Abstract
Twenty-five new derivatives of 8-hydroxycycloberberine (1) were synthesized and evaluated for their activities against Gram-positive bacteria, taking 1 as the lead. Part of them displayed satisfactory antibacterial activities against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA), as well as vancomycin-intermediate Staphylococcus aureus (VISA). Especially, compound 15a displayed an excellent anti-MRSA activity with MICs (minimum inhibitory concentrations) of 0.25–0.5 μg/mL, better than that of 1. It also displayed high stability in liver microsomes and whole blood, and the LD50 value of over 65.6 mg·kg−1 in mice via intravenous route, suggesting a good druglike feature. The mode of action showed that 15a could effectively suppress topo IV-mediated decatenation activity at the concentration of 7.5 μg/mL, through binding a different active pocket of bacterial topo IV from quinolones. Taken together, the derivatives of 1 constituted a promising kind of anti-MRSA agents with a unique chemical scaffold and a specific biological mechanism, and compound 15a has been chosen for the next investigation.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA), classified as serious threat pathogen, has become a main cause of hospital and community-acquired infections across the world [1,2,3,4]. In the US alone, at least two million illnesses and 23,000 deaths are caused by multidrug-resistant bacterial infections every year according to data released by the Centers for Disease Control and Prevention (CDC) [1]. Thereinto, around 19,000 deaths and 360,000 hospitalizations resulted from infections of MRSA along with $3–4 billion in healthcare costs [5]. Vancomycin, linezolid and daptomycin have been used as the last resort for MRSA infections in the clinic. Unfortunately, vancomycin-intermediate S. aureus (VISA), heteroresistant VISA (hVISA), vancomycin-resistant S. aureus (VRSA), linezolid-resistant S. aureus and daptomycin-resistant S. aureus have been successively reported [6,7,8,9,10]. Especially, MRSA/VISA have been listed as global priority pathogens of antibiotic-resistant bacteria released by the World Health Organization (WHO) on Feb 27th 2017 [11], and the treatment of MRSA/VISA infections has become severe concerns across the world. Therefore, great efforts must be made to explore alternative agents witH-Novel structure scaffold or mechanism of action for the treatment of invasive life-threatening infections arising from MRSA/VISA.
In the past several years, our team has been committed to the discovery and development of novel antibacterial agents against multidrug-resistant pathogens, witH-Novel chemical entities and biological mode of action [12,13]. We first discovered that the cycloberberine (CBBR, Figure 1), generated from berberine (BBR) in our lab [14], was a special molecular scaffold against both methicillin-susceptible S. aureus (MSSA) and MRSA, as well as VISA [15,16]. The primary structure–activity relationships (SAR) demonstrated that introducing a suitable mono-substituent at the 8- or 13-position of CBBR could significantly enhance the antibacterial activity against MSSA/MRSA [15,16], though CBBR itself had no bactericidal activity at all, as shown in Figure 1. The top compound 8-hydroxycycloberberine (1, Figure 1) displayed a promising effect against the tested strains such as MRSA with MIC values of 0.5−>64 μg/mL, which is much higher than that of levofloxacin (Lev) widely used in the clinic.
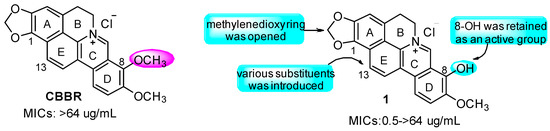
Figure 1.
Structures of CBBR and 1 as well as modification strategies.
Interestingly, compared with CBBR possessing a 8-OCH3 group, compound 1 with 8-OH showed a greatly significant improvement in activity against both MSSA and MRSA, indicating that the 8-OH function might play a critical role in enhancing the antibacterial effects. The unique chemical scaffold and biological activity of 1 encouraged us to continue a new round of SAR of its derivatives, aiming at developing these compounds into a new class of drug candidates against MRSA. Therefore, in the present study, as depicted in Figure 1, taking compound 1 as the lead, the 8-OH in CBBR was retained, and various substituents were respectively attached on the 13-position, by which a series of new 1 derivatives were designed, synthesized and then evaluated for their antibacterial effect against MSSA and MRSA. Additionally, the stability in liver microsomes and whole blood, toxicity in vivo as well as the bactericidal mechanism studies of the representative compounds were carried out as well.
2. Results and Discussion
2.1. Chemistry
The synthetic routes to all twenty-five target compounds are displayed in Scheme 1 and Scheme 2 respectively. As shown in Scheme 1, palmatine was selectively reduced to dihydropalmatine (2) in 89% yield using NaBH4 as a reducing agent in the presence of 5% NaOH/K2CO3 using a previous procedure [14]. Compound 2 was then reacted with 40% glyoxal in refluxing HOAc/CH3CN to give the intermediate 13-acetaldehyde palmatine (3), to which CH3OH/HCl (2/1 by vol.) was added directly to complete the cyclization reaction and the desired product cyclopalmatine (4) was obtained in an overall yield of 62%. Then, compound 4 was heated at 195–210 °C under vacuum (20–30 mmHg) to get the key intermediate 5. Finally, product 6 was acquired with a combined 90% conversion via acidification of compound 5 with concentrated HCl/EtOH (5/95 by vol.) by a classic keto-enol tautomerism.
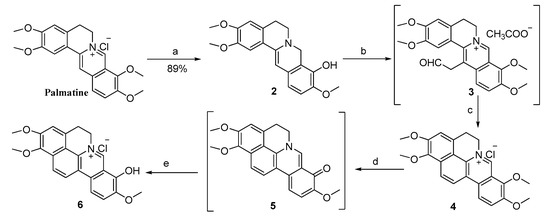
Scheme 1.
Reagents and conditions: (a) NaBH4, 5% NaOH/K2CO3, CH3OH, room temperature, 3 h; (b) 40% glyoxal, HOAc/CH3CN, reflux, 6 h; (c) CH3OH/HCl (2/1 by vol.), room temperature, 24 h, 62% (over two steps); (d) 20–30 mmHg, 195–210 °C, 40 min; (e) HCl/EtOH (5/95 by vol.), 90% (over two steps).
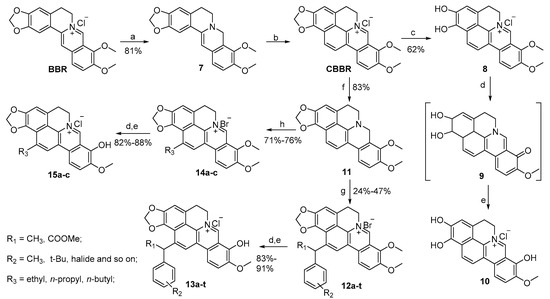
Scheme 2.
Reagents and conditions: (a) NaBH4, 5% NaOH, K2CO3, CH3OH, r.t., 3 h; (b) (i) 40% glyoxal, HOAc, CH3CN, reflux, 6 h; (ii) CH3OH/HCl (2/1 by vol.), room temperature, 24 h, 67% (over two steps); (c) benzene-1,3,5-triol, 60% H2SO4; (d) 20–30 mmHg, 195–210 oC, 40 min; (e) HCl/EtOH (5/95 by vol.), 39% (over two steps); (f) NaBH4, K2CO3, CH3OH, room temperature, 12 h; (g) substituted benzyl bromide, K2CO3 (2.0 eq.), CH3CN, reflux, 3–24 h; (h) aliphatic aldehyde, HOAc, 80% EtOH, reflux, 8 h.
As described in Scheme 2, CBBR was prepared from BBR using a previously reported procedure [14]. Compound 8 was prepared via demethylenation of CBBR with benzene-1,3,5-triol and 60% H2SO4 in a yield of 62%. The demethylation of 8 yielded intermediate 9, which was converted quickly to 1,2,8-trihydroxyl 10 in the presence of concentrated HCl/EtOH (5/95) in a combined 39% yield. Dihydrocycloberberine (11) was prepared in 83% yield via reduction of CBBR witH-NaBH4 as a reducing agent in CH3OH in the presence of K2CO3 [15]. The compounds in series 12 were generated in 24–47% yields from 11 and diverse substituted benzyl bromides at reflux temperature.
Similarly, compounds 12a–t were heated at 195–210 °C for 40 min under vacuum (20–30 mmHg) and acidified using concentrated HCl/EtOH (5/95) to produce the target compounds 13a–t in good yields. Meanwhile, the products in series 14 were obtained in good yields from 11 and diverse aliphatic aldehydes in HOAc and 80% EtOH at reflux temperature. The products 15a–c were generated from 14a–c using a similar synthetic method as for compounds 13a–t. All the desired products were purified with flash column chromatography on silica gel using CH2Cl2 and CH3OH as eluents or washed with 95% EtOH. The 1H-NMR, 13C-NMR and HRMS data of compound 15a is provided in Supplementary Materials.
