Mycosporine-Like Amino Acids (MAAs) in Time-Series of Lichen Specimens from Natural History Collections
Abstract
1. Introduction
2. Results
2.1. Validation of Analytical Procedure
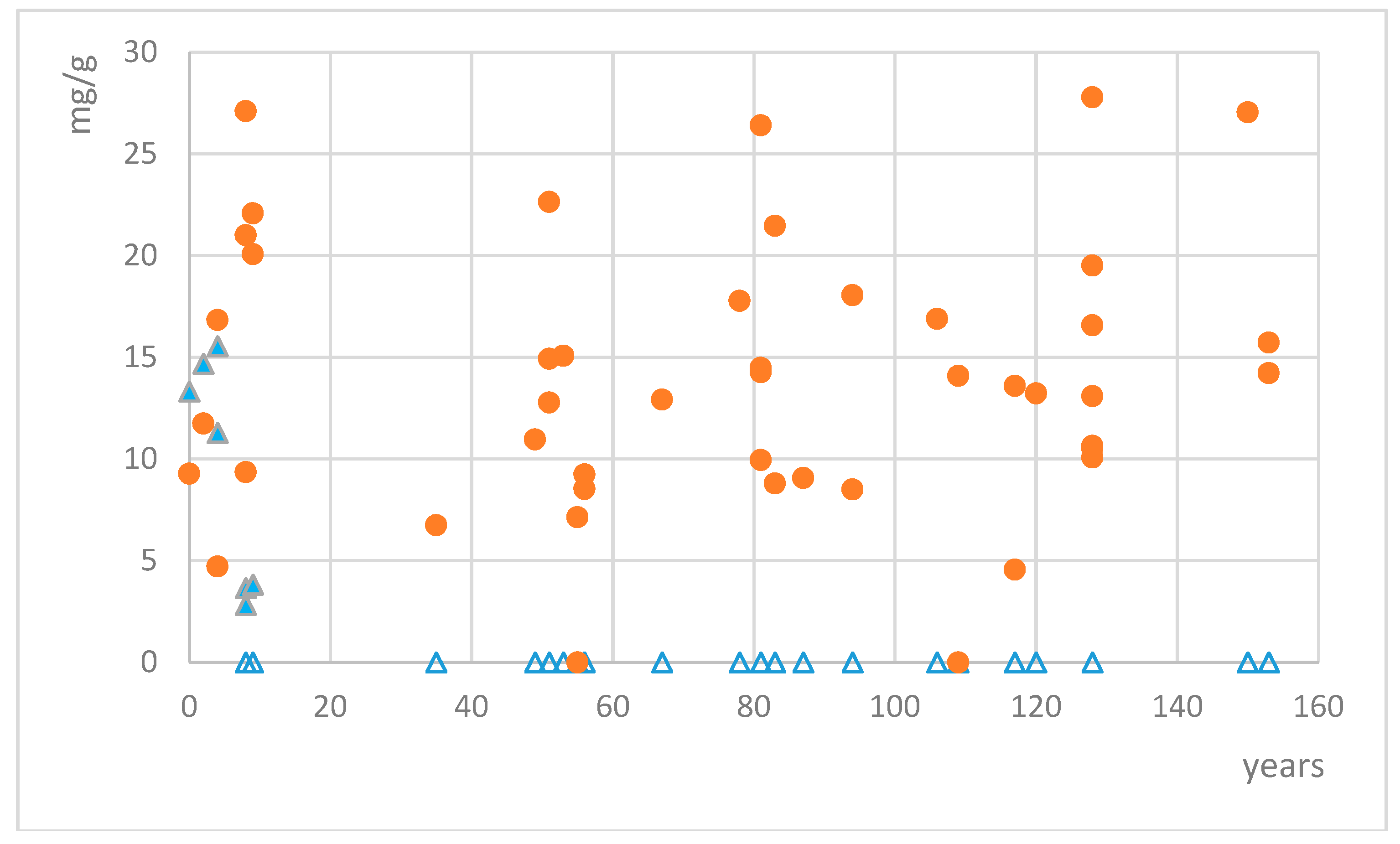
2.2. Analysis of Lichen Collections
3. Discussion
4. Materials and Methods
4.1. Specimen Selection
4.2. Sample Preparation
4.3. Method Validation
4.4. HILIC-HPLC-DAD Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Särkinen, T.; Staats, M.; Richardson, J.E.; Cowan, R.S.; Bakker, F.T. How to Open the Treasure Chest? Optimising DNA Extraction from Herbarium Specimens. PLoS ONE 2012, 7, e43808. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Carine, M.A.; Wood, J.R.I.; Wortley, A.H.; Harris, D.J.; Prance, G.T.; Davidse, G.; Paige, J.; Pennington, T.D.; Robson, N.K.B.; et al. Herbaria are a major frontier for species discovery. Proc. Natl. Acad. Sci. USA 2012, 107, 22169–22171. [Google Scholar] [CrossRef] [PubMed]
- Vitikainen, O. William Nylander (1822–1899) and Lichen Chemotaxonomy. Bryologist 2001, 104, 263–267. [Google Scholar] [CrossRef]
- Culberson, C.F.; Kristinsson, H. A standardized method for the identification of lichen products. J. Chromatogr. 1970, 46, 85–93. [Google Scholar] [CrossRef]
- Arup, U.; Søchting, U.; Frödén, P. A new taxonomy of the family Teloschistaceae. Nord. J. Bot. 2013, 31, 16–83. [Google Scholar] [CrossRef]
- Gadea, A.; Le Lamer, A.-C.; Le Gall, S.; Jonard, C.; Ferron, S.; Catheline, D.; Ertz, D.; Le Pogam, P.; Boustie, J.; Lohezic-Le Devehat, F. Intrathalline Metabolite Profiles in the Lichen Argopsis friesiana Shape Gastropod Grazing Patterns. J. Chem. Ecol. 2018, 44, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Higgins, N.F.; Connan, S.; Stengel, D.B. Factors influencing the distribution of coastal lichens Hydropunctaria maura and Wahlenbergiella mucosa. Mar. Ecol. 2015, 36, 1400–1414. [Google Scholar] [CrossRef]
- Gaya, E.; Fernández-Brime, S.; Vargas, R.; Lachlan, R.F.; Gueidan, C.; Ramírez-Mejía, M.; Lutzoni, F. The adaptive radiation of lichen-forming Teloschistaceae is associated with sunscreening pigments and a bark-to-rock substrate shift. Proc. Natl. Acad. Sci. USA 2015, 112, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Purvis, O.W.; Elix, J.A.; Broomhead, J.A.; Jones, G.C. The occurrence of copper-norstictic acid in lichens from cupriferous substrata. Lichenologist 1987, 19, 193–203. [Google Scholar] [CrossRef]
