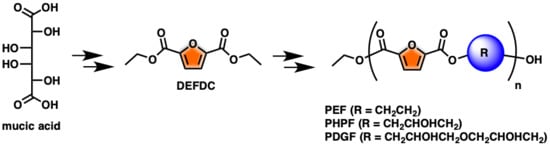
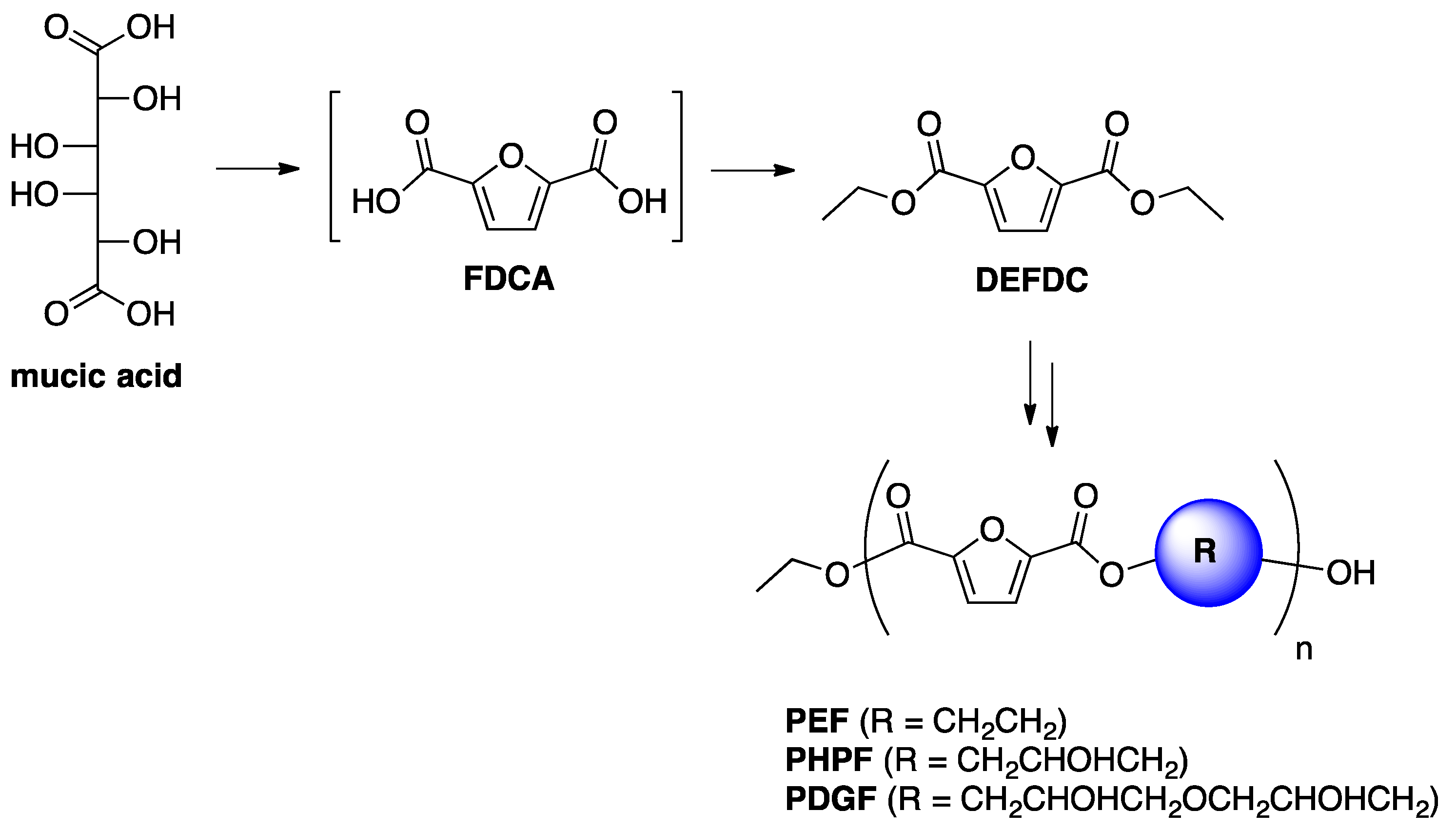
One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters
Abstract
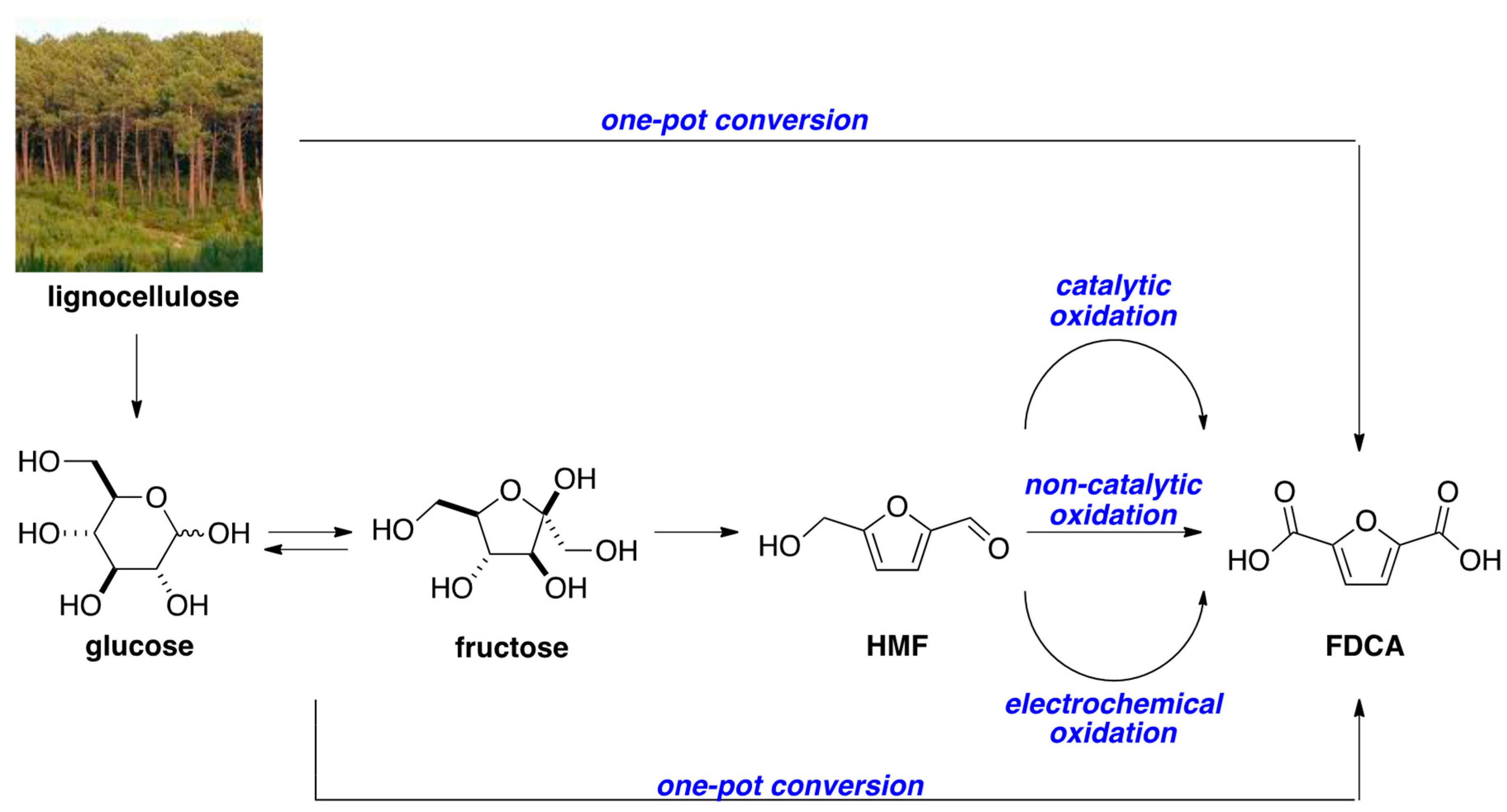
1. Introduction
2. Results and Discussion
2.1. Optimization of DEFDC Synthesis
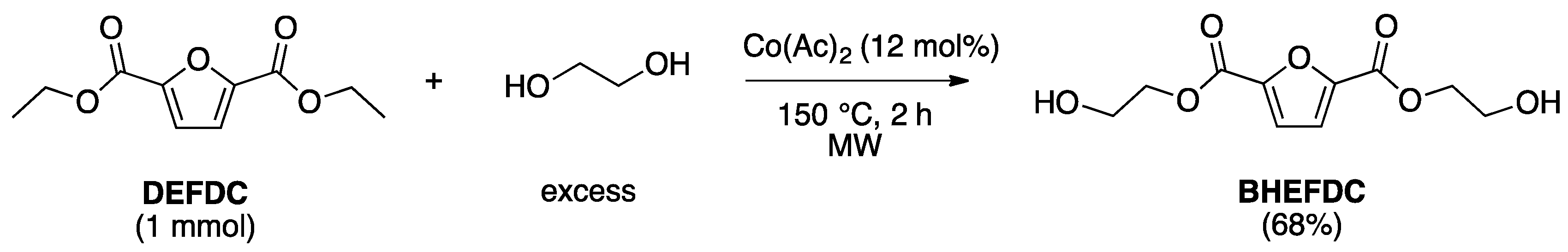
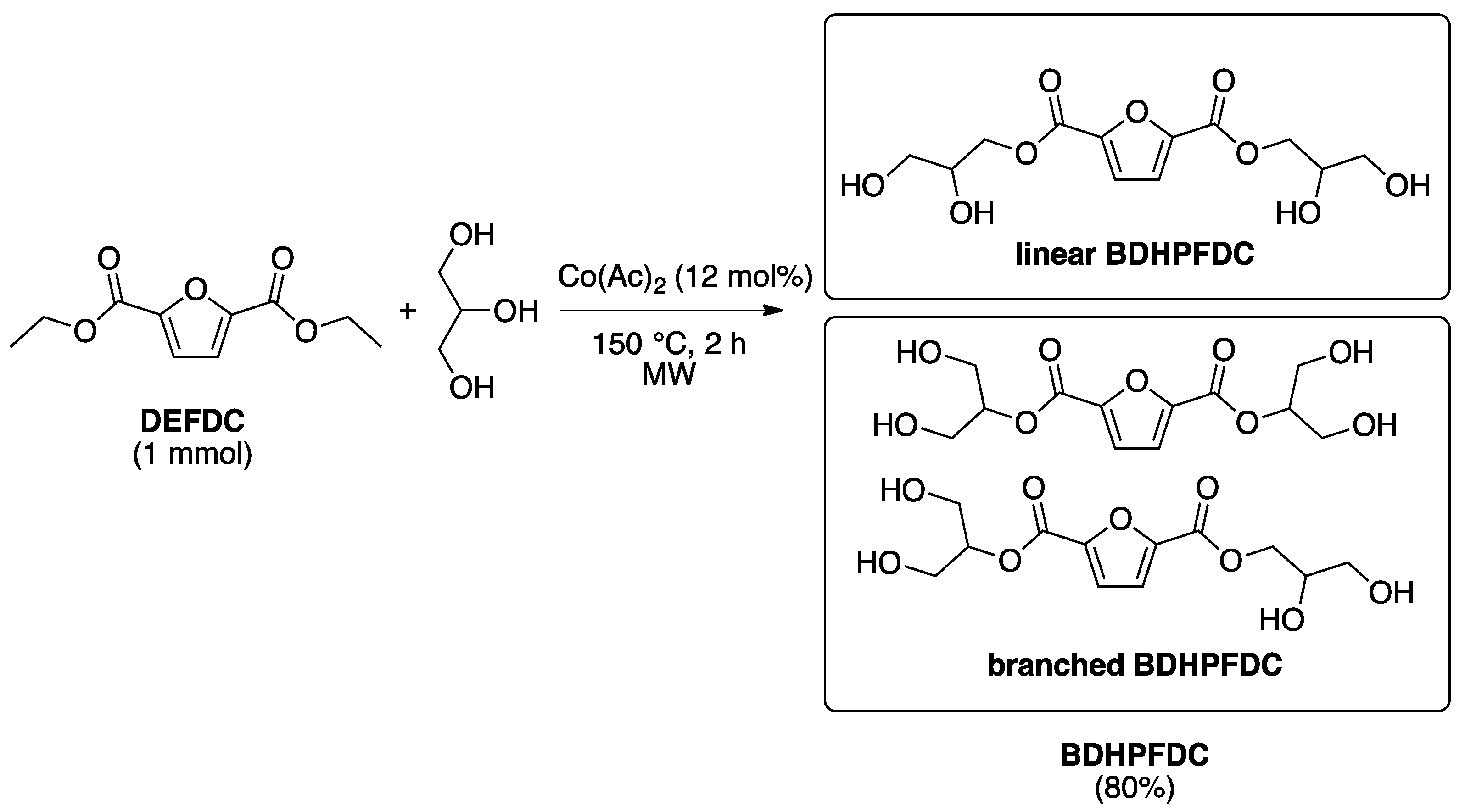
2.2. Optimization of the Prepolymers Synthesis and Additional Regioselective Acylation of Glycerol
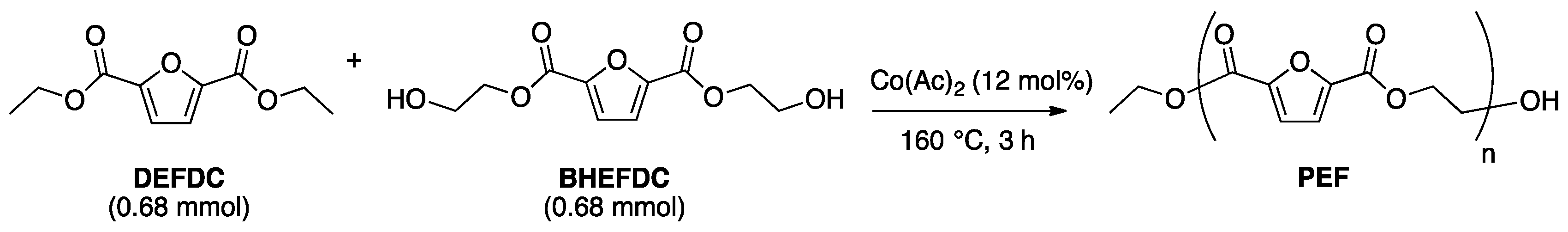
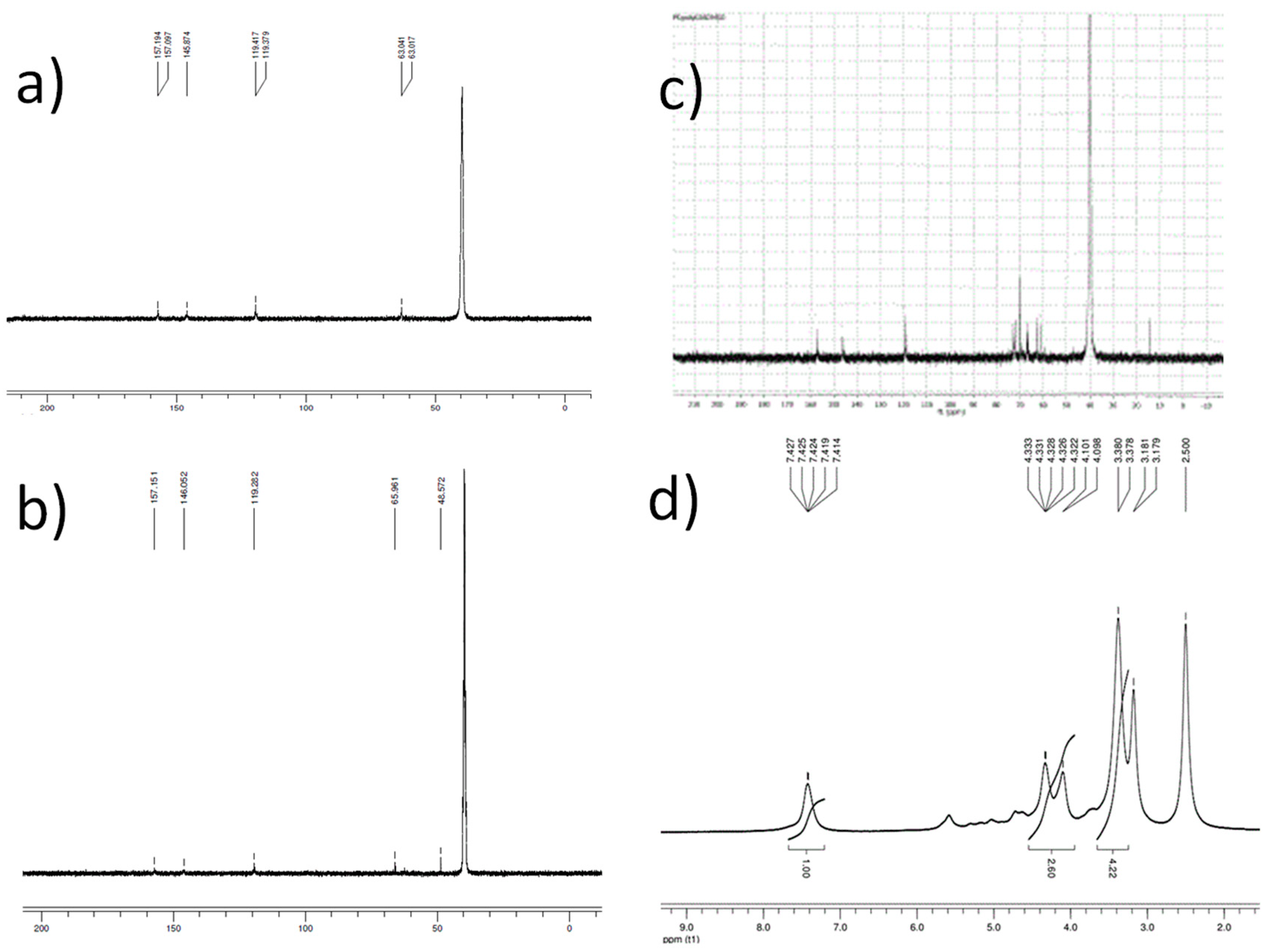
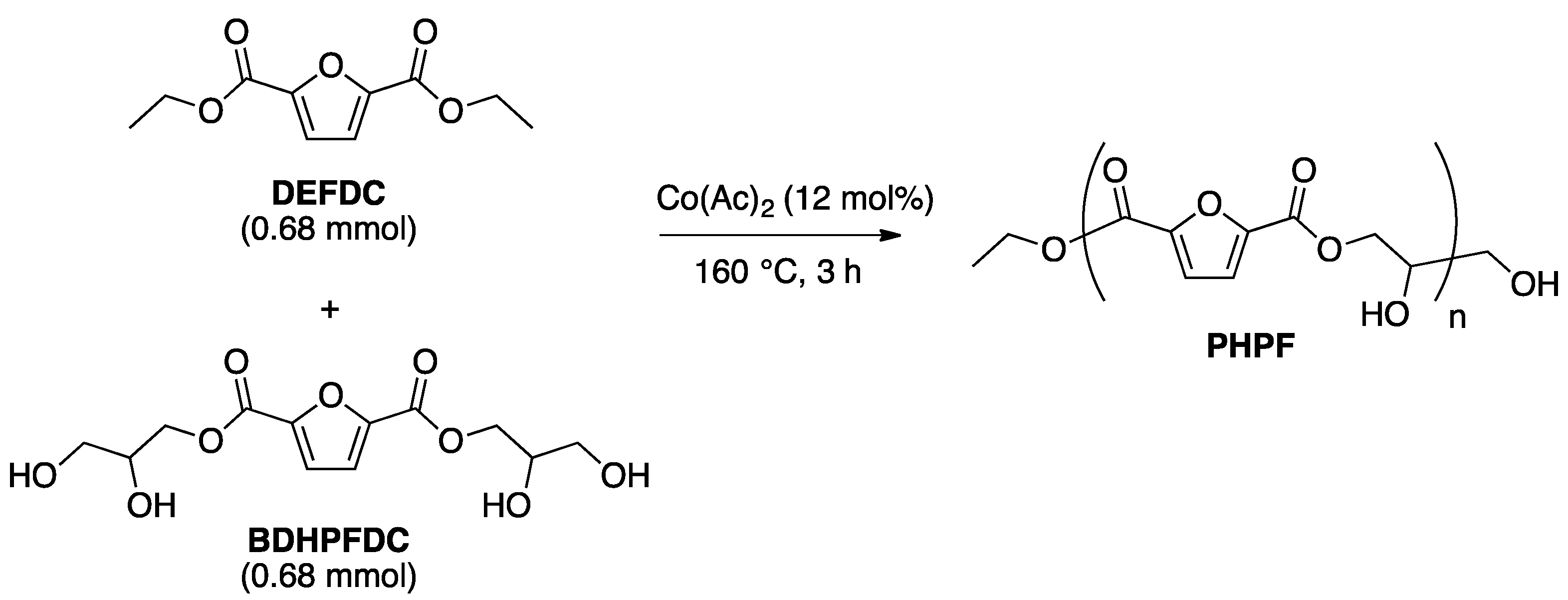