2.2. Pharmacological Evaluation
2.2.1. SAR for Anti-Bacterial Activity
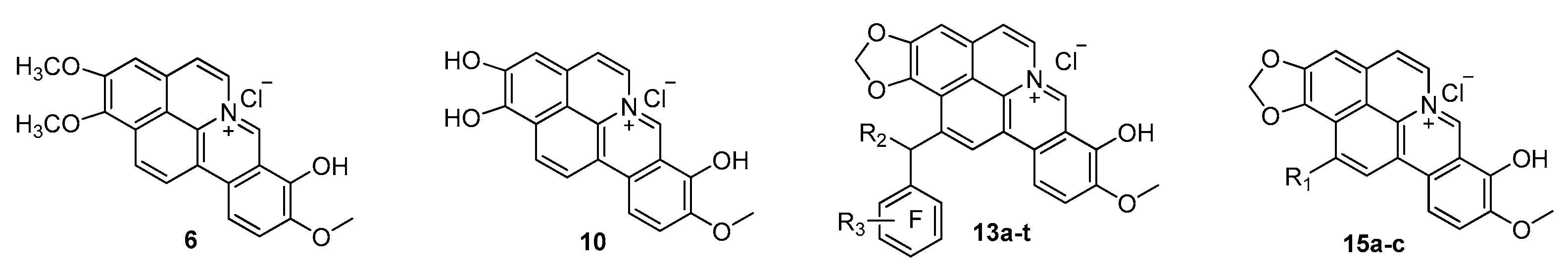
SAR study for antibacterial activity against drug-susceptible Gram-positive bacteria was initially carried out, taking Lev and compound 1 as reference drugs. The chemical structures of 25 target compounds and their MIC values against a panel of drug-susceptible strains [17], including MSSA, methicillin-susceptible S. epidermidis (MSSE), S. saprophyticus (S. s) and S. hominis (S. h), were summarized in Table 1. Bacteria strains used in this study were from the ATCC collection and clinical isolates in Chinese hospitals. The influence of the methylenedioxy ring was explored first, and compounds 6 and 10 were created and tested. As shown in Table 1, both of them lost the antimicrobial activities partially or completely, suggesting that methylenedioxy ring might be essential for the activity against Gram-positive strains.

Table 1.
Antimicrobial activities of the target compounds against drug-susceptible Gram-positive strains (MIC (μg/mL) a).
Then, we moved SAR analysis to the influence of substituent at the 13-position in compound 1. Our previous SAR results on CBBR indicated that the introduction of a 13-benzyl motif was beneficial for the activity [16], therefore, various benzyl moieties with electron-donating and electron-withdrawing groups on the benzene ring were respectively introduced into position 13, and 20 new analogs 13a–t were constructed and tested. As described in Table 1, compound 13a with a 13-benzyl displayed moderate antimicrobial activity with MICs of 8–16 μg/mL, while the compounds 13b–d with fluoro atoms as an isostere of the hydrogen atom lost their potency to varying degrees. However, the compounds 13e and 13f with p-chloro or p-bromo groups showed preferable antimicrobial activities with MIC values of 2–8 μg/mL. Compounds 13h and 13i bearing m-methyl and p-methyl gave potent activities with MICs of 2–16 μg/mL, less than that of lead 1. Next, compounds 13o and 13p with p-CN and o-NO2 respectively displayed moderate antimicrobial activities with most MIC values in the 4–16 μg/mL range, while other compounds with electron-withdrawing groups showed partially or completely diminished antimicrobial activities.
Furthermore, a methyl or methyloxycarbonyl group was introduced to the α-C atom of ring E to generate 13s and 13t, respectively, and both of them showed decreased activity to different degrees. Finally, an ethyl, n-propyl or n-butyl were respectively introduced at the 13-position of 1, whereby three new analogs 15a–c were prepared and investigated. All of them exhibited good activities against the tested strains with MIC values of 0.25–16 μg/mL, and compound 15a showed especially potent activities, with MIC values of 0.25–1.0 μg/mL, superior or similar to those of the lead compound 1. The results thus indicate that introducing a small-sized substituent at the 13-position might enhance the activity.
All target compounds were then measured for their antibacterial activities against drug-resistant organisms, such as MRSA/VISA and methicillin-resistant S. epidermidis strains (MRSE). As listed in Table 2, their potencies against MRSE/MRSA/VISA strains were basically consistent with those against drug-susceptible ones. Most of compounds showed potential anti-MRSA and anti-VISA effects with MIC values ranging from 1 to 64 μg/mL. Compounds 13e–f, 13h–i, 13o–p and 15a–c displayed promising effects, with MIC values ranging from 0.25 to 8 μg/mL. Especially, compound 15a displayed an excellent effect against MRSA and VISA with MIC values of 0.25–0.5 μg/mL, better than that of 1, and then it was selected as the representative compound for the next investigation.

Table 2.
Antibacterial activities of the target compounds against drug-resistant Gram-positive strains (MIC (μg/mL)).
2.2.2. Time-kill Curve Study of 15a
To further confirm the antibacterial activities of this kind of compounds, the time–kill curve assay of compound 15a for MRSA strain isolated from Chinese patients was carried out subsequently with DMSO as a negative control. As shown in Figure 2, distinct reductions of the viable cell counts were observed after 4 h exposure of the bacteria to 15a at concentrations of 2× and 8×MIC. Especially, it exhibited significant time-dependent action and the maximum reduction in viable counts was over 6 log10CFU/mL in 12 h for the 8× MIC, therefore, it was determined that 15a was a marginal bactericide [18] at the concentration of 8× MIC.
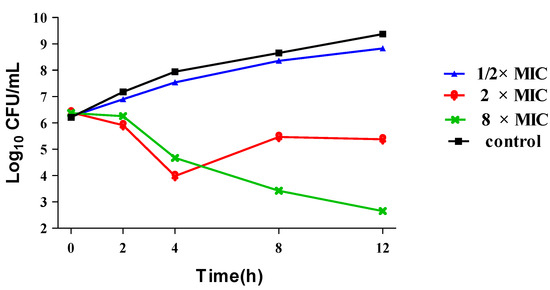
Figure 2.
Time-kill curve assay of compound 15a over a 12 h incubation period at 37 °C against MRSA 18-4 (MIC = 0.5 μg/mL). Samples were taken at 2, 4, 8 and 12 h to determine viable bacterial numbers.
2.2.3. Evaluation of Metabolic Stability and Acute Toxicity Assay of Compound 15a
The metabolic stability of compound 15a in liver microsomes was conducted in human and rat as well as mouse with the clinical reagent testosterone (Tes) as reference. As depicted in Table 3, compared to Tes, compound 15a showed an obviously longer half-life (T1/2) and residual ratio, as well as lower microsomal intrinsic clearance in all three species. Especially, the T1/2 of compound 15a was over 105 min and the remaining ratio was still 70.6% after 60 min in human liver microsomes, indicating a good characteristic of metabolic stability.

Table 3.
Metabolic stability of tested compound in human, rat and mouse liver microsomes a.
Next, a stability assay of compound 15a was carried out in whole blood in vitro, taking enalapril (Ems) [15,16] as the positive control. As indicated in Table 4, as expected, compound 15a gave an exciting stability profile and the remaining ratio was still over 85.4% after 24 h in blood, while Ems was completely decomposed within three hours. The results suggest that 15a might possess an ideal in vivo metabolic stability.

Table 4.
Metabolic stability of 15a in whole blood.
Then, acute toxicity test on compound 15a was performed in Kunming mice through intravenous route. The results indicated that compound 15a gave a half-lethal dose (LD50) value of 65.6 mg·kg−1, suggesting a good safety profile.
2.2.4. Preliminary Mechanism of Compound 15a
Taking into account the planar rigid structure similarity between 15a and quinolones, we initially examined its effects on gyrase and topoisomerase (topo) IV activities of S. aureus by gyrase-mediated supercoiling of relaxed DNA assay and topo IV-mediated decatenation of kinetoplast DNA (kDNA) assay [19,20], respectively. As shown in Figure 3, topo IV-mediated decatenation activity was significantly inhibited in a concentration-dependent manner after treatment of 15a. It could partially inhibit topo IV activity at the concentration of 7.5 μg/mL (lane 5), and block almost completely the decatenation reaction at the concentration of 15 μg/mL (lane 7). However, inhibition activity on gyrase-mediated supercoiling was not distinctly observed after treatment with 15a at even upped to 100 μg/mL. These results indicated that 15a could effectively suppress the topo IV-mediated decatenation activity, but a different mode of action from quinolones.