- Hauck, M.; Jürgens, S.R.; Huneck, S.; Leuschner, C. High acidity tolerance in lichens with fumarprotocetraric, perlatolic or thamnolic acids is correlated with low pKa1 values of these lichen substances. Environ. Pollut. 2009, 157, 2776–2780. [Google Scholar] [CrossRef] [PubMed]
- Hauck, M.; Jürgens, S.R.; Brinkmann, M.; Herminghaus, S. Surface hydrophobicity causes SO2 tolerance in lichens. Ann. Bot. 2008, 101, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Boustie, J.; Grube, M. Lichens—A promising source of bioactive secondary metabolites. Plant Gen. Resour. 2005, 3, 273–287. [Google Scholar] [CrossRef]
- Devkota, S.; Chaudhary, R.P.; Werth, S.; Scheidegger, C. Trade and legislation: Consequences for the conservation of lichens in the Nepal Himalaya. Biodivers. Conserv. 2017, 26, 2491–250510. [Google Scholar] [CrossRef]
- Fahselt, D.; Krol, M.; Alstrup, V.; Huner, N. Detection of pigments in specimens of recent and subfossil Umbilicaria from North Greenland. Bryologist 2001, 104, 593–599. [Google Scholar] [CrossRef]
- Erkens, R.H.J.; Cross, H.; Maas, J.W.; Hoenselaar, K.; Chatrou, L.W. Assessment of age and greenness of herbarium specimens as predictors for successful extraction and amplification of DNA. Blumea 2008, 53, 407–428. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Wilbraham, J.; Banack, S.A.; Metcalf, J.S.; Codd, G.A. Microcystins, BMAA and BMAA isomers in 100-year-old Antarctic cyanobacterial mats collected during Captain R.F. Scott’s Discovery Expedition. Eur. J. Phycol. 2018, 53, 115–121. [Google Scholar] [CrossRef]
- Roullier, C.; Chollet-Krugler, M.; Bernard, A.; Boustie, J. Multiple dual-mode centrifugal partition chromatographie as an efficient method for the purification of a mycosporine from a crude methanolic extract of Lichina pygmaea. J. Chromatogr. B 2009, 877, 2067–2073. [Google Scholar] [CrossRef]
