Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra
Abstract
:1. Introduction
2. Results and Discussion
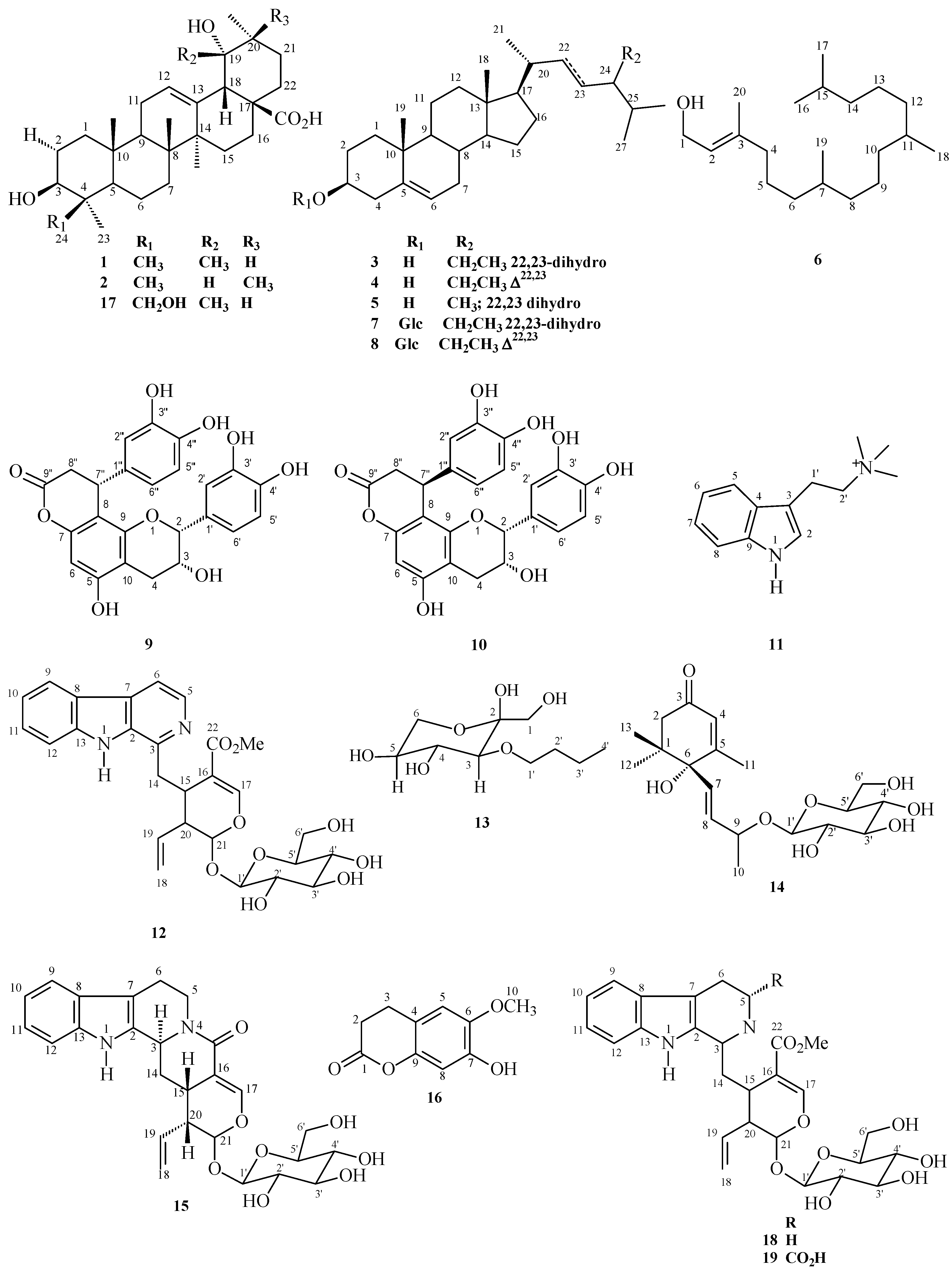
2.1. Chemical Study
2.2. Antimycobacterial Activity
2.3. Inhibition by Triterpenes and Alkaloids of LPS-induced NO Production and Cytotoxicity in RAW264.7 Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Preparation and Fractionation of Methanol Extract
3.4. Antimycobacterial Activity
3.5. Determination of Nitric Oxide Production by RAW 264.7 Macrophages
3.6. Cytotoxic Effect
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Barreira, D. The challenges to eliminating tuberculosis in Brazil. Epidemiol. Serv. Saude 2018, 27, 1–3. [Google Scholar]
- WHO. Global Tuberculosis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Kim, S.; Seo, H.; Al Mahmud, H.; Islam, M.I.; Lee, B.-E.; Cho, M.-L.; Song, H.-Y. In vitro activity of collinin isolated from the leaves of Zanthoxylum schinifolium against multidrug- and extensively drug-resistant Mycobacterium tuberculosis. Phytomedicine 2018, 46, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Tsubouchi, H.; Sasaki, H.; Shimokawa, Y.; Komatsu, M. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLos Med. 2006, 3, 2131–2144. [Google Scholar] [CrossRef]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009, 8, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.H.; Silva, I.C.V.; Oliveira, P.F.; Barreto, A.R.R.; Konno, T.U.P.; Esteves, F.D.A.; Barth, T.; Aguiar, F.A.; Lopes, N.P.; Dermenjian, R.K.; et al. Biological activities and phytochemical profile of Passiflora mucronata from the Brazilian restinga. Braz. J. Pharmacog. 2017, 27, 702–710. [Google Scholar]
- Garlanda, C.; Di Liberto, D.; Vecchi, A.; La Manna, M.P.; Buracchi, C.; Caccamo, N.; Salerno, A.; Dieli, F.; Mantovani, A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 Receptor 8/Single Ig IL-1-related Receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 2007, 179, 3119–3125. [Google Scholar] [CrossRef]
- Bernardes, N.R.; Heggdorne-Araújo, M.; Borges, I.F.J.C.; Almeida, F.M.; Amaral, E.P.; Lasunskaia, E.B.; Muzitano, M.F.; Oliveira, D.B. Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius. Braz. J. Pharmacog. 2014, 24, 644–650. [Google Scholar] [CrossRef]
- Verotta, L.; Pilati, T.; Tato, M.; Elisabetsky, E.; Amador, T.A. Pyrrolidinoindoline alkaloids from Psychotria colorata. J. Nat. Prod. 1998, 61, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Amador, T.A.; Elisabetsky, E.; De Souza, D.O. Effects of Psychotria colorata alkaloids in brain opioid system. Neurochem. Res. 1996, 21, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, H.-P.; Wang, Y.-H.; Hao, X.-J. A new dimeric alkaloid from the Leaf of Psychotria calocarpa. Helv. Chim. Acta 2010, 93, 1650–1652. [Google Scholar]