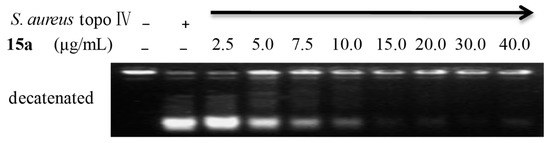
Figure 3.
Inhibition of S. aureus topo IV. In all panels, enzyme and 15a are absent (-) in lane 1, enzyme present (+) and 15a absent in lane 2, and enzyme (arrow) and 15a present in lanes 3–10 with different concentration. Decatenation activity of topo IV was partially blocked by 15a (lanes 4, 5, 6) and completely blocked by 15a (lanes 7, 8, 9, 10).
2.2.5. Molecular Docking Study of Key Compound 15a
It was reported that topo IV enzyme of Gram-positive bacteria possesses at least two active pockets, one is the well-known quinolone-binding cavity and the other is kibdelomycin-binding site [21,22,23]. In view of the good activity of compound 15a against quinolone-resistant MRSA or VISA, we performed molecular docking between the topo IV binding site of kibdelomycin and compound 15a interactions using Discovery Studio 4.5 software (Edition 4.5; BIOVIA: San Diego, CA, USA, 2015) [17,24]. As expected, compound 15a fit well in the active hydrophobic pocket of the binding site (Figure 4A, brown area). One conventional hydrogen bond with GLU53, two attractive charges formed by nitrogen ions with ASP76 and GLU53, van der Waals forces and hydrophobic interactions (Figure 4B) contributed together to the strong interactions. The results suggested that 15a might bind to the kibdelomycin-pocket site on topo IV, a different mode of action from the quinolones widely used in the clinic.
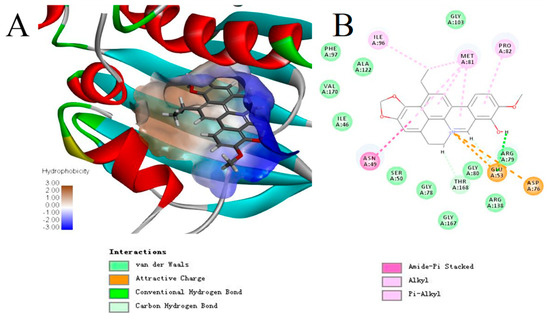
Figure 4.
(A) Solid surface map of the interaction pocket with compound 15a. Red, blue, and white colored regions correspond to negatively charged, positively charged, and neutral areas, respectively. (B) Binding modes within the receptor S. aureus topo IV active pocket. These figures were produced using the Discovery Studio 4.5 software. The receptor structure is shown in surface form. Key bonds are indicated by dashed lines between the atoms involved, and the colors of key bonds and residues are shown according to the interaction mode (van der Waals, light green; attractive charge, orange; hydrogen bond, green; carbon hydrogen bond, celadon; amide-π stacked, amaranth; alkyl and π-alkyl, mauve pink).
3. Experimental Section
3.1. Apparatus, Materials, and Analysis Reagents
Melting points (m.p.) were obtained with a CXM-300 melting point apparatus (Shanghai Changfang Optical Instrument Co., Ltd., Shanghai, China) and are uncorrected. The 1H-NMR spectra was recorded on an Inova 500 or 600 MHz spectrometer (Varian, San Francisco, CA, USA) and 13C-NMR on an Avance III 400, 500 or 600 spectrometer (Bruker, Zürich, Switzerland), with Me4Si as the internal standard. High-resolution mass spectra (HRMS-ESI) data was recorded on an Autospec UItima-TOF mass spectrometer (Micromass UK Ltd., Manchester, UK). Flash chromatography (particle size 0.038 mm) was performed on a CombiflashRf 200 system (Teledyne, Lincoln, NE, USA).
3.2. Chemistry
3.2.1. Synthesis of 1,2-Dimethoxy-8-hydroxy-9-methoxycycloberberine Chloride (6)
Palmatine (7.7 g, 20 mmol) and K2CO3 (8.3 g, 60 mmol) were dissolved in CH3OH (250 mL), and then 5% NaOH (10 mL) solution containing NaBH4 (0.83 g, 22 mmol) was added dropwise. The reaction mixture was stirred at room temperature for 3 h. After the reaction was complete, the precipitated solid was filtered, washed with distilled water (100 mL) and 80% EtOH (100 mL) to give yellow green solid 2. Intermediate 2 (5.6 g, 16 mmol) was then reacted with 40% glyoxal (3 mL) in a stirred solvent mixture of CH3CN (160 mL) and HOAc (40 mL), which was refluxed for 6 h to prepare compound 3. The solvent was evaporated under vacuum, and 2 N HCl/CH3OH (100 mL) was added to the residue. The reaction mixture was stirred at room temperature for 24 h, then evaporated under vacuum and recrystallized from 95% EtOH to obtain scarlet solid 4; Yield: 62%, m.p.: 180–182 °C. 1H- NMR (DMSO-d6) δ 10.15 (s, 1H), 8.87 (d, J = 9.2 Hz, 1H), 8.83 (d, J = 9.2 Hz, 1H), 8.40 (d, J = 9.2 Hz, 1H), 8.27 (d, J = 9.2 Hz, 1H), 7.70 (s, 1H), 5.26 (t, J = 6.8 Hz, 2H), 4.15 (s, 3H), 4.09 (s, 3H), 4.03 (s, 3H), 3.94 (s, 3H), 3.65 (t, J = 6.8 Hz, 2H). ESI-MS m/z: 376.15. Then, 4 (3.7 g, 9.1 mmol) was heated at 195–210 °C in a dry oven under vacuum (20–30 mmHg) for 40 min. After completion of the reaction, the crude material 5 was obtained, which was used without further purification. Next 5 was acidified with concentrated HCl/EtOH (5:95 by vol.) for 10 min. Then the mixture was evaporated under vacuum and purified with flash column chromatography on silica gel using a CH2Cl2 and CH3OH gradient as eluent to obtain the red solid compound 6; yield: 90%; m.p.: 176–178 °C; 1H-NMR (DMSO-d6) δ 10.07 (s, 1H), 8.78 (d, J = 9.6 Hz, 1H), 8.40–8.32 (m, 2H), 8.06 (d, J = 8.4 Hz, 1H), 7.67 (s, 1H), 5.18 (t, J = 6.6 Hz, 2H), 4.04 (s, 6H), 3.95 (s, 3H), 3.62 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 151.0, 146.9, 146.1, 141.3, 128.4, 128.0 (2), 127.4, 127.3, 123.8, 123.7, 121.8, 121.0, 116.0, 115.8, 114.5, 112.6, 61.2, 56.8, 56.6, 54.9, 26.3; HRMS: calcd for C22H20NO4Cl [M − Cl]+: 362.1387, found: 362.1390.
3.2.2. Synthesis of 1,2,8-Trihydroxy-9-methoxycycloberberine Chloride (10)
CBBR (3.95 g, 10 mmol) and phloroglucin (5.0 g, 40 mmol) were added into 60% H2SO4 (20 mL), and the reaction mixture was stirred at 90 °C for 10 h. Then the mixture was cooled, poured into saturated sodium chloride solution (10 mL) and filtered. The residue was washed with water and redissolved in 1 N NaOH (60 mL). After that, 2 N HCl (60 mL) was added and the mixture filtered. The residue was purified with flash column chromatography on silica gel using CH2Cl2 and CH3OH to obtain the intermediate 8 in 62% yield. Next 8 was heated at 195–210 °C in a dry oven under vacuum (20–30 mmHg) for 40 min to create the crude material 9, which was acidified in concentrated HCl/EtOH (5:95 by vol.) for 10 min. Then the mixture was filtered and the residue was washed with water, CH2Cl2 and EtOH to afford the title compound 10 as a black-purple solid; yield: 39%; m.p: 191–193 °C; 1H-NMR (DMSO-d6) δ 10.30 (s, 1H), 10.00 (s, 1H), 9.55 (s, 1H), 8.67 (d, J = 9.6 Hz, 1H), 8.44–8.35 (m, 2H), 8.08 (d, J = 9.0 Hz, 1H), 7.29 (s, 1H), 5.11 (t, J = 6.6 Hz, 2H), 4.03 (s, 3H), 3.48 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 146.2, 145.5, 144.1, 137.9, 128.6, 127.6, 124.2, 123.9 (3), 122.1, 121.2, 118.8, 117.3, 115.7, 114.8, 56.7, 55.9, 55.2, 25.6; HRMS: calcd for C20H16NO4Cl [M − Cl]+: 334.1074, found: 334.1079.
