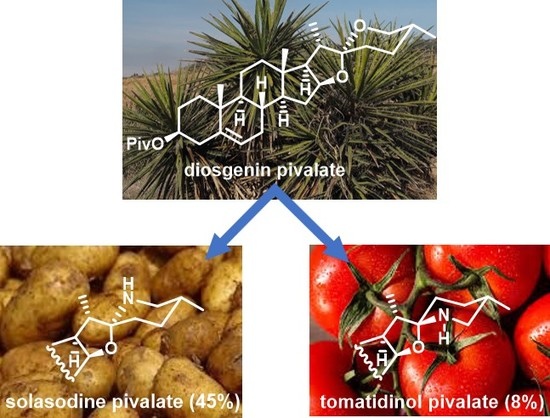
Two-step Synthesis of Solasodine Pivalate from Diosgenin Pivalate
Abstract
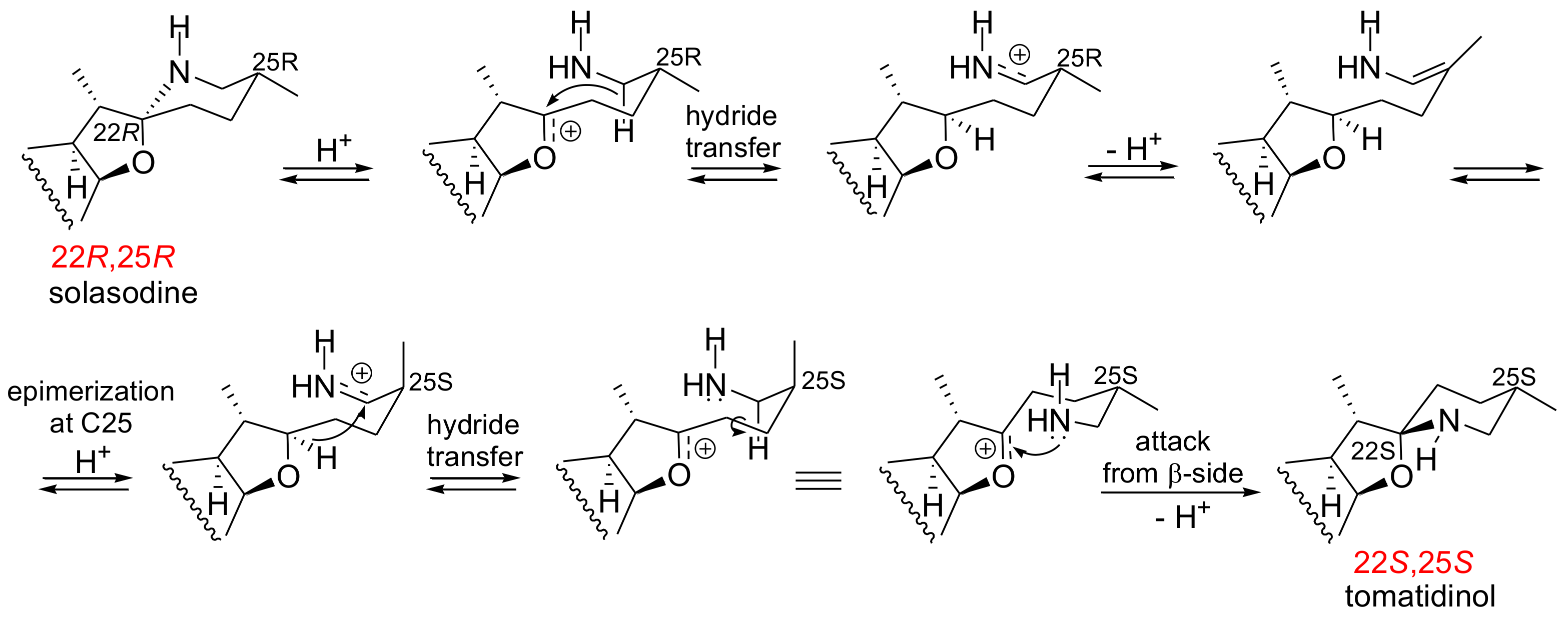
:1. Introduction
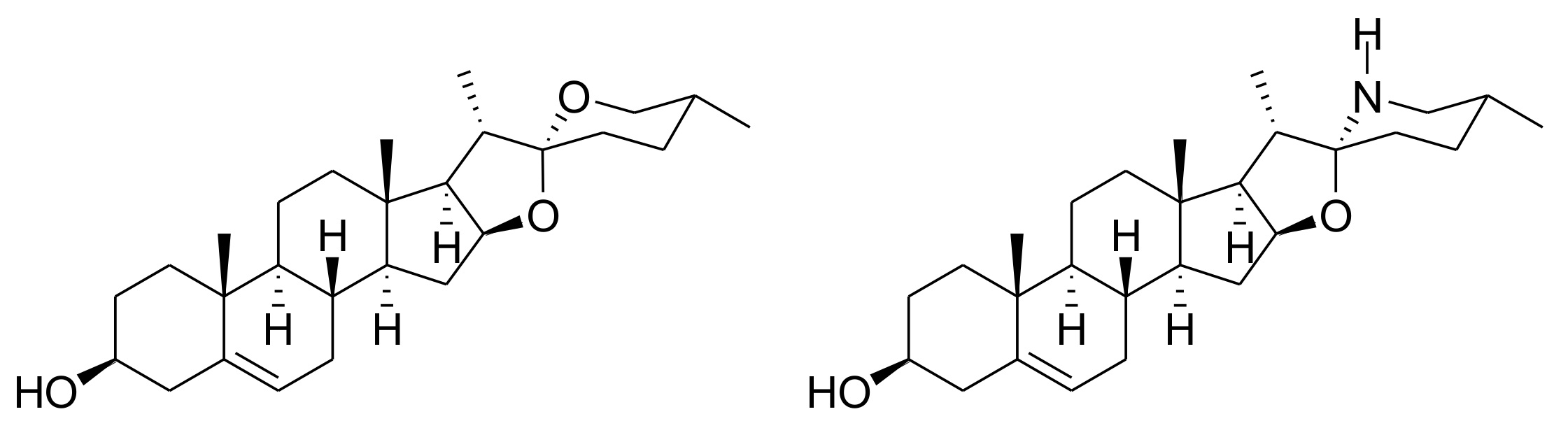
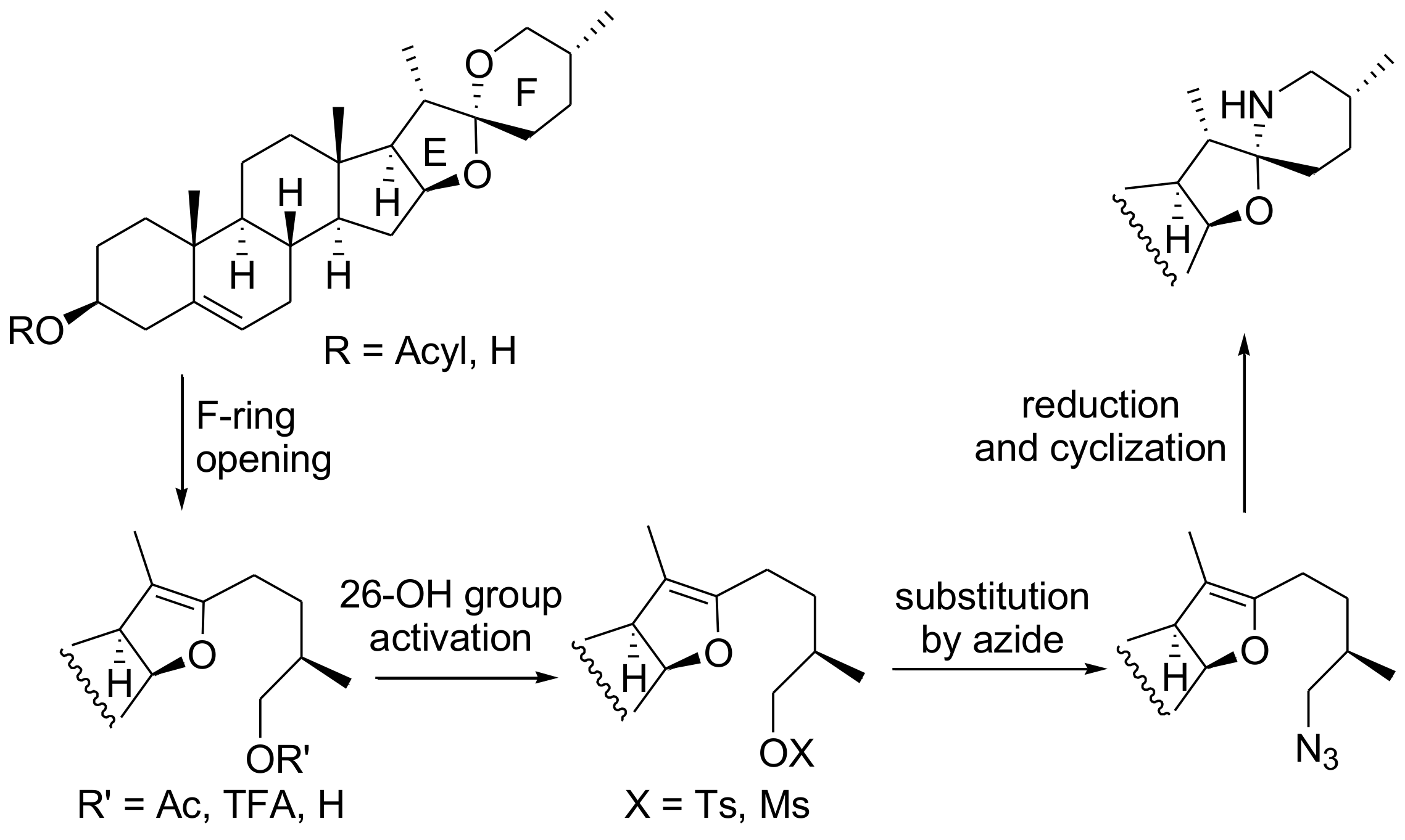
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Chemical Synthesis
3.2.1. Typical Procedure for the Reaction between Diosgenin Pivalate and Carbamate in the Presence of Lewis Acid
3.2.2. Deprotection of the 26-amino Group with AcBr/BuOH
3.2.3. Synthesis of Solasodine Pivalate from Diosgenin Pivalate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinder, A.R. Steroidal Alkaloids. In Supplements to the 2nd edition of Rodd’s Chemistry of Carbon Compounds, Vol. IV: Heterocyclic Compounds; Ansell, M.F., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; Chapter 35; pp. 393–427. [Google Scholar]
- Hale, K.J. Steroidal Alkaloids. In Second Supplements to the 2nd edition of Rodd’s Chemistry of Carbon Compounds, Vol. IV: Heterocyclic Compounds; Sainsbury, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; Chapter 35; pp. 65–112. [Google Scholar]
- Lee, K.R.; Kozukue, N.; Han, J.S.; Park, J.H.; Chang, E.Y.; Baek, E.J.; Chang, J.S.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food. Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Takanori, N.; Chieko, K.; Lee, Y.Y.; Fumio, H.; Shoji, Y.; Toshihiro, N.; Akio, E. Cytotoxic activities of Solanum steroidal glycosides. Biol. Pharm. Bull. 1996, 19, 564–566. [Google Scholar] [CrossRef]