- Benevides, P.J.C.; Young, M.C.M.; Bolzani, V.D.S. Biological activities of constituents from Psychotria spectabilis. Pharm. Biol. 2004, 42, 565–569. [Google Scholar] [CrossRef]
- Carvalho Junior, A.R.; Vieira, I.J.C.; Carvalho, M.G.; Braz-Filho, R.; Lima, M.A.S.; Ferreira, R.O.; Maria, E.J.; Oliveira, D.B. 13C-NMR Spectral Data of Alkaloids Isolated from Psychotria Species (Rubiaceae). Molecules 2017, 22, 103. [Google Scholar] [CrossRef]
- Lu, H.; Liu, L.; Li, D.; Li, J.; Xu, L. A new iridoid glycoside from the root of Psychotria rubra. Biochem. Syst. Ecol. 2014, 57, 133–136. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Zhang, D.-M.; Chen, M.-F.; Guan, S.-Y.; Yao, J.-H.; He, X.-X.; Lei, L.-F.; Zhong, Y.; Wang, Z.-F.; Ye, W.-C. Antiproliferative triterpenoid saponins from the stem of Psychotria sp. Planta Med. 2013, 79, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.O.; Dzoyem, J.P.; Eloff, J.N.; McGaw, L.J. Extracts of six Rubiaceae species combined with rifampicin have good in vitro synergistic antimycobacterial activity and good anti-inflammatory and antioxidant activities. BMC Complement. Altern. Med. 2016, 16, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Le, V.D.; Do, T.H.; Nguyen, T.L.; Nguyen, P.T.; Nguyen, T.T.; Nguyen, T.D. Anti-inflammatory constituents from Psychotria prainii H. Lév. Nat. Prod. Res. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Both, F.L.; Farias, F.M.; Nicoláo, L.L.; Misturini, J.; Henriques, A.T.; Elisabtsky, E.E. Avaliaçao da atividade analgésica de extratos alcaloídicos de espécies de Psychotria. Rev. Bras. Plantas Med. 2002, 5, 41–45. [Google Scholar]
- Jayasinghe, U.L.B.; Jayasooriya, C.P.; Bandara, B.M.R.; Ekanayake, S.P.; Merlini, L.; Assante, G. Antimicrobial activity of some Sri Lankan Rubiaceae and Meliaceae. Fitoterapia 2002, 73, 424–427. [Google Scholar] [CrossRef]
- Moraes, T.M.S.; Araújo, M.H.; Bernardes, N.R.; Oliveira, D.B.; Lasunskaia, E.B.; Muzitano, M.F.; Da Cunha, M. Antimycobacterial Activity and Alkaloid Prospection of Psychotria Species (Rubiaceae) from the Brazilian Atlantic Rainforest. Planta Med. 2011, 77, 964–970. [Google Scholar] [CrossRef]