3.2.3. General Procedure for the Synthesis of 13a–t
To a stirred solution of compound 11 (361 mg, 1.0 mmol, 1.0 equiv) and K2CO3 (2.0 equiv) in anhydrous CH3CN (20 mL) were added various benzyl bromides (15.0 equiv). The reaction mixture was refluxed for 3–24 h, cooled, the mixture was filtered and the filtrate was purified by flash column chromatography on silica gel using a gradient of CH2Cl2 and CH3OH as eluent to obtain the compounds 12a–t. Then 12a–t were heated at 195–210 °C in a dry oven under vacuum (20–30 mmHg) for 40 min to create the crude materials, which were acidified in concentrated HCl/EtOH (5:95 by vol.) for 10 min. Then the mixture was evaporated under vacuum and purified by flash column chromatography on silica gel using a CH2Cl2 and CH3OH gradient as eluent to obtain the desired compounds 13a–t.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-benzylcycloberberine chloride (13a): red solid; yield: 78%; m.p.: 248–250 °C; 1H-NMR (CDCl3/CD3OD) δ 9.91 (s, 1H), 8.20 (s, 1H), 8.11 (d, J = 9.0 Hz, 1H), 7.99 (d, J = 9.0 Hz, 1H), 7.39 (s, 1H), 7.36–7.31 (m, 2H), 7.29–7.24 (m, 3H), 6.22 (s, 2H), 5.14 (t, J = 6.6 Hz, 2H), 4.78 (s, 2H), 4.11 (s, 3H), 3.66 (t, J = 6.6 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 148.6, 147.2, 146.7, 143.3, 140.5, 140.0, 129.6 (2), 129.2 (2), 128.8, 128.3, 127.1 (2), 126.8, 124.7, 123.6, 121.5, 119.4, 118.1, 116.8, 114.2, 111.3, 102.9, 57.2, 56.7, 41.1, 28.1; HRMS: calcd for C28H22NO4Cl [M – Cl]+: 436.1543, found: 436.1535.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-fluorobenzylcycloberberine chloride (13b): red solid; yield: 83%; m.p.: 203–205 °C; 1H-NMR (CDCl3/CD3OD) δ 9.93 (s, 1H), 8.33 (s, 1H), 8.22 (d, J = 9.0 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.41 (s, 1H), 7.30–7.24 (m, 2H), 7.04–6.98 (m, 2H), 6.19 (s, 2H), 5.12 (t, J = 6.6 Hz, 2H), 4.74 (s, 2H), 4.11 (s, 3H), 3.64 (t, J = 6.7 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 162.8, 149.0, 147.6, 147.2, 146.7, 143.3, 140.3, 136.9, 131.5, 131.4, 129.4, 128.7, 127.9, 125.1, 123.9, 122.2, 119.5, 118.7, 117.2, 116.1, 116.0, 114.7, 111.6, 103.4, 57.4, 57.0, 40.5, 28.3; HRMS: calcd for C28H21NO4FCl [M − Cl]+: 454.1449, found: 454.1447.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-2′,4′-difluorobenzylcycloberberine chloride (13c): red solid; yield: 78%; m.p.: 218–220 °C; 1H-NMR (DMSO-d6) δ 11.56 (s, 1H), 10.09 (s, 1H), 8.54 (s, 1H), 8.29 (d, J = 9.0 Hz, 1H), 8.15 (d, J = 9.0 Hz, 1H), 7.56 (s, 1H), 7.30–7.26 (m, 1H), 7.00–6.93 (m, 2H), 6.19 (s, 2H), 5.17 (t, J = 6.6 Hz, 2H), 4.67 (s, 2H), 4.07 (s, 3H), 3.59 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 161.7, 160.1, 147.3, 147.0, 146.3, 146.2, 141.7, 136.0, 131.2, 128.6, 127.7, 127.4, 124.9, 123.7, 122.4, 122.3, 118.1, 117.6, 116.3, 114.0, 111.8, 111.0, 104.2, 102.5, 57.4, 55.4, 32.7, 26.9; HRMS: calcd for C28H20NO4F2Cl [M − Cl]+: 472.1355, found: 472.1346.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-2′,6′-difluorobenzylcycloberberine chloride (13d): red solid; yield: 73%; m.p.: 214–216 °C; 1H-NMR (DMSO-d6) δ 11.57 (s, 1H), 10.07 (s, 1H), 8.11 (d, J = 9.0 Hz, 1H), 7.90 (s, 1H), 7.84 (d, J = 9.0 Hz, 1H), 7.60 (s, 1H), 7.56–7.50 (m, 1H), 7.27–7.20 (m, 2H), 6.33 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.77 (s, 2H), 4.02 (s, 3H), 3.59 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 161.5 (2), 147.5, 147.0, 146.5, 146.2, 142.1, 136.3, 130.3, 128.5, 127.7, 127.1, 125.0, 121.8, 118.7, 117.9, 117.4, 116.3, 114.3, 113.1, 112.4, 112.3, 111.1, 102.6, 57.3, 55.4, 27.6, 26.9; HRMS: calcd for C28H20NO4F2Cl [M − Cl]+: 472.1355, found: 472.1342.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-chlorobenzylcycloberberine chloride (13e): red solid; yield: 88%; m.p.: 199–201 °C; 1H-NMR (CDCl3/CD3OD) δ 9.93 (s, 1H), 8.27 (s, 1H), 8.19 (d, J = 8.4 Hz, 1H), 8.02 (d, J = 8.4 Hz, 1H), 7.39 (s, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 6.21 (s, 2H), 5.14 (t, J = 6.6 Hz, 2H), 4.75 (s, 2H), 4.12 (s, 3H), 3.67 (t, J = 6.6 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 148.6, 147.3, 146.8, 146.1, 143.1, 139.7, 138.9, 132.9, 131.0 (2), 129.2 (2), 128.9, 128.3, 127.0, 124.9, 123.6, 121.9, 119.2, 118.2, 116.8, 114.4, 111.4, 103.0, 57.3, 56.8, 40.5, 28.1; HRMS: calcd for C28H21NO4Cl2 [M − Cl]+: 470.1154, found: 470.1144.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-bromobenzylcycloberberine chloride (13f): red solid; yield: 88%; m.p.: 207–209 °C; 1H-NMR (CDCl3/CD3OD) δ 9.93 (s, 1H), 8.22 (s, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 7.8 Hz, 2H), 7.38 (s, 1H), 7.15 (d, J = 8.4 Hz, 2H), 6.21 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.73 (s, 2H), 4.12 (s, 3H), 3.68 (t, J = 6.6 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 148.4, 147.1, 146.6, 145.7, 142.9, 139.4, 139.0, 132.1 (2), 131.1 (2), 128.6, 128.0, 126.6, 124.6, 123.4, 121.6, 120.8, 119.0, 117.9, 116.5, 114.1, 111.3, 102.8, 57.2, 56.6, 40.4, 28.0; HRMS: calcd for C28H21BrNO4Cl [M − Cl]+: 514.0648, found: 514.0653.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-o-methylbenzylcycloberberine chloride (13g): red solid; yield: 83%; m.p.: 203–205 °C; 1H-NMR (DMSO-d6) δ 11.54 (s, 1H), 10.09 (s, 1H), 8.34 (s, 1H), 8.15 (s, 2H), 7.57 (s, 1H), 7.29 (d, J = 7.8 Hz, 1H), 7.16 (t, J = 7.2 Hz, 1H), 7.05 (t, J = 7.2 Hz, 1H), 6.73 (d, J = 7.5 Hz, 1H), 6.16 (s, 2H), 5.18 (t, J = 6.6 Hz, 2H), 4.66 (s, 2H), 4.06 (s, 3H), 3.34 (s, 2H), 2.38 (s, 3H); 13C-NMR (DMSO-d6) δ 146.8, 146.2, 145.8, 145.7, 141.4, 138.2, 137.2, 135.8, 129.9, 129.5, 127.9, 127.7, 126.9, 126.1, 125.9, 124.4, 121.8, 121.1, 118.1, 117.1, 115.8, 113.2, 110.4, 101.9, 56.8, 54.9, 37.1, 26.4, 19.2; HRMS: calcd for C29H24NO4Cl [M − Cl]+: 450.1700, found: 450.1687.