- Cham, B.E.; Daunter, B. Solasodine glycosides. Selective cytotoxicity for cancer cells and inhibition of cytotoxicity by rhamnose in mice with sarcoma 180. Cancer Lett. 1990, 55, 221–225. [Google Scholar] [CrossRef]
- Daunter, B.; Cham, B.E. Solasodine glycosides. In vitro preferential cytotoxicity for human cancer cells. Cancer Lett. 1990, 55, 209–220. [Google Scholar] [CrossRef]
- Lecanu, L.; Hashim, A.I.; McCourty, A.; Giscos-Douriez, I.; Dinca, I.; Yao, W.; Vicini, S.; Szabo, G.; Erdeyli, F.; Greeson, J.; et al. The naturally occurring steroid solasodine induces neurogenesis in vitro and in vivo. Neuroscience 2011, 183, 251–264. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhou, Q.T.; Bai, D.L. Synthesis of several analogues of antifungal steroid alkaloids. Acta Pharm. Sin. 1998, 33, 436–441. [Google Scholar]
- Pandurangan, A.; Khosa, R.L.; Hemaltha, S. Anti-inflammatory activity of an alkaloid from Solanum trilobatum on acute, chronic inflammation models. Nat. Prod. Res. 2011, 25, 1132–1141. [Google Scholar] [CrossRef]
- Kim, Y.C.; Che, Q.M.; Gunatilaka, A.A.L.; Kingston, D.G.I. Bioactive steroidal alkaloids from Solanum umbelliferum. J. Nat. Prod. 1996, 59, 283–285. [Google Scholar] [CrossRef]
- Millward, M.; Powell, A.; Tyson, S.; Daly, P.; Ferguson, R.; Carter, S. Results of phase I clinical trials of Coramsine in patients with advanced solid tumors. J. Clin. Oncol. 2006, 24, 2070. [Google Scholar] [CrossRef]