2.3. Optimization of Solvent-Free Poly Trans-Esterification and Solids Characterizations
3. Experimental Section
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. Synthesis of Diethyl Furan-2,5-Dicarboxylate (DEFDC)
3.2.2. Synthesis of Bis(Hydroxyethyl)-2,5-Furandicarboxylate (BHEFDC)
3.2.3. Synthesis of Bis(2,3-Dihydropropyl)-2,5-Furandicarboxylate (BDHPFDC)
3.2.4. General Melt Polytransesterification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pal, P.; Saravanamurugan, S. Recent advances on development of 5-hydroxymethylfurfural oxidation with base (non-precious) metal-containing catalysts. ChemSusChem 2019, 12, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, material science, and synthetic building blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent advances in the catalytic synthesis of 2,5-furandicarboxylic acid and its derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Partenheimer, W.; Grushin, V.V. Synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by air-oxidation of 5-hydroxymethylfurfural. Unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal=bromide catalysts. Adv. Synth. Catal. 2001, 343, 102–111. [Google Scholar] [CrossRef]
- Saha, B.; Dutta, S.; Abu-Omar, M.M. Aerobic oxidation of 5-hydroxymethylfurfural with homogeneous and nanoparticulate catalysts. Catal. Sci. Technol. 2012, 2, 79–81. [Google Scholar] [CrossRef]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Carani, F. Insights into the reaction mechanism for 5-hydroxymethylfurfural oxidation to FDCA on bimetallic Pd-Au nanoparticles. Appl. Catal. A Gen. 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Abonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J. Selective oxidation of 5-hydroxymethyl-2-furfural over TiO2-supported gold-copper catalysts prepared from preformed nanoparticles: Effect of Au/Cu ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Rass, M.A.; Essayem, N.; Besson, M. Selective aerobic oxidation of 5-HMF into 2,5-furandicarboxylic acid with Pt catalysts supported on TiO2- and ZrO2-based supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Delbecq, F.; Len, C. Recent advances in the microave-assisted production of hydroxymethylfurfural by hydrolysis of cellulose derivatives—A review. Molecules 2018, 23, 1973. [Google Scholar] [CrossRef] [PubMed]
- Gaset, A.; Rigal, L.; Sene, B.; Ralainirina, R. 2,5-furan dicarboxylic ester preparation. France Patent FR 2723945B1.