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-m-methylbenzylcycloberberine chloride (13h): red solid; yield: 79%; m.p.: 203–205 °C; 1H-NMR (DMSO-d6) δ 11.52 (s, 1H), 10.07 (s, 1H), 8.68 (s, 1H), 8.44 (d, J = 9.0 Hz, 1H), 8.17 (d, J = 9.0 Hz, 1H), 7.55 (s, 1H), 7.20–7.10 (m, 2H), 7.06 (d, J = 7.8 Hz, 1H), 6.99 (d, J = 7.2 Hz, 1H), 6.27 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.68 (s, 2H), 4.08 (s, 3H), 3.58 (t, J = 6.6 Hz, 2H), 2.23 (s, 3H); 13C-NMR (DMSO-d6) δ 146.7, 146.2, 145.7, 145.7, 141.2, 140.0, 137.8, 137.3, 129.5, 129.0, 128.2, 127.8, 127.0, 126.7, 125.5, 124.3, 122.0, 121.8, 117.7, 117.1, 115.8, 113.7, 110.4, 101.8, 56.9, 54.9, 39.7, 26.4, 21.0; HRMS: calcd for C29H24NO4Cl [M − Cl]+: 450.1700, found: 450.1687.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-methylbenzylcycloberberine chloride (13i): red solid; yield: 81%; m.p.: 229–231 °C; 1H-NMR (DMSO-d6) δ 11.51 (s, 1H), 10.06 (s, 1H), 8.67 (s, 1H), 8.43 (d, J = 9.0 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 7.55 (s, 1H), 7.17 (d, J = 7.8 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 6.26 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.66 (s, 2H), 4.08 (s, 3H), 3.57 (t, J = 6.6 Hz, 2H), 2.22 (s, 3H); 13C-NMR (DMSO-d6) δ 147.3, 146.7, 146.2, 146.2, 141.7, 138.6, 137.6, 135.5, 129.4 (2), 128.8 (2), 128.4, 127.6, 127.6, 124.8, 122.5, 122.2, 118.2, 117.7, 116.3, 114.3, 110.9, 102.4, 57.4, 55.4, 39.3, 27.0, 21.0; HRMS: calcd for C29H24NO4Cl [M − Cl]+: 450.1700, found: 450.1704.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-3′,5′-dimethylbenzylcycloberber ine chloride (13j): red solid; yield: 83%; m.p.: 218–220 °C; 1H-NMR (DMSO-d6) δ 11.48 (s, 1H), 10.03 (s, 1H), 8.64 (s, 1H), 8.42 (d, J = 9.0 Hz, 1H), 8.15 (d, J = 9.0 Hz, 1H), 7.53 (s, 1H), 6.87 (s, 2H), 6.78 (s, 1H), 6.25 (s, 2H), 5.12 (t, J = 6.6 Hz, 2H), 4.61 (s, 2H), 4.06 (s, 3H), 3.55 (t, J = 6.6 Hz, 2H), 2.15 (s, 6H); 13C-NMR (DMSO-d6) δ 146.7, 146.1, 145.7 (2), 141.2, 139.9, 137.9, 137.1, 129.5, 127.8, 127.5, 127.1, 127.0, 126.2 (2), 124.2, 122.0, 121.8, 117.7, 117.1, 115.8, 113.8, 110.4, 101.8, 56.9, 54.9, 39.5, 26.4, 20.9 (2); HRMS: calcd for C30H26NO4Cl [M − Cl]+: 464.1856, found: 464.1848.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-tert-butylmethylbenzylcyclo berberine chloride (13k): red solid; yield: 77%; m.p.: 212–214 °C; 1H-NMR (DMSO-d6) δ 11.51 (s, 1H), 10.06 (s, 1H), 8.73 (s, 1H), 8.48 (d, J = 9.0 Hz, 1H), 8.17 (d, J = 9.0 Hz, 1H), 7.56 (s, 1H), 7.30–7.21 (m, 4H), 6.29 (s, 2H), 5.14 (t, J = 6.6 Hz, 2H), 4.68 (s, 2H), 4.09 (s, 3H), 3.54 (t, J = 6.6 Hz, 2H), 1.21 (s, 9H); 13C-NMR (DMSO-d6) δ 148.3, 146.7, 146.1, 145.7, 141.1, 138.1, 137.2, 132.5, 128.0 (2), 127.8, 127.2, 127.0, 125.0 (2), 124.2, 122.0, 121.8, 117.6, 117.2, 115.8, 113.8, 110.3, 101.8, 56.9, 54.9, 48.5, 38.5, 31.0 (3), 26.4; HRMS: calcd for C32H30NO4Cl [M − Cl]+: 492.2169, found: 492.2158.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-trifluoromethoxybenzylcyclo berberine chloride (13l): red solid; yield: 77%; m.p.: 185–187 °C; 1H-NMR (DMSO-d6) δ 11.53 (s, 1H), 10.08 (s, 1H), 8.78 (s, 1H), 8.48 (d, J = 9.0 Hz, 1H), 8.17 (d, J = 9.0 Hz, 1H), 7.56 (s, 1H), 7.42 (d, J = 7.8 Hz, 2H), 7.26 (d, J = 8.4 Hz, 2H), 6.26 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.73 (s, 2H), 4.09 (s, 3H), 3.58 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 146.8, 146.6, 146.3, 145.7 (2), 141.0, 139.8, 137.1, 130.1 (2), 129.5, 127.9, 127.2, 127.0, 124.3, 122.2, 122.0, 120.9 (2), 117.4, 117.2, 115.8, 113.8, 110.4, 101.9, 56.9, 54.9, 38.4, 26.4; HRMS: calcd for C29H21NO5F3Cl [M − Cl]+: 520.1366, found: 520.1349.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-o-cyanbenzylcycloberberine chloride (13m): red solid; yield: 83%; m.p.: 209–211 °C; 1H-NMR (CDCl3/CD3OD) δ 9.96 (s, 1H), 8.27 (s, 1H), 8.14 (d, J = 9.0 Hz, 1H), 8.01 (d, J = 9.0 Hz, 1H), 7.80 (dd, J = 7.8, 1.2 Hz, 1H), 7.54 (td, J = 7.8, 1.2 Hz, 1H), 7.44 (t, J = 7.8 Hz, 1H), 7.40 (s, 1H), 7.19 (d, J = 7.8 Hz, 1H), 6.19 (s, 2H), 5.16 (t, J = 6.6 Hz, 2H), 4.98 (s, 2H), 4.12 (s, 3H), 3.68 (t, J = 6.6 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 148.6, 147.4, 146.8, 146.3, 144.0, 142.9, 137.5, 133.9, 133.8, 130.1, 129.0, 128.2, 128.0, 127.0, 124.9, 123.4, 122.2, 118.9, 118.6, 118.1, 116.7, 114.2, 113.2, 111.5, 103.0, 57.2, 56.7, 39.5, 28.0; HRMS: calcd for C29H21N2O4Cl [M − Cl]+: 461.1496, found: 461.1502.
1,2-Methylenedioxy-8-hydroxy-9-dimethoxy-13-m-cyanbenzylcycloberberine chloride (13n): red solid; yield: 87%; m.p.: 189–191 °C; 1H-NMR (CD3OD) δ 9.95 (s, 1H), 8.50 (s, 1H), 8.33 (d, J = 9.0 Hz, 1H), 8.07 (d, J = 9.0 Hz, 1H), 7.58 (td, J = 9.0, 1.2 Hz, 2H), 7.55 (s, 1H), 7.45 (t, J = 7.8 Hz, 1H), 7.42 (s, 1H), 6.15 (s, 2H), 5.13 (t, J = 6.6 Hz, 2H), 4.81 (s, 2H), 4.11 (s, 3H), 3.64 (t, J = 6.6 Hz, 2H); 13C-NMR (CD3OD) δ 151.0, 148.4, 146.9, 145.8, 142.5, 141.3, 138.2, 133.8, 132.3, 130.6, 129.8, 129.2, 128.5, 126.9, 126.3, 123.2, 121.7, 120.7, 119.0, 118.7, 118.5, 117.5, 112.6, 111.4, 102.5, 57.2, 56.8, 40.3, 27.6; HRMS: calcd for C29H21N2O4Cl [M − Cl]+: 461.1496, found: 461.1495.