- Cham, B.E.; Daunter, B.; Evans, R.A. Topical treatment of malignant and premalignant skin lesions by very low concentrations of a standard mixture (BEC) of solasodine glycosides. Cancer Lett. 1991, 59, 183–192. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Kamdem, J.P.; Kade, I.J.; Rocha, J.B.T.; Adanlawo, I.G. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. S. Afr. J. Bot. 2013, 88, 56–61. [Google Scholar] [CrossRef]
- Zha, X.M.; Sun, H.B.; Hao, J.; Zhang, Y.H. Efficient synthesis of solasodine, O-acetylsolasodine, and soladulcidine as anticancer steroidal alkaloids. Chem. Biodivers. 2007, 4, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Uhle, F.C. The transformation of kryptogenin to solasodine. J. Am. Chem. Soc. 1953, 75, 2280–2281. [Google Scholar] [CrossRef]
- Uhle, F.C. The synthesis of azaoxaspirane steroid alkaloids. J. Am. Chem. Soc. 1961, 83, 1460–1472. [Google Scholar] [CrossRef]
- Kessar, S.V.; Gupta, Y.P.; Singh, M.; Mahajan, R.K. Synthetic studies in steroidal sapogenins and alkaloids-X: Syntheses of tomatid-5-ene-3β-ol and solasodine. Tetrahedron 1971, 27, 2869–2875. [Google Scholar] [CrossRef]
- Tang, X.M.; Xu, Q.H.; Wang, J.; Lin, J.R.; Jin, R.H.; Tian, W.S. Synthesis of solasodine by using the intact skeleton of diosgenin. Acta Chim. Sin. 2007, 20, 2315–2319. [Google Scholar]
- Wei, G.; Wang, J.; Du, Y. Total synthesis of solamargine. Bioorg. Med. Chem. Lett. 2011, 21, 2930–2933. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Koag, M.C.; Cheun, Y.; Shin, A.; Lee, S. Application of hypoiodite-mediated aminyl radical cyclization to synthesis of solasodine acetate. Steroids 2012, 77, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-P.; Shen, S.