- Chama, M.A.; Dziwornu, G.A.; Waibel, R.; Osei-Safo, D.; Addae-Mensah, I.; Otchere, J.; Wilson, M. Isolation, characterization, and anthelminthic activities of a novel dichapetalin and other constituents of Dichapetalum filicaule. Pharm. Biol. 2016, 54, 1179–1188. [Google Scholar]
- Wang, X.; Hay, A.; Matheeussen, A.; Gupta, M.P.; Hostettmann, K. Structure elucidation and NMR assignments of two new triterpenoids from the stems of Paragonia pyramidata (Bignoniaceae). Magn. Reson. Chem. 2011, 49, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.D.; Souza, A.F.; Rodrigues, E.D.; Garcez, F.R.; Garcez, W.S. Constituintes Químicos das Folhas de Riedeliella graciliflora. Quim. Nova 2012, 35, 1306–1311. [Google Scholar] [CrossRef]
- Kojima, H.; Sato, N.; Hatano, A.; Ogura, H. Sterol glucosides from Prunella Vulgaris. Phytochemistry 1990, 29, 2351–2355. [Google Scholar] [CrossRef]
- Nonaka, G.-I.; Nishioka, I. Phenylpropanoid-substituted epicatechins, cincochanains from Cinchona succirubra. Chem. Pharm. Bull. 1982, 30, 4268–4276. [Google Scholar] [CrossRef]
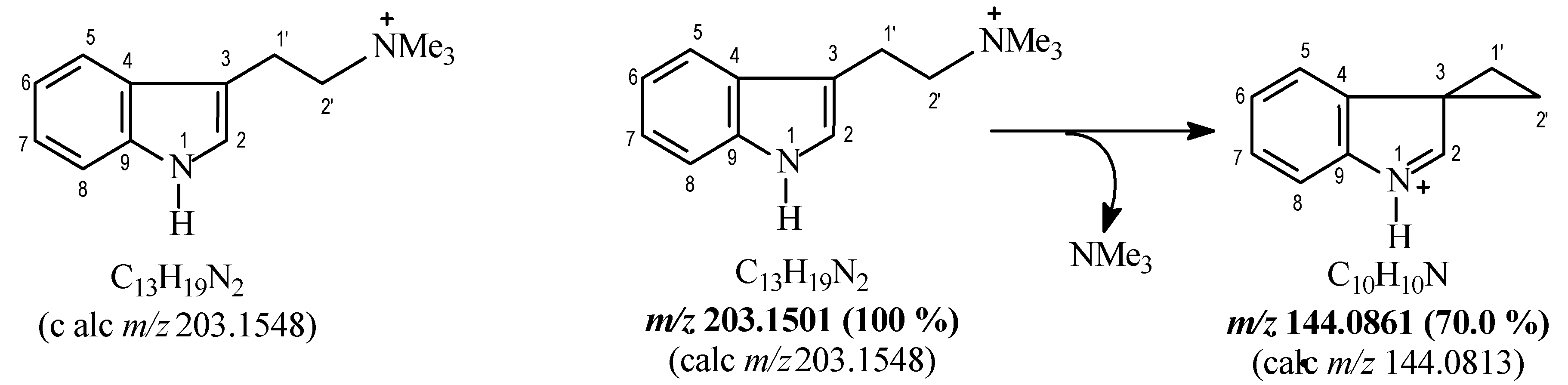
- Martins, C.P.B.; Freeman, S.; Alder, J.F.; Brandt, S.D. Characterisation of a proposed internet synthesis of N,N-dimethyltryptamine using liquid chromatography/electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 6119–6123. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Kostyan, M.K.; Klose, S.I.; Gastegger, M.; Lorbeer, E.; Brecker, L.; Schinnerl, J. Loganin and secologanin derived tryptamine-iridoid alkaloids from Palicourea crocea and Palicourea padifolia (Rubiaceae). Phytochemistry 2015, 116, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Siddiqui, B.S.; Begum, S.; Ali, M.I.; Marasini, B.P.; Khan, A.; Choudhary, M.I. Bioassay-guided isolation of urease and α-chymotrypsin inhibitory constituents from the stems of Lawsonia alba Lam. (Henna). Fitoterapia 2013, 84, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Yao, M.; Kamada, K.; Takeda, Y. Alangiosides GM: Glycosides of megastigmane Derivatives from the leaves of Alangium premnifolium. Chem. Pharm. Bull. 1995, 43, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Elsohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. New indole alkaloids from the bark of Nauclea orientalis. J. Nat. Prod. 2001, 64, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, A.; Kosela, S.; Kardono, L.B.S.; Syah, Y.M. Scopoletin, a coumarin derivative compound isolated from Macaranga gigantifolia Merr. J. Appl. Pharm. Sci. 2012, 2, 175–177. [Google Scholar]
- Nakatani, M.; Miyazaki, Y.; Iwashita, T.; Naoki, H.; Hase, T. Triterpenes from Ilex rotunda Fruits. Phytochemistry 1989, 28, 1479–1482. [Google Scholar] [CrossRef]