- Roullier, C.; Chollet-Krugler, M.; Pferschy-Wenzig, E.-M.; Maillard, A.; Rechberger, G.N.; Legouin-Gargadennec, B.; Bauer, R.; Boustie, J. Characterization and identification of mycosporines-like compounds in cyanolichens. Isolation of mycosporine hydroxyglutamicol from Nephroma laevigatum Ach. Phytochemistry 2011, 72, 1348–1357. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- LaBarre, S.; Roullier, C.; Boustie, J. Mycosporine-Like Amino Acids (MAAs) in Biological Photosystems. In Outstanding Marine Molecules: Chemistry, Biology, Analysis. Mycosporine-Like Amino Acids (MAAs) in Biological Photosystems; LaBarre, S., Kornprobst, J.-M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 333–359. [Google Scholar]
- Gröninger, A.; Sinha, R.P.; Klisch, M.; Häder, D.-P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Phytobiol. B 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Llewyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of Microalgae across 13 Classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in Green Algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J. Phycol. 2005, 557–566. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar. Biol. 2002, 619–627. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Chollet-Krugler, M.; Lohézic-Le Dévéhat, F.; Rouaud, I.; Boustie, J. Mycosporine-Like Compounds in Chlorolichens: Isolation from Dermatocarpon luridum and Dermatocarpon miniatum, and their Photoprotective Properties. Planta Med. Lett. 2015, 2, e1–e5. [Google Scholar] [CrossRef]
- Thüs, H.; Muggia, L.; Pérez-Ortega, S.; Favero-Longo, S.E.; Joneson, S.; O’Brien, H.; Nelsen, M.P.; Duque-Thüs, R.; Grube, M.; Friedl, T.; et al. Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). Eur. J. Phycol. 2011, 46, 399–415. [Google Scholar] [CrossRef]
- Shivarov, V.; Denchev, C.; Thüs, H. Ecology and distribution of Dermatocarpon (Verrucariaceae/Ascomycota) in the catchment areas of two Bulgarian rivers. Lichenologist 2018, 50, 679–690. [Google Scholar] [CrossRef]
- Fontaine, K.M.; Beck, A.; Stocker-Wörgötter, E.; Piercey-Normore, M.D. Photobiont Relationships and Phylogenetic History of Dermatocarpon luridum var. luridum and Related Dermatocarpon Species. Plants 2012, 1, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.M.; Stocker-Wörgötter, E.; Booth, T.; Piercey-Normore, M.D. Genetic diversity of the lichen-forming alga, Diplosphaera chodatii, in North America and Europe. Lichenologist 2013, 45, 799–813. [Google Scholar] [CrossRef]
