Synthesis and In Vitro Evaluation of Caffeoylquinic Acid Derivatives as Potential Hypolipidemic Agents
Abstract
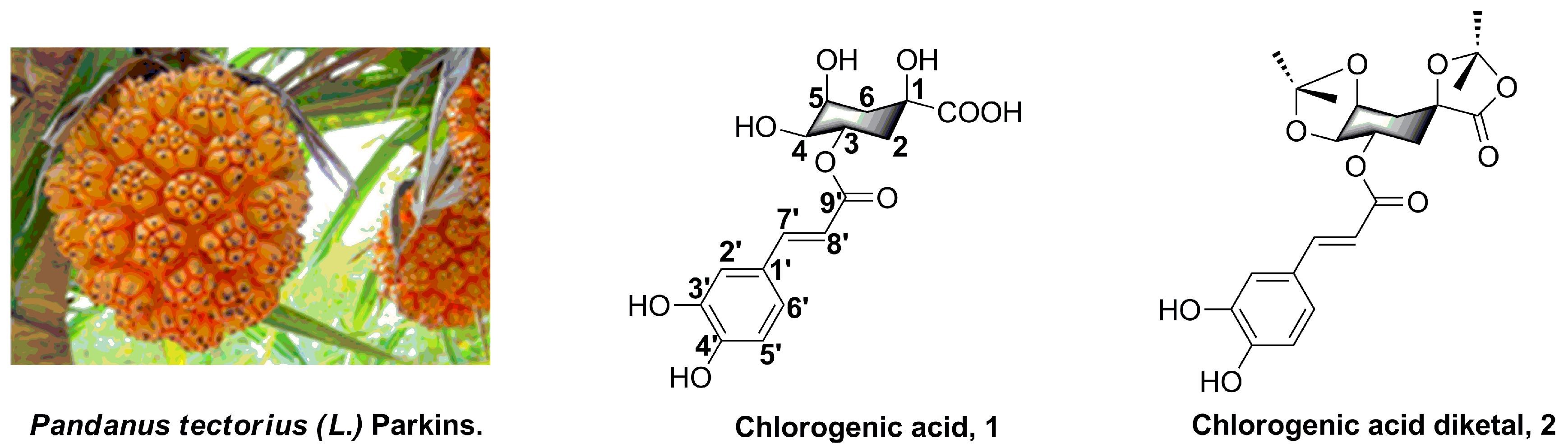
:1. Introduction
2. Results and Discussion
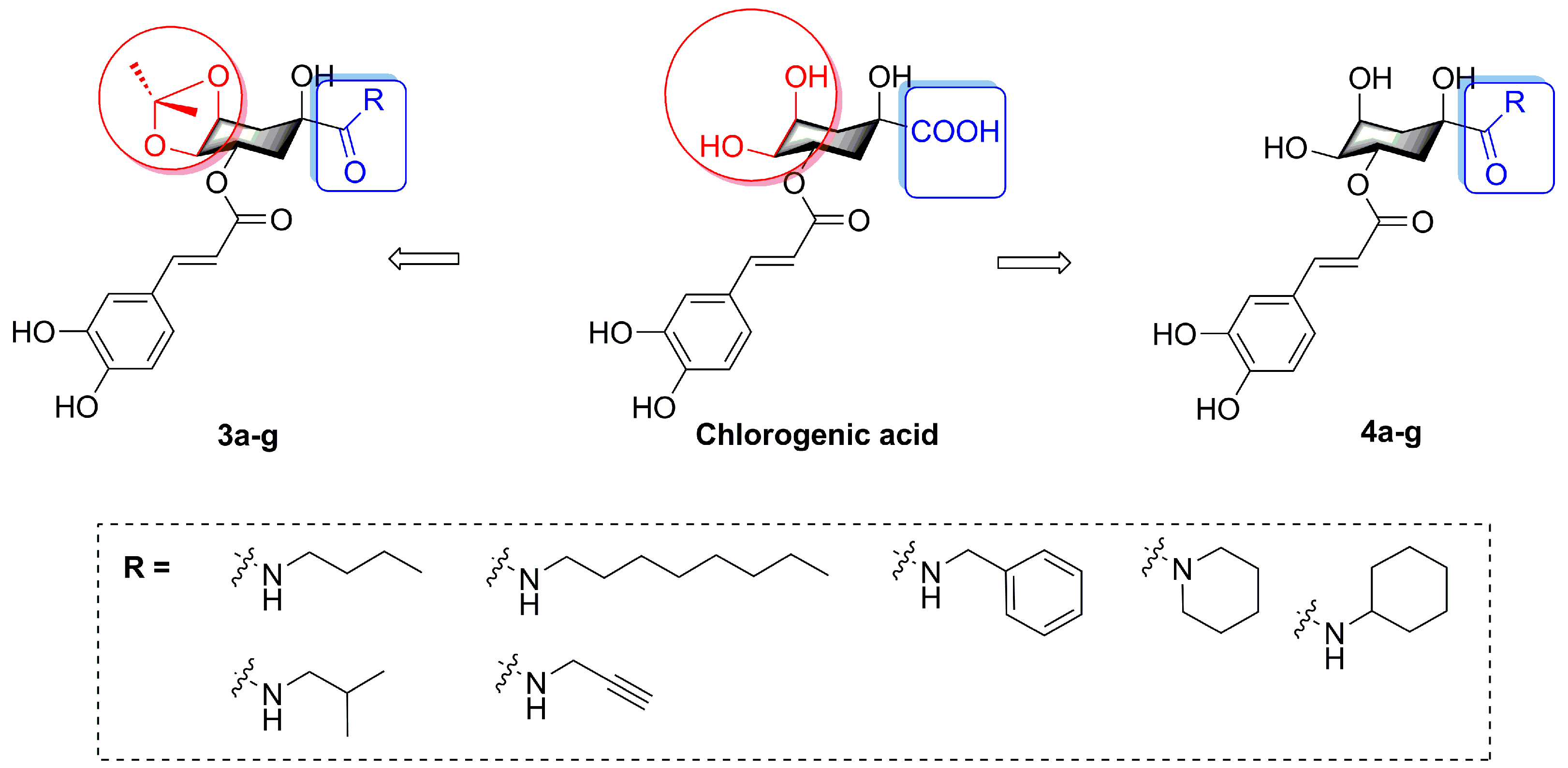
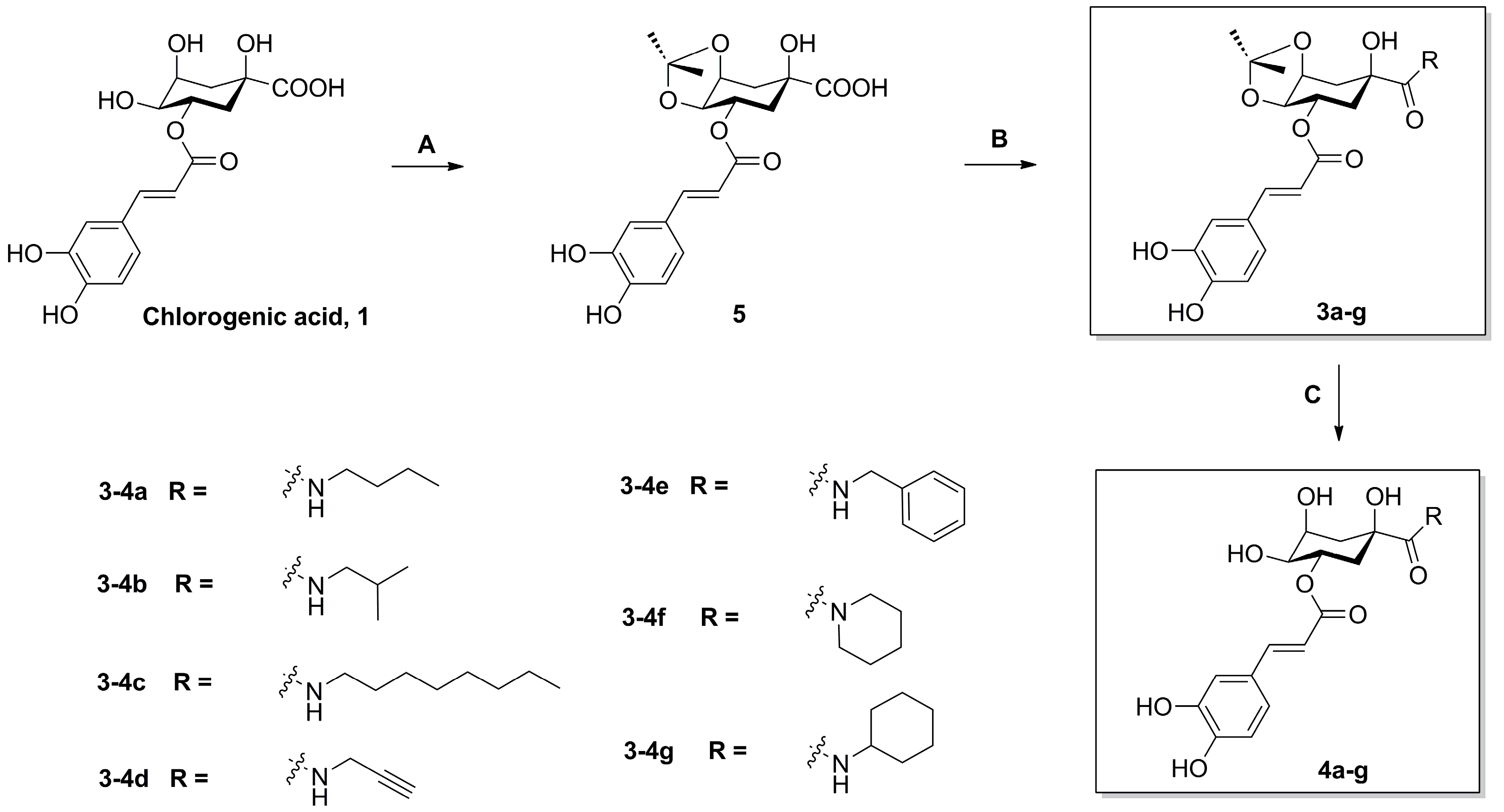
2.1. Chemistry
2.2. Biological Results and Discussion
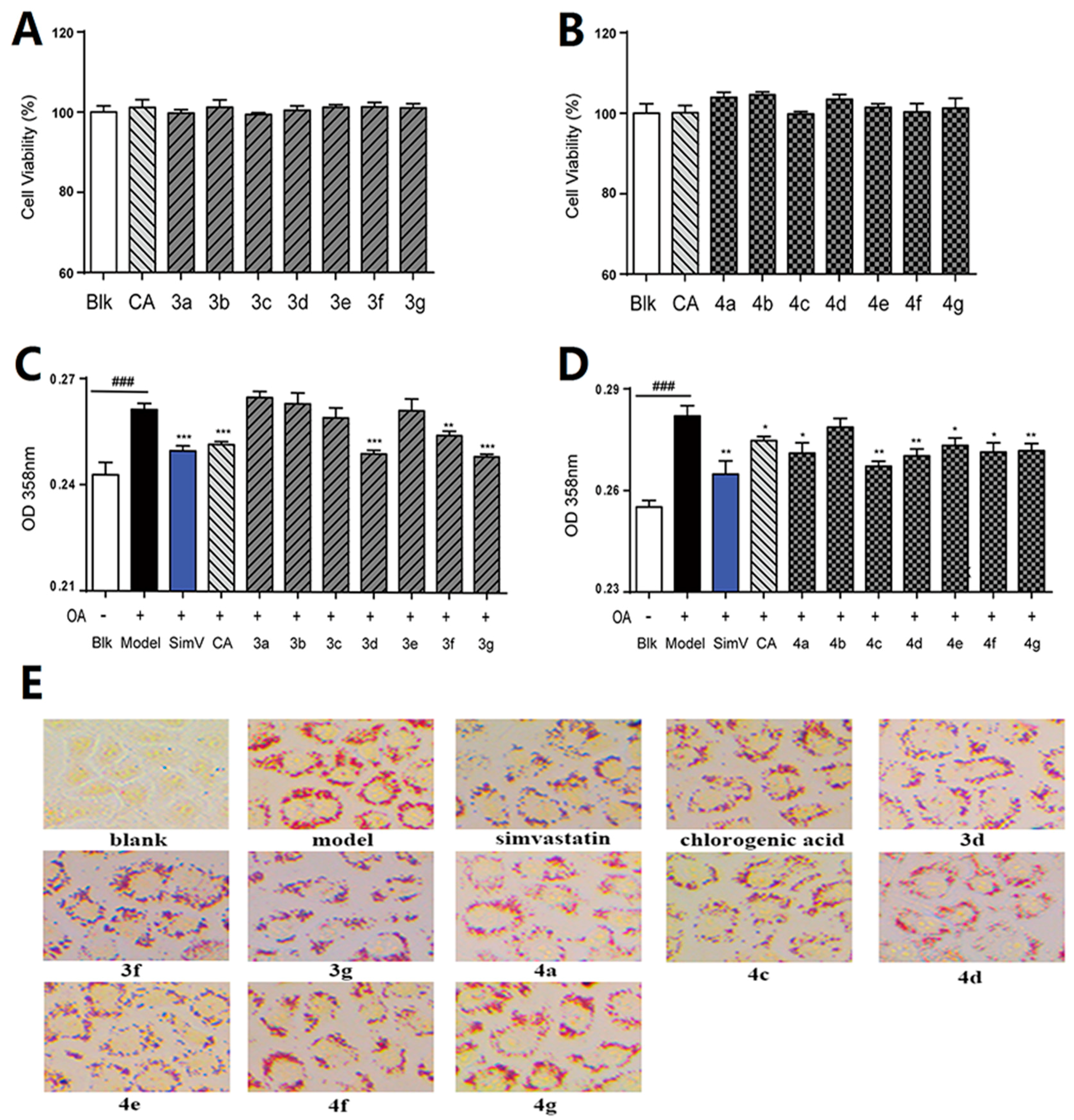
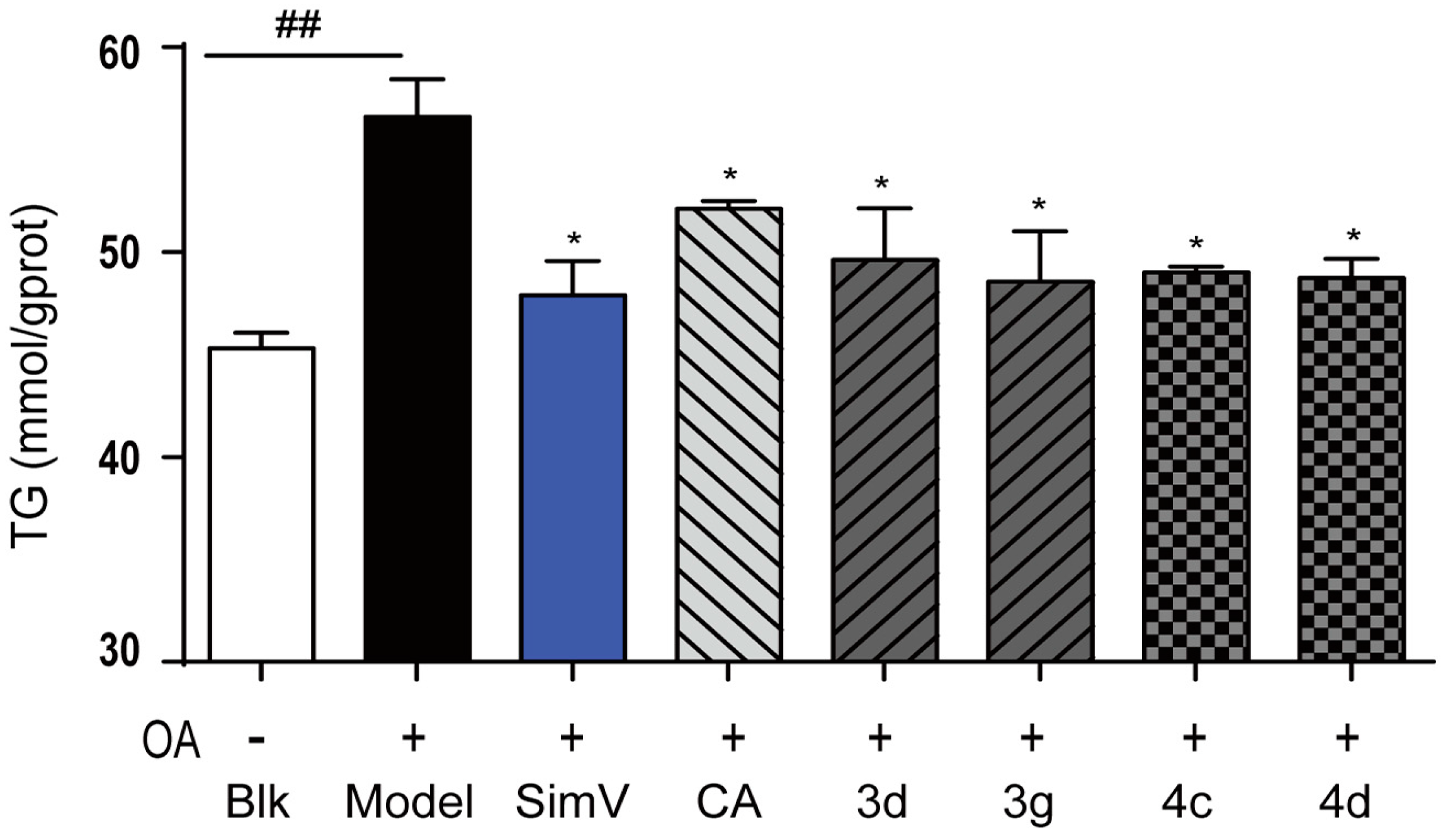
2.2.1. Lipid-Lowering Effects of Chlorogenic Acid and Compounds 3a–g and 4a–g on Oleic Acid-Elicited Lipid Accumulation in HepG2 Liver Cells
2.2.2. Inhibitory Effects of Compounds 3d, 3g, 4c, and 4d Toward Triglycerides
3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. Procedure for the Synthesis of Intermediate 5
3.2.2. General Procedure for the Synthesis of Compounds 3a–g
3.2.3. General Procedure for the Synthesis of Compounds 4a–g
3.3. Evaluation of the Biological Activity
3.3.1. Cell Culture
3.3.2. Cell Viability Assay
3.3.3. Oil red O staining
3.3.4. Intracellular TG Quantification