- Patthy-luka, Á.; Károlyházy, L.; Szabó, L.F.; Podányi, B. First direct and detailed stereochemical analysis of strictosidine. J. Nat. Prod. 1997, 60, 69–75. [Google Scholar] [CrossRef]
- Ferrari, F.; Messana, I.; Botta, B.; Mello, J.F. Constituents of Guettarda Platypoda. J. Nat. Prod. 1986, 49, 1150–1151. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Balestrieri, M.L.; Cautela, D.; Castaldo, D. N-Methylated Tryptamine Derivatives in Citrus Genus Plants: Identification of N,N,N-Trimethyltryptamine in Bergamot. J. Agric. Food Chem. 2012, 60, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.A. Alcaloides e o chá de ayahuasca: Uma correlação dos “estados alterados da consciência” induzido por alucinógenos. Rev. Bras. Plants Med. 2011, 13, 349–358. [Google Scholar] [CrossRef]
- Pires, A.P.S.; Oliveira, C.D.R.; Yonamine, M. Ayahuasca: Uma revisão dos aspectos farmacológicos e toxicológicos. Rev. Ciênc. Farm. Básica Apl. 2010, 31, 15–23. [Google Scholar]
- Camacho-Corona, M.d.R.; Favela-Hernández, J.M.J.; González-Santiago, O.; Garza-González, E.; Molina-Salinas, G.M.; Said-Fernández, S.; Delgado, G.; Luna-Herrera, J. Evaluation of Some Plant-derived Secondary Metabolites Against Sensitive and Multidrug-resistant Mycobacterium tuberculosis. J. Mex. Chem. Soc. 2009, 53, 71–75. [Google Scholar]
- Cantrel, C.L.; Franzblau, S.G.; Fischer, N.H. Antimycobacterial plants terpenoids. Planta Med. 2001, 67, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Wachter, G.A.; Valcic, S.; Flagg, M.L.; Franzblau, S.G.; Montenegro, G.; Suárez, E.; Timmermann, B.N. Antitubercular activity of pentacyclic triterpenoids from plants of Argentina and Chile. Phytomedicine 1999, 6, 341–345. [Google Scholar] [CrossRef]
- Macabeo, A.P.; Vidar, W.S.; Chen, X.; Decker, M.; Heilmann, J.; Wan, B.; Franzblau, S.G.; Galvez, E.V.; Aguinaldo, M.A.; Cordell, G.A. Mycobacterium tuberculosis and cholinesterase inhibitors from Voacanga globosa. Eur. J. Med. Chem. 2011, 46, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Krohn, K.; Gehle, D.; Read, R.W.; Brophy, J.J.; Franzblau, S.G.; Aguinaldo, M.A.M. Activity of the Extracts and Indole Alkaloids from Alstonia scholaris Against Mycobacterium tuberculosis H37Rv. Phillipp Agric. Sci. 2008, 91, 1–4. [Google Scholar]