- Amtoft, A.; Lutzoni, F.; Miadlikowska, J. Dermatocarpon (Verrucariaceae) in the Ozark Highlands, North America. Bryologist 2008, 111, 1–40. [Google Scholar] [CrossRef]
- Heiðmarsson, S. Dermatocarpon. In Nordic Lichen Flora; Museum of Evolution: Uppsala, Sweden, 2018; Volume 6, pp. 19–25. [Google Scholar]
- Thüs, H.; Schultz, M. Freshwater Flora of Central Europe, Vol. 21, Part 1: Lichen; Spektrum: Heidelberg, Germany, 2008; 223p. [Google Scholar]
- Le Pogam, P.; Legouin, B.; Le Lamer, A.-C.; Boustie, J.; Rondeau, D. Analysis of the cyanolichen Lichina pygmaea metabolites using in situ DART-MS: From detection to thermochemistry of mycosporine serinol. J. Mass Spectrom. 2015, 50, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Le Pogam, P.; Schinkovitz, A.; Legouin, B.; Le Lamer, A.-C.; Boustie, J.; Richomme, P. Matrix-Free UV-Laser Desorption Ionization Mass Spectrometry as a Versatile Approach for Accelerating Dereplication Studies on Lichens. Anal. Chem. 2015, 87, 10421–10428. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Murauer, A.; Ganzera, M. A quantitative analysis of mycosporine-like amino acids in marine algae by capillary electrophoresis with diode-array detection. J. Pharm. Biomed. 2017, 138, 153–157. [Google Scholar] [CrossRef]
- Przeslawski, R.; Benkendorff, K.; Davis, A.R. A quantitative survey of mycosporine-like amino acids (MAAs) in intertidal egg masses from temperate rocky shores. J. Chem. Ecol. 2005, 31, 2417–2438. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like Amino Acids in selected algae and cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a novel MAA from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ludsin, S.A.; Martin, J.F.; Dittmann, E.; Lee, J. Mycosporine-like amino acids (MAAs)-producing Microcystis in Lake Erie: Development of a qPCR assay and insight into its ecology. Harmful Algae 2018, 77. [Google Scholar] [CrossRef] [PubMed]