3.3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barry, V.W.; Caputo, J.L.; Kang, M. The joint association of fitness and fatness on cardiovascular disease mortality: A meta-analysis. Prog. Cardiovasc. Dis. 2018, 61, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2018, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Menegatti, M.; Ferrari, C.; Carnevale Schianca, G.P.; Pirisi, M. Ultrasound-assessed visceral fat and associations with glucose homeostasis and cardiovascular risk in clinical practice. Nutr. Metab. Cardiovas. 2018, 28, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Berry, J.D.; Dyer, A.; Cai, X.; Garside, D.B.; Ning, H.Y.; Thomas, A.; Greenland, P.; Horn, L.V.; Tracy, R.P.; Lloyd-Jones, D.M. Lifetime risks of cardiovascular disease. N. Engl. J. Med. 2012, 366, 321–329. [Google Scholar] [CrossRef]
- Jones, A. Triglycerides and cardiovascular risk. Heart 2013, 99, 1–2. [Google Scholar] [CrossRef]
- Pilz, S.; Scharnagl, H.; Tiran, B.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; Schaefer, J.R.; März, W. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J. Clin. Endocrinol. Metab. 2006, 91, 2542–2547. [Google Scholar] [CrossRef]
- Zhang, X.P.; Wu, C.M.; Wu, H.F.; Sheng, L.H.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.B.; Sun, X.B.; Tian, Y.; et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef]