- Lewkowski, J. Convenient synthesis of furan-2,5-dicarboxylic acid and its derivatives. Polish J. Chem. 2001, 75, 1943–1946. [Google Scholar]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.J.M.; Coehlo, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5982. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.D.; Nam, B.U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5151. [Google Scholar] [CrossRef]
- Storbeck, R.; Ballauff, M. Synthesis and properties of polyesters based on 2,5-furandicarboxylic acid and 1,4:3,6-dianhydrohexitols. Polymer 1993, 34, 5003–5006. [Google Scholar] [CrossRef]
- Arnaud, S.P.; Wu, L.; Wong Chang, M.A.; Comerfod, J.W.; Farmer, T.J.; Schmid, M.; Chang, F.; Li, Z.; Mascal, M. New bio-based monomers: Tuneable polyester properties using branched diols from biomass. Faraday Discuss. 2017, 202, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.I.J.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957–1965. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Fonseca, A.C.; Freire, C.S.R.; Coelho, J.F.J.; Silvestre, A.J.D. A new generation of furanic copolyesters with enhanced degradability: Poly(ethylene 2,5-furandicarboxylate)-co-poly(lactic acid) copolyesters. Macromol. Chem. Phys. 2014, 215, 2175–2184. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martinez de Ilarduya, A.; Mufioz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coehlo, J.F.J. New copolyesters derived from terephthalic and 2,5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, Y.; Liu, F.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) with 1,4-cyclohexanedimethylene: Influence of composition on mechanical and barrier properties. Polymer 2016, 103, 1–8. [Google Scholar] [CrossRef]
- Gandini, A.; Sivestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly(ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Razzaq, A.; Bonham, P. Synthesis and characterization of all renewable resources based branched polyesters: Poly(2,5-furandicarboxylic acid-co-glycerol). ISRN Polym. Sci. 2013, 2013, 645169. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Narayan-Sarathy, S.; Ghosh, T.; Mahajan, K.; Naceur Belgaum, M. Synthesis and characterization of bio-based furanic polyesters. J. Polym. Res. 2014, 21, 340. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Liu, Y.; Sun, L.; Zhu, J. Synthesis of bio-based poly(ethylene 2,5-furandicarboxylate) copolyesters: Higher glass transition temperature, better transparency, and good barrier properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3298–3307. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H.; Wu, L.; Li, B.G.; Dubois, P. DBU-catalyzed biobased poly(ethylene 2,5-furandicarboxylate) polyester with rapid melt crystallization: Synthesis, crystallization kinetics and melting behavior. RSC Adv. 2016, 6, 101578–101586. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; De Roo, J.; Storti, G.; Morbidelli, M. Diffusion (DOSY) 1H NMR as an alternative method for molecular weight determination of poly(ethylene furanoate) (PEF) polyesters. Macromol. Chem. Phys. 2017, 218, 1600436. [Google Scholar] [CrossRef]
- Taguchi, Y.; Oishi, A.; Iida, H. One-step synthesis of dibutyl furandicarboxylates from galactaric acid. Chem. Lett. 2008, 37, 50–51. [Google Scholar] [CrossRef]
- Slavko, E.; Taylor, M.S. Catalyst-controlled polycondensation of glycerol with diacyl chlorides: Linear polyesters from a trifunctional monomer. Chem. Sci. 2017, 8, 7106–7111. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Billamboz, M.; Leonard, E.; Len, C.; Bottcher, C.; Prasad, A.K.; Haag, R.; Sharma, S.K. Self-assembly, photoresponsive behavior and transport potential of azobenzene grafted dendronized polymeric amphiphiles. RSC Adv. 2015, 5, 48301–48310. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, A.; Malhotra, S.; Mosurkal, R.; Dhawan, A.; Pandey, M.K.; Singh, B.K.; Kumar, R.; Prasad, A.K.; Sharma, S.K.; et al. Synthesis of macromolecular systems via lipase catalyzed biocatalytic reactions: Applications and future perspectives. Chem. Soc. Rev. 2016, 45, 6855–6887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. Ecoscale, a semi-quantitative tool to select an organic preparation based on economical and acological parameters. Beilstein J. Org. Chem. 2006, 2, 3–9. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
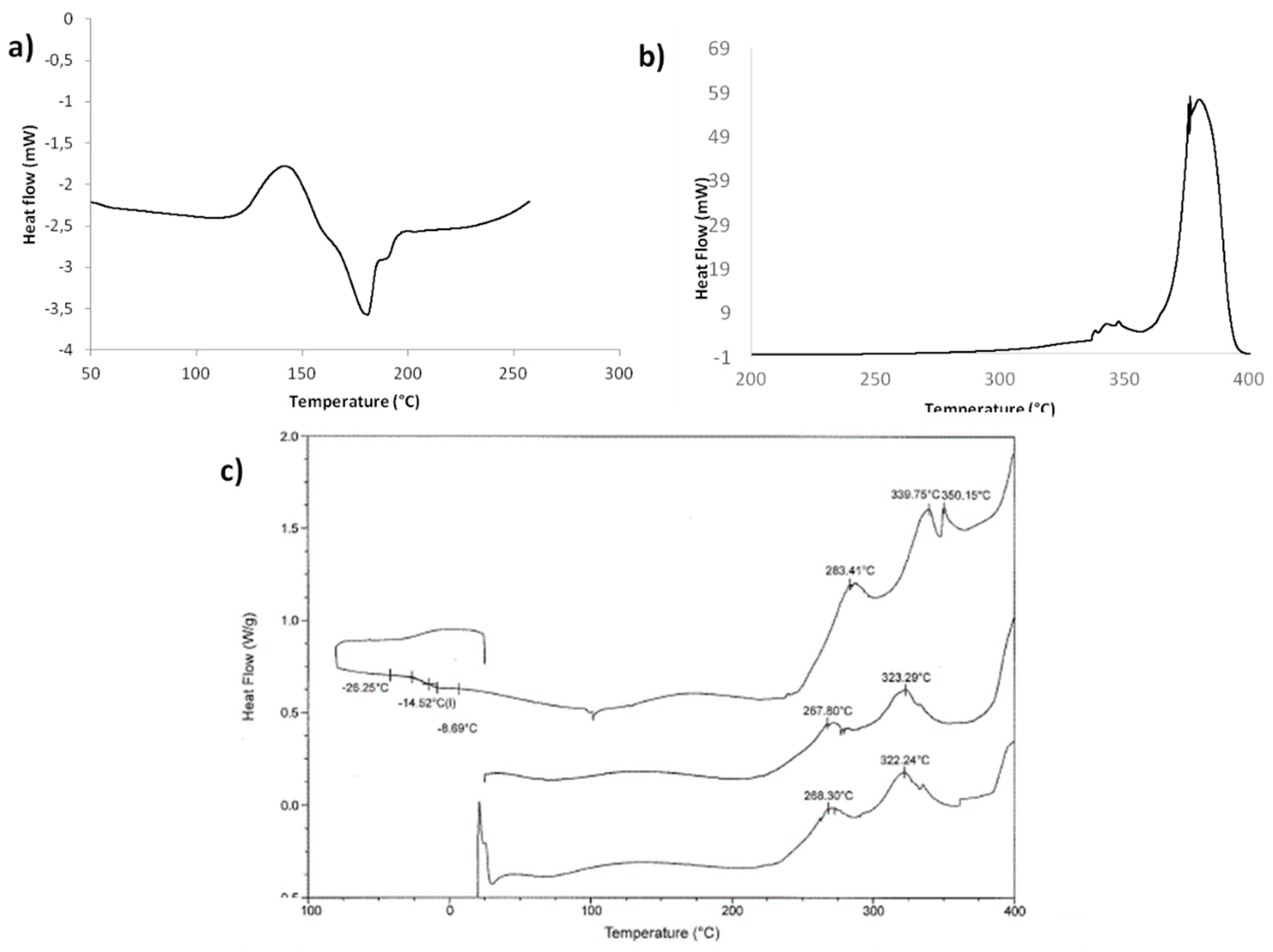

