem. Toxicol. 2014, 65, 205–212. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.Y.; Yuan, C.M.; Hao, X.J.; Li, Y. A reporter gene system for screening inhibitors of Wnt signaling pathway. Nat. Prod. Bioprospect. 2013, 3, 24–28. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Concordet, J.P.; Lassot, I.; Albert, I.; del los Santos, R.; Durand, H.; Perret, C.; Rubinfeld, B.; Margottin, F.; Benarous, R.; et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 1999, 9, 207–210. [Google Scholar] [CrossRef]
- Sadot, E.; Simcha, I.; Iwai, K.; Ciechanover, A.; Geiger, B.; Ben-Ze’ev, A. Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system. Oncogene 2000, 19, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, X.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J. Biol. Chem. 2006, 281, 10865–10875. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T.; Brown, J.D.; Torres, M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997, 13, 157–162. [Google Scholar] [CrossRef]
- Gao, C.; Chen, G.; Romero, G.; Moschos, S.; Xu, X.; Hu, J. Induction of Gsk3beta-beta-TrCP interaction is required for late phase stabilization of beta-catenin in canonical Wnt signaling. J. Biol. Chem. 2014, 289, 7099–7108. [Google Scholar] [CrossRef]
- Ohishi, K.; Toume, K.; Arai, M.A.; Koyano, T.; Kowithayakorn, T.; Mizoguchi, T.; Itoh, M.; Ishibashi, M. 9-Hydroxycanthin-6-one, a beta-Carboline Alkaloid from Eurycoma longifolia, Is the First Wnt Signal Inhibitor through Activation of Glycogen Synthase Kinase 3beta without Depending on Casein Kinase 1alpha. J. Nat. Prod. 2015, 78, 1139–1146. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Baarsma, H.A.; Konigshoff, M.; Gosens, R. The WNT signaling pathway from ligand secretion to gene transcription: Molecular mechanisms and pharmacological targets. Pharmacol. Ther. 2013, 138, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.N.; Yu, M.Y.; Kong, L.M.; Wang, W.H.; Yang, Y.F.; Liu, J.Q.; Qiu, M.H.; Li, Y. Biflavone Ginkgetin, a Novel Wnt Inhibitor, Suppresses the Growth of Medulloblastoma. Nat. Prod. Bioprospect. 2015, 5, 91–97. [Google Scholar] [CrossRef]
- Dong, X.L.; Zhang, J.X.; Zhou, Z.L.; Ye, Z.N.; Chen, J.H.; Yuan, J.F.; Cao, F.J.; Wang, X.B.; Liu, W.C.; Yu, W.X.; et al. Maslinic acid promotes autophagy by disrupting the interaction between Bcl2 and Beclin1 in rat pheochromocytoma PC12 cells. Oncotarget 2017, 8, 74527–74538. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.M.; Feng, T.; Wang, Y.Y.; Li, X.Y.; Ye, Z.N.; An, T.; Qing, C.; Luo, X.D.; Li, Y. Bisleuconothine A, a bisindole alkaloid, inhibits colorectal cancer cell in vitro and in vivo targeting Wnt signaling. Oncotarget 2016, 7, 10203–10214. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the 4βHWE are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.-N.; Yuan, F.; Liu, J.-Q.; Peng, X.-R.; An, T.; Li, X.; Kong, L.-M.; Qiu, M.-H.; Li, Y. Physalis peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules 2019, 24, 1146. https://doi.org/10.3390/molecules24061146
Ye Z-N, Yuan F, Liu J-Q, Peng X-R, An T, Li X, Kong L-M, Qiu M-H, Li Y. Physalis peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules. 2019; 24(6):1146. https://doi.org/10.3390/molecules24061146
Chicago/Turabian StyleYe, Zhen-Nan, Feng Yuan, Jie-Qing Liu, Xing-Rong Peng, Tao An, Xue Li, Ling-Mei Kong, Ming-Hua Qiu, and Yan Li. 2019. "Physalis peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo" Molecules 24, no. 6: 1146. https://doi.org/10.3390/molecules24061146
APA StyleYe, Z.-N., Yuan, F., Liu, J.-Q., Peng, X.-R., An, T., Li, X., Kong, L.-M., Qiu, M.-H., & Li, Y. (2019). Physalis peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules, 24(6), 1146. https://doi.org/10.3390/molecules24061146