- Andriani, Y.; Ramli, N.M.; Syamsumir, D.F.; Kassim, M.N.I.; Jaafar, J.; Aziz, N.A.; Marlina, L.; Musa, N.S.; Mohamad, H. Phytochemical analysis, antioxidant, antibacterial and cytotoxicity properties of keys and cores part of Pandanus tectorius fruits. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Wu, C.M.; Zhang, X.P.; Zhang, X.; Luan, H.; Sun, G.B.; Sun, X.B.; Wang, X.L.; Guo, P.; Xu, D.D. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J. Nutr. Biochem. 2014, 25, 412–419. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Wang, H.Y.; Yang, B.; Tao, H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010, 120, 1138–1142. [Google Scholar] [CrossRef]
- Gao, R.F.; Yang, H.D.; Jing, S.F.; Liu, B.; Wei, M.; He, P.F.; Zhang, N.S. Protective effect of chlorogenic acid on lipopolysaccharide-induced inflammatory response in dairy mammary epithelial cells. Microb. Pathog. 2018, 124, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Zhao, X.M.; Liu, Y.L. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017, 102, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Bender, O.; Llorent-Martínez, E.J.; Zengin, G.; Mollica, A.; Ceylan, R.; Molina-García, L.; Córdova, M.L.F.; Atalay, A. Integration of in vitro and in silico perspectives to explain chemical characterization, biological potential and anticancer effects of Hypericum salsugineum: A pharmacologically active source for functional drug formulations. PLoS ONE 2018, 13, e0197815. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Zengin, G.; Córdova, M.L.F.; Bender, O.; Atalay, A.; Ceylan, R.; Mollica, A.; Mocan, A.; Uysal, S.; Guler, G.O.; et al. Traditionally used Lathyrus species: Phytochemical composition, antioxidant activity, enzyme inhibitory properties, cytotoxic effects, and in silico studies of L. czeczottianus and L. nissolia. Front. Pharmacol. 2017, 8, 83. [Google Scholar] [CrossRef]
- Jabeur, I.; Tobaldini, F.; Martins, N.; Barros, L.; Martins, I.; Calhelha, R.C.; Henriques, M.; Silva, S.; Achour, L.; Santos-Buelga, C.; et al. Bioactive properties and functional constituents of Hypericum androsaemum L.: A focus on the phenolic profile. Food Res. Int. 2016, 89, 422–431. [Google Scholar] [CrossRef]