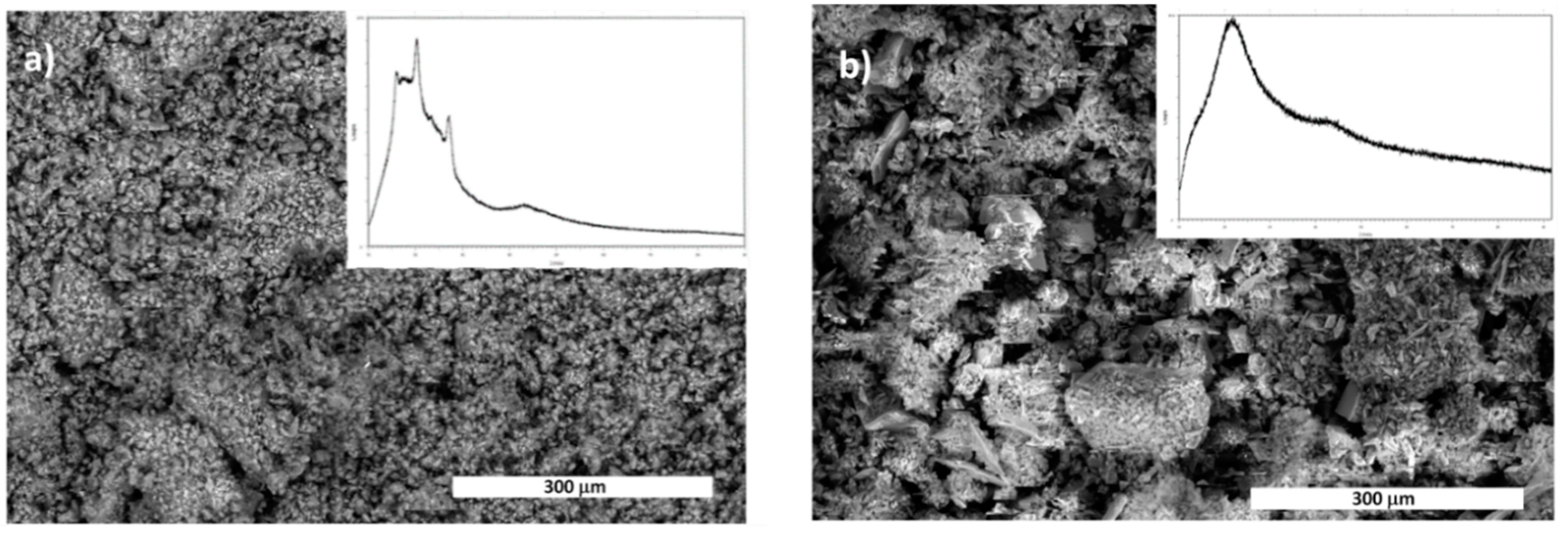
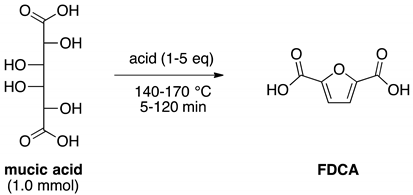
| Entry | Acid | [Acid] [eq] | Temperature [°C] | Time [min] | Yield of FDCA [%] a |
|---|---|---|---|---|---|
| 1 | PTSA | 1 | 160 | 60 | 32 |
| 2 | PTSA | 2 | 160 | 60 | 41 |
| 3 | PTSA | 3 | 160 | 60 | 36 |
| 4 | PTSA | 4 | 160 | 60 | 34 |
| 5 | PTSA | 5 | 160 | 60 | 37 |
| 6 | MSA | 1 | 160 | 60 | 34 |
| 7 | MSA | 2 | 160 | 60 | 39 |
| 8 | MSA | 3 | 160 | 60 | 35 |
| 9 | MSA | 4 | 160 | 60 | 35 |
| 10 | MSA | 5 | 160 | 60 | 30 |
| 11 | CSA | 1 | 160 | 60 | 4 |
| 12 | CSA | 2 | 160 | 60 | 11 |
| 13 | CSA | 3 | 160 | 60 | 15 |
| 14 | CSA | 4 | 160 | 60 | 12 |
| 15 | CSA | 5 | 160 | 60 | 9 |
| 16 | PTSA | 2 | 140 | 60 | 12 |
| 17 | PTSA | 2 | 170 | 60 | 28 |
| 18 | PTSA | 2 | 160 | 5 | 8 |
| 19 | PTSA | 2 | 160 | 15 | 20 |
| 20 | PTSA | 2 | 160 | 30 | 30 |
| 21 | PTSA | 2 | 160 | 90 | 40 |
| 22 | PTSA | 2 | 160 | 120 | 37 |
| 23 | MSA | 2 | 140 | 60 | 15 |
| 24 | MSA | 2 | 170 | 60 | 25 |
| 25 | MSA | 2 | 160 | 5 | 8 |
| 26 | MSA | 2 | 160 | 15 | 30 |
| 27 | MSA | 2 | 160 | 30 | 39 |
| 28 | MSA | 2 | 160 | 90 | 35 |
| 29 | MSA | 2 | 160 | 120 | 32 |

| Entry | Temperature [°C] | Time [h] | Yield of DEFDC [%] a |
|---|---|---|---|
| 1 | 70 | 4 | 15 |
| 2 | 70 | 8 | 23 |
| 3 | 70 | 16 | 29 |
| 4 | 90 | 4 | 27 |
| 5 | 90 | 8 | 29 |
| 6 | 90 | 16 | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Delbecq, F.; Len, C. One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters. Molecules 2019, 24, 1030. https://doi.org/10.3390/molecules24061030
Zhao D, Delbecq F, Len C. One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters. Molecules. 2019; 24(6):1030. https://doi.org/10.3390/molecules24061030
Chicago/Turabian StyleZhao, Deyang, Frederic Delbecq, and Christophe Len. 2019. "One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters" Molecules 24, no. 6: 1030. https://doi.org/10.3390/molecules24061030
APA StyleZhao, D., Delbecq, F., & Len, C. (2019). One-Pot FDCA Diester Synthesis from Mucic Acid and Their Solvent-Free Regioselective Polytransesterification for Production of Glycerol-Based Furanic Polyesters. Molecules, 24(6), 1030. https://doi.org/10.3390/molecules24061030