- Fernandesm, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Yuri, T.; Shingo, M.; Hiroo, O. Researches on the stability of porphyra-334 solution and its influence factors. J. Ocean. Univ. China 2004, 3, 166–170. [Google Scholar] [CrossRef]
- Grönigner, A.; Häder, D.P. Stability of mycosporine-like amino acids. Recent J. Photochem. Photobiol. 2000, 4, 247–252. [Google Scholar]
- Rastogi, R.P.; Sonani, R.R.; Madamawar, D.; Incharoenssakdi, A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016, 1, 110–118. [Google Scholar] [CrossRef]
- Pittet, J.-L.; Bouillant, M.-L.; Bernillon, J.; Arpin, N. Sur la presence de mycosporines-glutamine reduites, nouvelles molecules, chez plusieurs Deuteromycetes. Tetrahedron Lett. 1983, 24, 65–68. [Google Scholar] [CrossRef]
- Bernillon, J.; Bouillant, M.-L.; Pittet, J.-L.; Favre-Bonvin, J.; Arpin, N. Mycosporine glutamine and related mycosporines in the fungus Pyronema omphalodes. Phytochemistry 1984, 23, 1083–1087. [Google Scholar] [CrossRef]
- Duque-Thüs, R.; Fulcher, T.K. Enhancing accessibility and conservation of plant tissue samples stored in silica gel, and developing a disaster plan for this collection at Royal Botanic Gardens, Kew. J. Nat. Sci. Collect. 2018, 5, 35–40. [Google Scholar]
- Shahani, C.J.; Harrison, G. Spontaneous formation of acids in the natural aging of paper. Stud. Conserv. 2002, 47, 189–192. [Google Scholar] [CrossRef]
- Ainsworth, M. Brief Biographies of British Mycologists; British Mycological Society: London, UK, 1996; 203p. [Google Scholar]
- Burkin, A.A.; Tolpysheva, T. Yu.; Kononenko, G.P. Safety of fungal secondary metabolites in herbarial lichen specimens. Vestn. Mosk. Univ. 2012, 3, 28–32. [Google Scholar]
- Tahereh Jafari, T.; Alanne, A.-L.; Issakainen, J.; Pihlaja, K.; Sinkkonen, J. Suitability of dried herbarium specimens for NMR metabolomics of mushrooms. A comparison of four species of the genera Kuehneromyces and Hypholoma (Strophariaceae). Fungal Biol. 2018, 122, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Thunig, J.; Hansen, S.H.; Janfelt, C. Analysis of Secondary Plant Metabolites by Indirect Desorption Electrospray Ionization Imaging Mass Spectrometry. Anal. Chem. 2011, 83, 3256–3259. [Google Scholar] [CrossRef] [PubMed]