- Truong, V.D.; Mcfeeters, R.F.; Thompson, R.T.; Dean, L.L.; Shofran, B. Phenolic acid content and composition in leaves and roots of common commercial sweetpotato (Ipomea batatas L.) cultivars in the United States. J. Food Sci. 2007, 72, c343–c349. [Google Scholar] [CrossRef]
- Stefanucci, A.; Macedonio, G.; Dvorácskó, S.; Tömböly, C.; Mollica, A. Novel fubinaca/rimonabant hybrids as endocannabinoid system modulators. Amino Acids 2018, 50, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.; Stefanucci, A.; Pieretti, S.; Marzoli, F.; Fidanza, L.; Mollica, A.; Mirzaie, S.; Carradori, S.; Petrocellis, L.D.; Moriello, A.S.; et al. Evaluation of the analgesic effect of 4-anilidopiperidine scaffold containing ureas and carbamates. J. Enzyme. Inhib. Med. Chem. 2016, 31, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Costante, R.; Akdemir, A.; Carradori, S.; Stefanucci, A.; Macedonio, G.; Ceruso, M.; Supuran, C.T. Exploring new probenecid-based carbonic anhydrase inhibitors: Synthesis, biological evaluation and docking studies. Bioorg. Med. Chem. 2015, 23, 5311–5318. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3a–g and 4a–g are available or not from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Cao, X.-X.; Shang, H.; Wu, C.-M.; Zhang, X.; Guo, P.; Zhang, X.-P.; Xu, X.-D. Synthesis and In Vitro Evaluation of Caffeoylquinic Acid Derivatives as Potential Hypolipidemic Agents. Molecules 2019, 24, 964. https://doi.org/10.3390/molecules24050964
Tian Y, Cao X-X, Shang H, Wu C-M, Zhang X, Guo P, Zhang X-P, Xu X-D. Synthesis and In Vitro Evaluation of Caffeoylquinic Acid Derivatives as Potential Hypolipidemic Agents. Molecules. 2019; 24(5):964. https://doi.org/10.3390/molecules24050964
Chicago/Turabian StyleTian, Yu, Xiao-Xue Cao, Hai Shang, Chong-Ming Wu, Xi Zhang, Peng Guo, Xiao-Po Zhang, and Xu-Dong Xu. 2019. "Synthesis and In Vitro Evaluation of Caffeoylquinic Acid Derivatives as Potential Hypolipidemic Agents" Molecules 24, no. 5: 964. https://doi.org/10.3390/molecules24050964
APA StyleTian, Y., Cao, X.-X., Shang, H., Wu, C.-M., Zhang, X., Guo, P., Zhang, X.-P., & Xu, X.-D. (2019). Synthesis and In Vitro Evaluation of Caffeoylquinic Acid Derivatives as Potential Hypolipidemic Agents. Molecules, 24(5), 964. https://doi.org/10.3390/molecules24050964