1,2-Methylenedioxy-8-hydroxy-9-dimethoxy-13-p-cyanbenzylcycloberberine chloride (13o): red solid; yield: 83%; m.p.:197–199 °C; 1H-NMR (DMSO-d6) δ 11.54 (s, 1H), 10.09 (s, 1H), 8.78 (s, 1H), 8.46 (d, J = 9.0 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 7.74 (d, J = 8.4 Hz, 2H), 7.55 (s, 1H), 7.46 (d, J = 7.8 Hz, 2H), 6.21 (s, 2H), 5.16 (t, J = 6.6 Hz, 2H), 4.78 (s, 2H), 4.09 (s, 3H), 3.58 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 146.8, 146.4, 146.2, 145.8, 145.7, 140.9, 136.0, 132.2 (2), 129.31 (2), 128.0, 127.2, 127.0, 124.3, 122.5, 121.9, 118.8, 117.4, 117.1, 115.8, 113.8, 110.5, 108.9, 101.9, 56.9, 54.9, 39.2, 26.4; HRMS: calcd for C29H21N2O4Cl [M − Cl]+: 461.1496, found: 461.1500.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-o-nitrobenzylcycloberberine chloride (13p): red solid; yield: 82%; m.p.: 199–201 °C; 1H-NMR (DMSO-d6) δ 11.57 (s, 1H), 10.11 (s, 1H), 8.69 (s, 1H), 8.35 (d, J = 9.0 Hz, 1H), 8.18 (d, J = 9.0 Hz, 1H), 8.05 (dd, J = 7.8, 1.2 Hz, 1H), 7.56–7.46 (m, 3H), 7.07 (dd, J = 7.8, 1.2 Hz, 1H), 6.04 (s, 2H), 5.17 (t, J = 6.6 Hz, 2H), 4.88 (s, 2H), 4.08 (s, 3H), 3.58 (t, J = 6.6 Hz, 2H); 13C-NMR (DMSO-d6) δ 149.7, 147.3, 147.1, 146.4, 146.3, 141.4, 135.9, 134.8, 133.7, 131.0, 130.1, 128.6, 128.1, 127.8, 127.5, 124.9, 123.1, 122.3, 118.0, 117.6, 116.4, 114.2, 111.0, 102.3, 57.4, 55.4, 36.7, 26.9; HRMS: calcd for C28H21N2O6Cl [M − Cl]+: 481.1394, found: 481.1381.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-m-nitrobenzylcycloberberine chloride (13q): red solid; yield: 89%; m.p.: 186–188 °C; 1H-NMR (CDCl3/CD3OD) δ 9.97 (s, 1H), 8.47 (s, 1H), 8.31 (d, J = 8.4 Hz, 1H), 8.12–8.08 (m, 2H), 8.05 (d, J = 9.0 Hz, 1H), 7.69 (d, J = 7.8 Hz, 1H), 7.54 (t, J = 8.4 Hz, 1H), 7.40 (s, 1H), 6.20 (s, 2H), 5.16 (t, J = 6.6 Hz, 2H), 4.90 (s, 2H), 4.13 (s, 3H), 3.68 (t, J = 6.6 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 149.2, 148.7, 147.5, 146.9, 146.4, 142.8 (2), 138.2, 135.9, 130.2, 129.2, 128.4, 127.2, 125.0, 124.0, 123.7, 122.7, 122.2, 119.0, 118.3, 116.9, 114.5, 111.6, 103.0, 57.3, 56.8, 40.7, 28.1; HRMS: calcd for C28H21N2O6Cl [M − Cl]+: 481.1394, found: 481.1392.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-p-nitrobenzylcycloberberine chloride (13r): red solid; yield: 85%; m.p.: 186–188 °C; 1H-NMR (CDCl3/CD3OD) δ 9.98 (s, 1H), 8.49 (s, 1H), 8.33 (d, J = 9.0 Hz, 1H), 8.16 (s, 1H), 8.15 (s, 1H), 8.07 (d, J = 9.0 Hz, 1H), 7.47 (d, J = 8.4 Hz, 2H), 7.41 (s, 1H), 6.17 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.91 (s, 2H), 4.13 (s, 3H), 3.66 (t, J = 6.6 Hz, 2H); 13C-NMR (CDCl3/CD3OD) δ 148.9, 148.8, 147.6 (2), 147.1, 146.7, 143.0, 138.2, 130.4 (2), 129.4, 128.6, 127.6, 125.1, 124.4 (2), 123.8, 123.0, 119.2, 118.5, 117.1, 114.7, 111.7, 103.2, 57.4, 56.9, 41.1, 28.2; HRMS: calcd for C28H21N2O6Cl [M − Cl]+: 481.1394, found: 481.1390.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-1′-phenylethylcycloberberine chloride (13s): orange solid; yield: 82%; m.p.: 213–215 °C; 1H-NMR (DMSO-d6) δ 11.53 (s, 1H), 10.07 (s, 1H), 8.49 (s, 1H), 8.43 (d, J = 9.0 Hz, 1H), 8.13 (d, J = 9.0 Hz, 1H), 7.56 (s, 1H), 7.41–7.37 (m, 2H), 7.30 (t, J = 7.8 Hz, 2H), 7.19 (tt, J = 7.2, 1.2 Hz, 1H), 6.35 (s, 1H), 6.23–6.20 (m, 1H), 5.48 (q, J = 6.6 Hz, 1H), 5.20–5.06 (m, 2H), 4.08 (s, 3H), 3.57 (t, J = 6.6 Hz, 2H), 1.87 (d, J = 7.2 Hz, 3H); 13C-NMR (DMSO-d6) δ 147.4, 146.8, 146.3, 146.2, 145.7, 144.0, 141.6, 128.8 (2), 128.3, 128.2 (2), 127.8, 127.5, 126.7, 124.7, 122.2, 119.0, 118.2, 117.7, 116.3, 114.3, 111.0, 102.2, 57.4, 55.4, 41.0, 27.0, 22.7; HRMS: calcd for C29H24NO4Cl [M − Cl]+: 450.1700, found: 450.1706.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-2′-methoxy-2′-oxo-1′-phenylethylcycloberberine chloride (13t): orange solid; yield: 84%; m.p.: 173–175 °C; 1H-NMR (DMSO-d6) δ 11.62 (s, 1H), 10.10 (s, 1H), 8.14 (d, J = 9.0 Hz, 1H), 7.97 (s, 1H), 7.76 (s, 1H), 7.61 (s, 1H), 7.53–7.41 (m, 5H), 6.35 (s, 1H), 6.32 (s, 1H), 6.24 (s, 1H), 5.20–5.09 (m, 2H), 4.04 (s, 3H), 3.73 (s, 3H), 3.63–3.57 (m, 2H); 13C-NMR (DMSO-d6) δ 171.9, 147.0, 146.9, 146.0, 145.7, 141.0, 137.0, 136.2, 129.5, 129.1 (2), 128.9 (2), 128.1, 127.7, 127.4, 126.7, 124.6, 120.9, 117.6, 117.0, 115.8, 112.3, 110.6, 102.1, 56.8, 54.8, 54.1, 52.4, 26.4; HRMS: calcd for C30H24NO6Cl [M − Cl]+: 494.1598, found: 494.1591.