- Castellar, A.; Coelho, T.S.; Silva, P.E.A.; Ramos, D.F.; Lourenço, M.C.S.; Lage, C.L.S.; Julião, L.S.; Barbosa, Y.G.; Leitão, S.G. The activity of flavones and oleanolic acid from Lippia lacunosa against susceptible and resistant Mycobacterium tuberculosis strains. Braz. J. Pharmacogn. 2011, 21, 835–840. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiler assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Calixto, N.O.; Pinto, M.E.F.; Ramalho, S.D.; Burger, M.C.M.; Bobey, A.F.; Young, M.C.M.; Bolzani, V.S.; Pinto, A.C. The Genus Psychotria: Phytochemistry, Chemotaxonomy, Ethnopharmacology and Biological Properties. J. Braz. Chem. Soc. 2016, 27, 1355–1378. [Google Scholar]
- Wang, C.Y.; Jang, H.-J.; Han, Y.K.; Su, X.D.; Lee, S.W.; Rho, M.-C.; Wang, H.-S.; Yang, S.Y.; Kim, Y.H. Alkaloids from Tetrastigma hemsleyanum and Their Anti-Inflammatory Effects on LPS-Induced RAW264.7 Cells. Molecules 2018, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cao, L.; Cheng, Y.; Meng, Z.-Q.; Tang, Z.-H.; Liu, W.-J.; Wang, Z.-Z.; Ding, G.; Xiao, W. In vivo anti-inflammatory and analgesic activities of strictosamide from Nauclea officinalis. Pharm. Biol. 2014, 52, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, Y.-Z.; Chen, J.-X.; Tang, Y.-Z. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7,8-dihydroxyflavone. J. Pharm. Pharmacol. 2017, 69, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.C.M.; Gomes, L.L.; Amaral, E.P.; Andrade, M.R.M.; Almeida, F.M.; Rezende, A.L.; Lanes, V.R.; Carvalho, E.C.Q.; Suffys, P.N.; Mokrousov, I.; et al. Mycobacterium tuberculosis Strains of the Modern Subline age of the Beijing Family Are More Likely to Display Increased Virulence than Strains of the Ancient Sublineage. J. Clin. Microbiol. 2014, 52, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- Moodley, S.; Koorbanally, N.A.; Moodley, T.; Ramjugernath, D.; Pillay, M. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalcones. J. Microbiol. Methods 2014, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Park, P.-H.; Kim, H.S.; Jin, X.Y.; Jin, F.; Hur, J.; Ko, G.; Sohn, D.H. KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. Eur. J. Pharmacol. 2009, 606, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Muzitano, M.F.; Cruz, E.A.; De Almeida, A.P.; Da Silva, S.A.; Kaiser, C.R.; Guette, C.; Bergmann, B.R.; Costa, S.S. Quercitrin: An Antileishmanial Flavonoid Glycoside from Kalanchoe pinnata. Planta Med. 2006, 72, 81–83. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of some compounds are available from the authors. |
| 11 | Literature * | ||||
|---|---|---|---|---|---|
| HSQC | HMBC | ||||
| C | δC | δH | 2JCH | 3JCH | δC ** |
| 3 | 107.97 | - | 2H-1′; H-2 | 2H-2′; H-5 | 110.8 |