-D.; Lei, M.; Hu, L.-H. A facile and efficient method for the synthesis of solasodine from diosgenin. Tetrahedron 2011, 67, 5894–5896. [Google Scholar] [CrossRef]
- Wu, J.J.; Gao, R.; Shi, Y.; Tian, W.S. Facile synthesis of solasodine based on a mild halogenation-ring opening reaction of spiroketals in steroidal sapogenins. Tetrahedron Lett. 2015, 56, 1215–1217. [Google Scholar] [CrossRef]
- Wu, J.J.; Gao, R.; Shi, Y.; Tian, W.S. Direct amination of EF spiroketal in steroidal sapogenins: An efficient synthetic strategy and method for related alkaloids. Tetrahedron Lett. 2015, 56, 6639–6642. [Google Scholar] [CrossRef]
- Wojtkielewicz, A.; Kiełczewska, U.; Banel, B.; Morzycki, J.W. Study on the reaction of diosgenin acetate with trimethylsilylazide catalyzed by Lewis acids. Steroids. In press. [CrossRef] [PubMed]
- Kozikowski, A.P.; Xia, Y.; Reddy, E.R.; Tückmantel, W.; Hanin, I.; Tang, X.C. Synthesis of huperzine A and Its analogues and their anticholinesterase activity. J. Org. Chem. 1991, 56, 4636–4645. [Google Scholar] [CrossRef]
- Brenner, E.; Baldwin, R.M.; Tamagnan, G. Synthesis of a new precursor to the nicotinic receptor tracer 5-IA-85380 precursor using trimethylsilyl iodide as deblocking agent. Tetrahedron Lett. 2004, 45, 3607–3610. [Google Scholar] [CrossRef]
- Movassaghi, M.; Tjandra, M.; Qi, J. Total Synthesis of (−)-Himandrine. J. Am. Chem. Soc. 2009, 131, 9648–9650. [Google Scholar] [CrossRef] [PubMed]