3.2.4. General Procedure for the Synthesis of 15a–c
To a stirred solution of intermediate 11 [15] (361 mg, 1.0 mmol, 1.0 equiv) in 80% EtOH (20 mL), various aliphatic aldehydes (5.0 equiv) and HOAc (4 mL) were added. The reaction mixture was heated to reflux for 8 h. The solvent was removed by evaporation to afford a red oil. The red oil was acidified by 2% HCl, stirred at room temperature for 0.5 h. The solvent was removed and the residue was purified by flash chromatography over silica gel using CH2Cl2/CH3OH (5:1) as eluent to obtain the intermediate compounds 14a–c. Next 14a–c were heated at 195–210 oC in a dry oven under vacuum (20–30 mmHg) for 40 min to create the crude materials, to were acidified in concentrated HCl/EtOH (5:95 by vol.) for 10 min. Then the mixture was evaporated under vacuum and purified by flash column chromatography on silica gel using a gradient of CH2Cl2 and CH3OH as eluent to obtain the desired compounds 15a–c.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-ethylcycloberberine chloride (15a): orange solid; yield: 82%; m.p.: 166–168 °C; 1H-NMR (DMSO-d6) δ 11.49 (s, 1H), 10.05 (s, 1H), 8.54 (s, 1H), 8.49 (d, J = 9.0 Hz, 1H), 8.12 (d, J = 9.0 Hz, 1H), 7.55 (s, 1H), 6.34 (s, 2H), 5.14 (t, J = 6.6 Hz, 2H), 4.08 (s, 3H), 3.57 (t, J = 6.6 Hz, 2H), 3.30 (q, J = 7.2 Hz, 2H), 1.36 (t, J = 7.2 Hz, 3H); 13C-NMR (DMSO-d6) δ 146.8, 145.9, 145.7 (2), 141.5, 141.2, 127.6, 127.1 (2), 124.2, 122.2, 119.8, 117.5, 117.1, 115.9, 114.1, 110.3, 102.0, 57.0, 55.0, 28.0, 26.6, 16.1; HRMS: calcd for C23H20NO4Cl [M − Cl]+: 374.1387, found: 374.1396.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-propylcycloberberine chloride (15b): orange solid; yield: 85%; m.p.: 165–167 °C; 1H-NMR (DMSO-d6) δ 11.49 (s, 1H), 10.04 (s, 1H), 8.58 (s, 1H), 8.53 (d, J = 9.0 Hz, 1H), 8.15 (d, J = 9.0 Hz, 1H), 7.55 (s, 1H), 6.34 (s, 2H), 5.15 (t, J = 6.6 Hz, 2H), 4.09 (s, 3H), 3.58 (t, J = 6.6 Hz, 2H), 3.28 (t, J = 7.8 Hz, 2H), 1.80–1.72 (m, 2H), 1.02 (t, J = 7.2 Hz, 3H); 13C-NMR (DMSO-d6) δ 146.6, 145.8, 145.6 (2), 141.2, 139.7, 127.6, 127.1, 127.0, 124.1, 122.0, 120.5, 117.6, 117.1, 115.8, 114.0, 110.2, 101.9, 56.9, 54.9, 36.5, 26.4, 24.3, 13.72; HRMS: calcd for C24H22NO4Cl [M − Cl]+: 388.1543, found: 388.1553.
1,2-Methylenedioxy-8-hydroxy-9-methoxy-13-butylcycloberberine chloride (15c): orange solid; yield: 88%; m.p.: 162–164 °C; 1H-NMR (DMSO-d6) δ 11.45 (s, 1H), 10.02 (s, 1H), 8.55 (s, 1H), 8.50 (d, J = 9.0 Hz, 1H), 8.12 (d, J = 9.0 Hz, 1H), 7.53 (s, 1H), 6.31 (s, 2H), 5.12 (t, J = 6.6 Hz, 2H), 4.06 (s, 3H), 3.55 (t, J = 6.6 Hz, 2H), 3.27 (t, J = 7.8 Hz, 2H), 1.68 (t, J = 7.8 Hz, 2H), 1.46–1.38 (m, 2H), 0.93 (t, J = 7.2 Hz, 3H); 13C-NMR (DMSO-d6) δ 146.7, 145.8, 145.6 (2), 141.2, 140.0, 127.6, 127.1, 127.0, 124.1, 122.1, 120.4, 117.6, 117.1, 115.8, 114.0, 110.2, 101.9, 56.9, 54.9, 34.3, 33.3, 25.4, 22.0, 13.7; HRMS: calcd for C25H24NO4Cl [M − Cl]+: 402.1700, found: 402.1692.
3.3. Antimicrobial Assay
Minimum inhibitory concentrations (MICs) of the target compounds were determined by using the agar dilution assay at various concentrations of 64, 32, 16, 8.0, 4.0, 2.0, 1.0, 0.5, 0.25, 0.125, 0.06 and 0.03 mg/mL described by the Clinical Laboratory Standards Institute [17]. Bacterial strains were purchased from the ATCC collection or isolated from Chinese hospitals. The medium was Mueller-Hinton agar, and the inoculum was 104 colony forming units (cfu)/spot. Culture plates were incubated at 35 °C for 18 h, and MICs were defined as the lowest concentrations that prevented visible growth of the bacteria.
3.4. Time-kill Curves of 15a
Kill kinetics of 15a was determined by time-kill experiments for MRSA strain (clinical isolate MRSA 18-4) according to the method described by Verma et al. with slight modifications [25]. An overnight culture was diluted with CAMH broth in a total volume of 30 mL containing an inoculum of 2×106 CFU/mL in a 250 mL flask for 18-4. Distilled water or 15a was added to yield concentrations of 0×, 1/2×, 2× and 8× MIC in the broth at standard inocula. Viability counts were performed at 0, 2, 4, 8 and 12 h of incubation at 37 °C by plating 0.1 mL undiluted and 10-fold serial diluted samples onto TSA plates in duplicate. Drug carryover effect was eliminated by saline and agar dilution. The experiments were performed three times on different days and the results were presented as mean and standard deviation.
3.5. Stability Assay of Key Compound in Liver Microsomes
The assay was performed with liver microsomes from CD-1 mouse, SD rat and human. Microsomes in 100 mM potassium phosphate buffer (0.5 mg/mL microsomal protein), cofactor MgCl2 (10 mM), tested compound (1 μM, cosolvent (0.01% DMSO), 0.99% CH3OH), and then NADPH (1 mM) at 37 °C for 60 min. The reaction can be started by the addition of liver microsomes or the tested compound or NADPH. Aliquots were sampled at 0, 5, 10, 20, 30 and 60 min incubation, and enzymatic reaction was stopped by protein precipitation in cold acetonitrile including 100 ng/mL tolbutamide and 100 ng/mL labetalol as internal standard. After centrifugation, samples were then analyzed by LC/MS/MS [26].
3.6. Stability Assay of Key Compound in SD Rat Whole Blood
Fresh blood was collected on the day of experiment from SD rats and pre-warmed in a water bath at 37 °C. 10 mM test compound or Ems stock solutions were prepared in DMSO, and then diluted with 45% MeOH/H2O to achieve 100 μM dosing solutions. Each dosing solution (2 μL) was incubated with 98 µL of blank blood at 37 °C in water bath for 0, 0.5, 1, 3, 7 and 24 h, respectively. At the end of incubation, for each sample, 100 μL water and 800 μL of stop solution (200 ng/mL tolbutamide plus 20 ng/mL buspirone in ACN) were immediately added to to precipitate protein and centrifuge at 4,000 rpm for 20 min. An aliquot of supernatant (100 μL) was then extracted, mixed with 200 μL H2O and then shook at 800 rpm for about 10 min before submitting to LC-MS/MS analysis. The experiment was repeated two times [18].
3.7. Acute Toxicity Assay
Female Kunming mice with weight of 20.0 (±1.0 g) were fed with regular rodent chow and housed in an air conditioned room. The mice were randomly divided into different groups with 6 mice each. Each compound was given intravenously in a single-dosing experiment at 40, 60, or 80 mg·kg−1 (ddH2O as control), respectively. The mice were closely monitored for 7 days. Body weight as well as survival was monitored [18].
3.8. Enzymatic Assay
The gyrase supercoling assay kit and topo IV decatenation kit were the tools for detection the target compound inhibited the activity of DNA gyrase and topo IV from MRSA (both kits obtained from Inspiralis, Norwich, United Kingdom). Briefly, supercoiled pBR322 plasmid DNA (0.5 mg) and 13a of varying concentrations (2.5–100 μg/mL) were incubated with 1 U gyrase in the dedicated supercoiling assay buffer supplied by the manufacturer. Reactions were carried out at 37 °C for 30 min and then terminated by the addition of equal volume of STEB buffer (40% sucrose, 100 mM Tris-HCl pH 7.5, 1 mM EDTA, 0.5 mg/ml bromophenol blue) and chloroform/isoamyl alcohol. Samples were vortexed, centrifuged and run through a 15 cm 1% agarose gel in TAE buffer (40 mM Tris-acetate, 2 mM EDTA) for 3 h at 50 V. Gels were stained with ethidium bromide and visualized under UV light. The decatenation assay was performed using MRSA topo IV decatenation kit. The operational approach was similar to above. Bands were visualized by UV light and photographed [27].
3.9. Molecular Docking Assay
The topo IV crystal structure in complex with kibdelomycin (PDB code 4URL, resolution: 2.29 Å) was selected as the template to generate the binding modes [24]. Discovery Studio 4.5 was used to create structures of the ligands and to perform energy minimization [16]. The LibDock software was employed. Before docking, protein structure and ligands were processed [28]. In the course of protein preparation, ligands and water molecules were removed from the structure. The docking conformation with the highest docking score was finally chosen to analyze the receptor ligand interaction in the Discovery Studio 4.5 software.