| 4 | 126.65 | - | 2H-1′; H-2; H-6; H-8 | 127.0 | |
| 9 | 136.78 | - | H-2; H-5; H-7 | 136.5 | |
| CH | |||||
| 2 | 122.95 | 7.25 (s) | 2H-1′ | 123.8 | |
| 5 | 118.77 | 7.64 (d, 8.0 Hz) | H-7 | 118.9 | |
| 6 | 117.53 | 7.09 (t, 7.5 Hz) | H-8 | 118.7 | |
| 7 | 121.44 | 7.15 (t, 7.5 Hz) | H-5 | 121.7 | |
| 8 | 111.20 | 7.41 (d, 8.0 Hz) | H-6 | 111.9 | |
| CH2 | |||||
| 1′ | 18.95 | 3.27 (m) | 2H-2′ | 19.0 | |
| 2′ | 66.47 | 3.62 (m) | 2H-1′ | NMe3 | 65.5 |
| CH3 | - | ||||
| N-M3 | 52.27 | 3.23 (s) | 52.5 | ||
| Substances | MIC (µg/mL) | |
|---|---|---|
| H37Rv | M299 | |
| Pomolic acid (1) and spinosic acid (2) | 19.2 ± 0.2 | Inactive at 100 µg/mL |
| Strictosidine (18) | 7.1 ± 0.6 | 33.1 ± 0.8 |
| 5α-carboxystrictosidine (19) | 26.3 ± 1.9 | Inactive at 100 µg/mL |
| Rifampicin 1 | 0.2 ± 0.1 | 1.1 ± 0.1 |
| IC50 (µg/mL) | ||
|---|---|---|
| Compound | NO | MTT |
| 1 + 2 | 25.5 ± 0.1 | 13.17±0.1 |
| 18 | 3.22 ± 0.1 | 90.7±0.1 |
| 19 | 3.44 ± 0.1 | >500 |
| L-NMMA1 | 78.3 ± 6.5 | >100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho Junior, A.R.; Oliveira Ferreira, R.; de Souza Passos, M.; da Silva Boeno, S.I.; Glória das Virgens, L.d.L.; Ventura, T.L.B.; Calixto, S.D.; Lassounskaia, E.; de Carvalho, M.G.; Braz-Filho, R.; et al. Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra. Molecules 2019, 24, 1026. https://doi.org/10.3390/molecules24061026
de Carvalho Junior AR, Oliveira Ferreira R, de Souza Passos M, da Silva Boeno SI, Glória das Virgens LdL, Ventura TLB, Calixto SD, Lassounskaia E, de Carvalho MG, Braz-Filho R, et al. Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra. Molecules. 2019; 24(6):1026. https://doi.org/10.3390/molecules24061026
Chicago/Turabian Stylede Carvalho Junior, Almir Ribeiro, Rafaela Oliveira Ferreira, Michel de Souza Passos, Samyra Imad da Silva Boeno, Lorena de Lima Glória das Virgens, Thatiana Lopes Biá Ventura, Sanderson Dias Calixto, Elena Lassounskaia, Mario Geraldo de Carvalho, Raimundo Braz-Filho, and et al. 2019. "Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra" Molecules 24, no. 6: 1026. https://doi.org/10.3390/molecules24061026
APA Stylede Carvalho Junior, A. R., Oliveira Ferreira, R., de Souza Passos, M., da Silva Boeno, S. I., Glória das Virgens, L. d. L., Ventura, T. L. B., Calixto, S. D., Lassounskaia, E., de Carvalho, M. G., Braz-Filho, R., & Curcino Vieira, I. J. (2019). Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids from Psychotria nuda (Cham. & Schltdl.) Wawra. Molecules, 24(6), 1026. https://doi.org/10.3390/molecules24061026