- Jacquemard, U.; Bénéteau, V.; Lefoix, M.; Routier, S.; Mérour, J.-Y.; Coudert, G. Mild and selective deprotection of carbamates with Bu4NF. Tetrahedron 2004, 60, 10039–10047. [Google Scholar] [CrossRef]
- Boeckman, R.K.; Potenza, J.C. Catechol boron halides: Mild and selective reagents for cleavage of common protecting groups. Tetrahedron Lett. 1985, 26, 1411–1414. [Google Scholar] [CrossRef]
- Yajima, H.; Fujii, N.; Ogawa, H.; Kawatani, H. Trifluoromethanesulphonic acid, as a deprotecting reagent in peptide chemistry. J. Chem. Soc. Chem. Commun. 1974, 107–108. [Google Scholar] [CrossRef]
- Bose, D.S.; Thurston, D.E. Boron trifluoride promoted cleavage of benzyl carbamtes. Tetrahedron Lett. 1990, 31, 6903–6906. [Google Scholar] [CrossRef]
- Yotapan, N.; Paptchikhine, A.; Bera, M.; Avula, S.K.; Vilaivan, T.; Andersson, P.G. Simple proline-derived phosphine-thiazole iridium complexes for asymmetric hydrogenation of trisubstituted olefins. Asian J. Org. Chem. 2013, 2, 674–680. [Google Scholar] [CrossRef]
- Lesk, A.; Nudelman, A. Acetyl bromide-alcohols as convenient reaction systems for: a) removal of N-tert-Boc, N-Cbz And N-Ac protective groups, b) esterifications and transesterifications, c) debenzylation of Aryl-O-Benzyl ethers. Synth. Comm. 1999, 29, 1405–1408. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
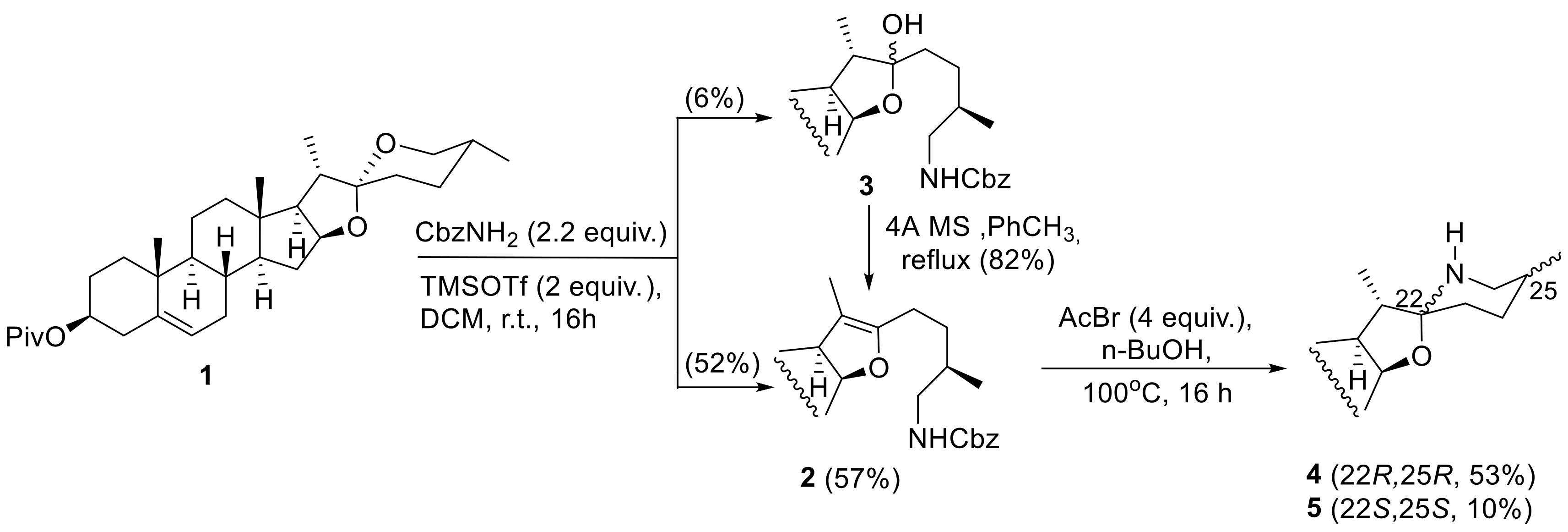
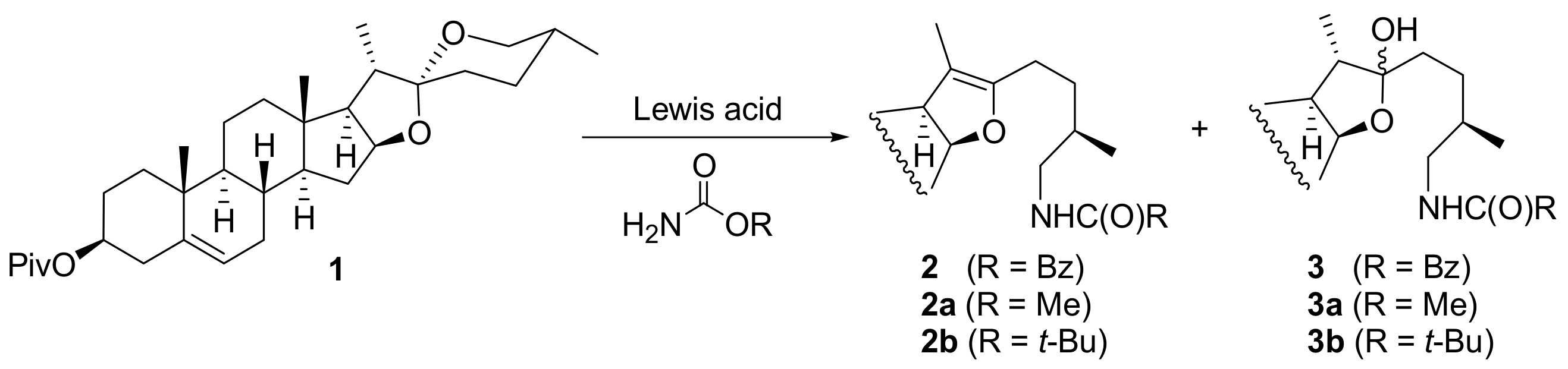 | |||||
|---|---|---|---|---|---|