- Kaoa, D.; Henkin, J.M.; Soejartob, D.D.; Kinghorn, A.D.; Oberlies, N.H. Non-destructive chemical analysis of a Garcinia mangostana L. (Mangosteen) herbarium voucher specimen. Phytochem. Lett. 2018, 28, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; de Felicio, R.; Fenner, A.; et al. Molecular Networking as a Dereplication Strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef]
- De la Parra, J. Herbariomics: Expanding possibilities for herbaria-based research pipelines by defining the herbariome. In Proceedings of the 255th ACS National Meeting & Exposition, New Orleans, LA, USA, 18–22 March 2018. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
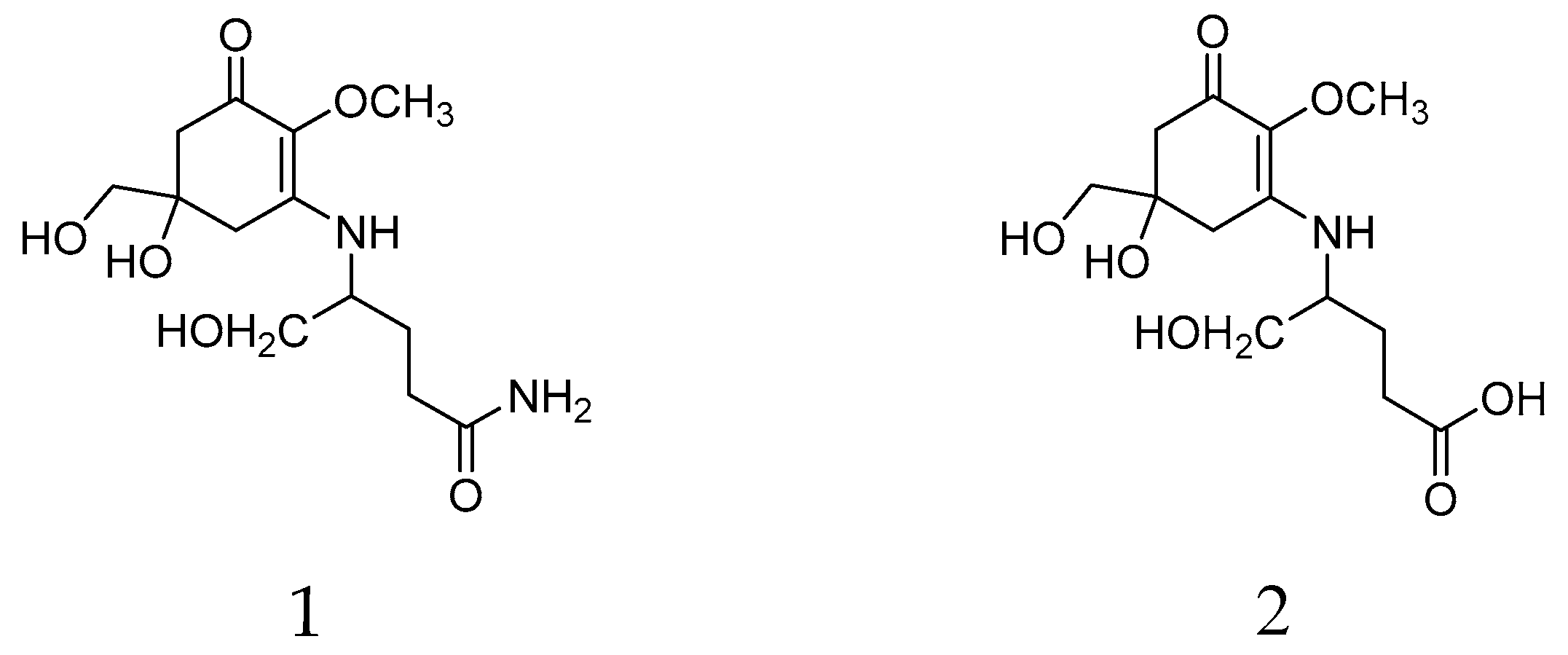
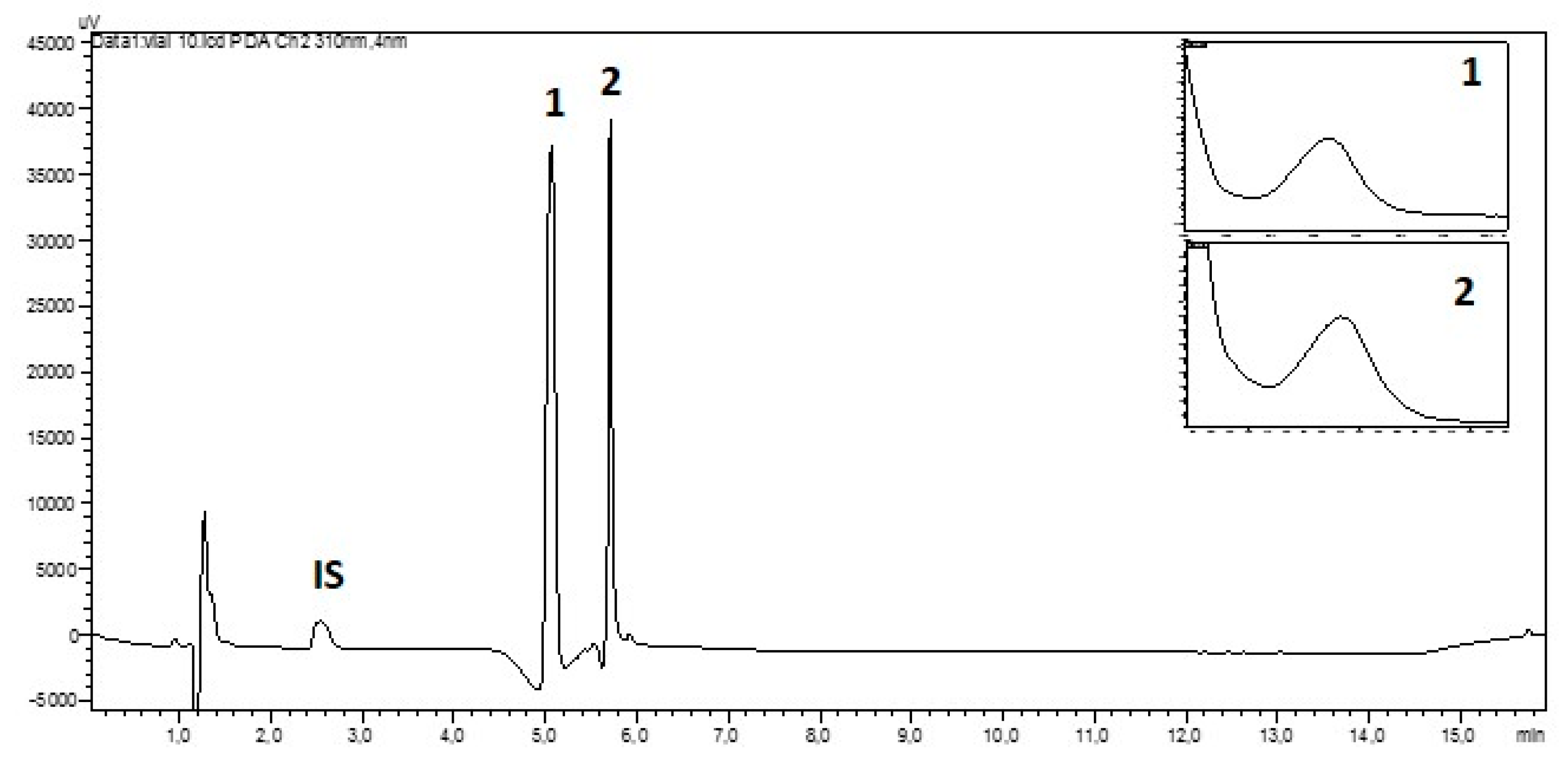
| Mycosporine Glutaminol (1) | Mycosporine Glutamicol (2) | |
|---|---|---|
| Concentration range (µg/mL) | 5.00–40.00 | 5.00–40.00 |
| Regression equation | y = 0.04x − 0.03 | y = 0.03x + 0.09 |
| Correlation coefficient (R2) | 0.9984 | 0.9922 |
| Precision (%) | 4.21 | 2.27 |
| LOD (µg/mL) | 1.41 | 4.49 |
| LOQ (µg/mL) | 2.93 | 8.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chollet-Krugler, M.; Nguyen, T.T.T.; Sauvager, A.; Thüs, H.; Boustie, J. Mycosporine-Like Amino Acids (MAAs) in Time-Series of Lichen Specimens from Natural History Collections. Molecules 2019, 24, 1070. https://doi.org/10.3390/molecules24061070
Chollet-Krugler M, Nguyen TTT, Sauvager A, Thüs H, Boustie J. Mycosporine-Like Amino Acids (MAAs) in Time-Series of Lichen Specimens from Natural History Collections. Molecules. 2019; 24(6):1070. https://doi.org/10.3390/molecules24061070
Chicago/Turabian StyleChollet-Krugler, Marylène, Thi Thu Tram Nguyen, Aurelie Sauvager, Holger Thüs, and Joël Boustie. 2019. "Mycosporine-Like Amino Acids (MAAs) in Time-Series of Lichen Specimens from Natural History Collections" Molecules 24, no. 6: 1070. https://doi.org/10.3390/molecules24061070
APA StyleChollet-Krugler, M., Nguyen, T. T. T., Sauvager, A., Thüs, H., & Boustie, J. (2019). Mycosporine-Like Amino Acids (MAAs) in Time-Series of Lichen Specimens from Natural History Collections. Molecules, 24(6), 1070. https://doi.org/10.3390/molecules24061070