4. Conclusions
A total of 25 new derivatives of 1 were designed, synthesized and evaluated for their anti-Gram-positive microbes activities with 1 as the lead. SAR studies revealed that introducing a small-sized substituent at the 13-position might be beneficial for activity. Part of them showed good activities against MRSA and VISA. Compound 15a bearing a 13-ethyl group exhibited the most potent antibacterial activities, with MIC values of 0.25–0.5 μg/mL, superior to those of the lead 1. Time-kill curve analysis of 15a further confirmed its potential bactericidal activity. Meanwhile, compound 15a displayed high liver microsomal metabolic stability and reasonable whole blood stability. It also showed a good in vivo safety profile with an LD50 value over 65.6 mg·kg−1 in mice via the intravenous route. A preliminary mechanism study showed that compound 15a could inhibit topo IV activity through binding with the active site of kibdelomycin, a different mechanism of action from the quinolone antibiotics currently used in hospitals. Taken together, these results provide powerful information for developing these compounds into promising anti-MRSA candidates with novel chemical scaffolds and a specific biological mechanism of action, and compound 15a has been chosen for further investigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/5/984/s1, the 1H-NMR, 13C-NMR and HRMS data of compound 15a.
Author Contributions
Y.-S.Y., W.W. and S.T. performed part of synthetic experiments and wrote the paper, X.-X.H. and J.P. performed the biological assay, D.-Q.S. conceived and designed the chemistry experiments, X.-F.Y. conceived and designed the biology experiments, T.-Y.F. and Y.-X.W. designed the target compounds and chemistry experiments.
Funding
This work was supported by the National Natural Science Foundation of China (81621064 and 81361138020), Beijing Natural Science Foundation (7172136), and the CAMS Innovation Fund for Medical Sciences (2017-12M-1-012).
Acknowledgments
The authors thank center for analysis and testing of Institute of Materia Medica and Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences for their contributions to the determination of HR-MS, 1H-NMR, and 13C-NMR.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Antibiotic Resistance: The Global Threat (U.S.); Centers for Disease Control and Prevention: Atlanta, GA, USA. Available online: http://stacks.cdc.gov/view/cdc/31340 (accessed on 27 February 2015).
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Filice, G.A.; Nyman, J.A.; Lexau, C.; Lees, C.H.; Bockstedt, L.A.; ComoSabetti, K.; Lesher, L.J.; Lynfield, R. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect. Control Hosp. Epidemiol. 2010, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Fischbach, M.A. Squashing superbugs—The race for new antibiotics. Sci. Am. 2009, 301, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Hanaki, H.; Ino, T.; Yabuta, K.; Oquri, T.; Tenover, F.C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 1997, 40, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Aritaka, N.; Hanaki, H.; Kawasaki, S.; Hosoda, Y.; Hori, S.; Fukuchi, Y.; Kobayashi, I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997, 350, 1670–1673. [Google Scholar] [CrossRef]
- Auckland, C.; Teare, L.; Cooke, F.; Kaufmann, M.E.; Warner, M.; Jones, G.; Bamford, K.; Ayles, H.; Johnson, A.P. Linezolid-resistant enterococci: Report of the first isolates in the United Kingdom. J. Antimicrob. Chemother. 2002, 50, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Yeh, W.W.; Wennersten, C.B.; Venkataraman, L.; Albano, E.; Alyea, E.P.; Gold, H.S.; Baden, L.R.; Pillai, S.K. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 2006, 44, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Basuino, L.; Diep, B.; Hamilton, S.; Chatterjee, C.C.; Chambers, H.F. Ceftobiprole- and ceftaroline-resistant methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2960–2963. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Liu, Y.X.; Xiao, C.L.; Wang, Y.X.; Li, Y.H.; Yang, Y.H.; Li, Y.B.; Bi, C.W.; Gao, L.M.; Jiang, J.D.; Song, D.Q. Synthesis, structure-activity relationship and in vitro anti-mycobacterial evaluation of 13-n-octylberberine derivatives. Eur. J. Med. Chem. 2012, 52, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.G.; Hu, X.X.; Li, C.R.; Li, Y.H.; Wang, Y.X.; Jiang, J.D.; Bi, C.W.; Tang, S.; You, X.F.; Song, D.Q. Design, synthesis and biological evaluation of monobactams as antibacterial agents against gram-negative bacteria. Eur. J. Med. Chem. 2016, 110, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Zhao, W.L.; Wang, Y.X.; Zhang, C.X.; Jiang, J.D.; Bi, C.W.; Tang, S.; Chen, R.X.; Shao, R.G.; Song, D.Q. Discovery, synthesis and biological evaluation of cycloprotoberberine derivatives as potential antitumor agents. Eur. J. Med. Chem. 2013, 68, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.Y.; Hu, X.X.; Tang, S.; Liu, X.J.; Wang, Y.X.; Deng, H.B.; You, X.F.; Jiang, J.D.; Li, Y.H.; Song, D.Q. Discovery and development of 8-substituted cycloberberine derivatives as novel antibacterial agents against MRSA. ACS Med. Chem. Lett. 2018, 9, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.Y.; Wang, Y.X.; Tang, S.; Hu, X.X.; Zeng, Q.X.; Pang, J.; Yang, Y.S.; You, X.F.; Song, D.Q. Synthesis and antibacterial evaluation of 13-substituted cycloberberine derivatives as a novel class of anti-MRSA agents. Eur. J. Med. Chem. 2018, 157, 877–886. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. M07–M10. [Google Scholar]
- Lu, X.; Yang, X.Y.; Li, X.; Lu, Y.; Ren, Z.T.; Zhao, L.Y.; Hu, X.X.; Jiang, J.D.; You, X.F. In vitro activity of sodium new houttuyfonate alone and in combination with oxacillin or netilmicin against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e68053. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Search for factors affecting antibacterial activity and toxicity of 1,2,4-triazole-ciprofloxacin hybrids. Eur. J. Med. Chem. 2015, 97, 94–103. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, H.; Dean, E.; Shannon, K.R.; Chandupatla, V.D.; Juntyma, J.E.; Ye, Z.Q.; Jones, S.M.; O’Brien, C.F.; Nicolau, D.P.; Tessier, P.R.; et al. Discovery and characterization of a water-soluble prodrug of a dual inhibitor of bacterial DNA gyrase and topoisomerase IV. ACS Med. Chem. Lett. 2015, 6, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Ullah, A. New developments in non-quinolone-based antibiotics for the inhibition of bacterial gyrase and topoisomerase IV. Eur. J. Med. Chem. 2018, 152, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J. DNA topoisomerases: Structure, function and mechanism. J. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.W.; Goetz, M.A.; Smith, S.K.; Zink, D.L.; Polishook, J.; Onishi, R.; Salowe, S.; Wiltsie, J.; Allocco, J.; Sigmund, J.; et al. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem. Biol. 2011, 18, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Patel, S.; Sharma, N.; Soisson, S.M.; Kishii, R.; Takei, M.; Fukuda, Y.; Lumb, K.J.; Singh, S.B. Structures of kibdelomycin bound to Staphylococcus aureus GyrB and ParE showed a novel U-shaped binding mode. ACS Chem. Biol. 2014, 9, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Verma, P. Methods for determining bactericidal activity and antimicrobial interactions synergy testing, time-kill curves, and population analysis. In Antimicrobial Susceptibility Testing Protocols; Schwalbe, R., Steele-Moore, L., Goodwin, A.C., Eds.; CRC Press: Roca Raton, FL, USA, 2017; pp. 275–298. [Google Scholar]
- Wang, B.; Wang, K.; Meng, P.; Hu, Y.; Yang, F.; Liu, K.; Lei, Z.; Chen, B.; Tian, Y. Design, synthesis, and evaluation of carboxyl-modified oseltamivir derivatives with improved lipophilicity as neuraminidase inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3477–3482. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Stefańska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Madeddu, S.; Boi, S.; Giliberti, G.; Wrzosek, M.; et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, M.; Shakeel, E.; Jamal, Q.M.; Kamal, M.A.; Sayeed, U.; Khan, M.K.; Siddiqui, M.H.; Arif, J.M.; Akhtar, S. Molecular interaction and computational analytical studies of pinocembrin for its antiangiogenic potential targeting VEGFR-2: A persuader of metastasis. Med. Chem. 2018, 14, 626–640. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6, 10, 13a–t and 15a–c are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).