| Entry | Lewis Acid (Equiv.) | Carbamate (Equiv.) | Solvent | Temp., Time | Product (Yield) |
| 1 | TMSOTf (2) | R = Bn (2.2) | DCM | r.t., 16h | 2 (52%), 3 (6%) |
| 2 | TMSOTf (2) | R = Bn (2.2) | benzene | 40 °C, 16h | 2 (20%), 3 (10%) |
| 3 | BF3xOEt2 (4) | R = Bn (4) | DCM | r.t., 16h | 2 (35%), 3 (15%) |
| 4 | Tf2NH (0.2) | R = Bn (1.5) | DCM | r.t., 16h | 2 (20%), 3 (20%) |
| 5 | TiCl4 (2) | R = Bn (2.2) | DCM | r.t., 16h | 2 (0%), 3 (0%) |
| 6 | TMSOTf (2) | R = Me (2.2) | DCM | r.t., 16h | 2a (36%), 3a (20%) |
| 7 | TMSOTf (2) | R = t-Bu (2.2) | DCM | r.t.-reflux, 48 h | conversion < 10%1 |
| 8 | BF3xOEt2 (4) | R = t-Bu (4) | DCM | r.t., 16h | conversion < 10%1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtkielewicz, A.; Kiełczewska, U.; Morzycki, J.W. Two-step Synthesis of Solasodine Pivalate from Diosgenin Pivalate. Molecules 2019, 24, 1132. https://doi.org/10.3390/molecules24061132
Wojtkielewicz A, Kiełczewska U, Morzycki JW. Two-step Synthesis of Solasodine Pivalate from Diosgenin Pivalate. Molecules. 2019; 24(6):1132. https://doi.org/10.3390/molecules24061132
Chicago/Turabian StyleWojtkielewicz, Agnieszka, Urszula Kiełczewska, and Jacek W. Morzycki. 2019. "Two-step Synthesis of Solasodine Pivalate from Diosgenin Pivalate" Molecules 24, no. 6: 1132. https://doi.org/10.3390/molecules24061132
APA StyleWojtkielewicz, A., Kiełczewska, U., & Morzycki, J. W. (2019). Two-step Synthesis of Solasodine Pivalate from Diosgenin Pivalate. Molecules, 24(6), 1132. https://doi.org/10.3390/molecules24061132