Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice
Abstract
1. Introduction
2. Results
2.1. Identification of Pumpkin Polysaccharides
2.1.1. Determination of Molecular Weight
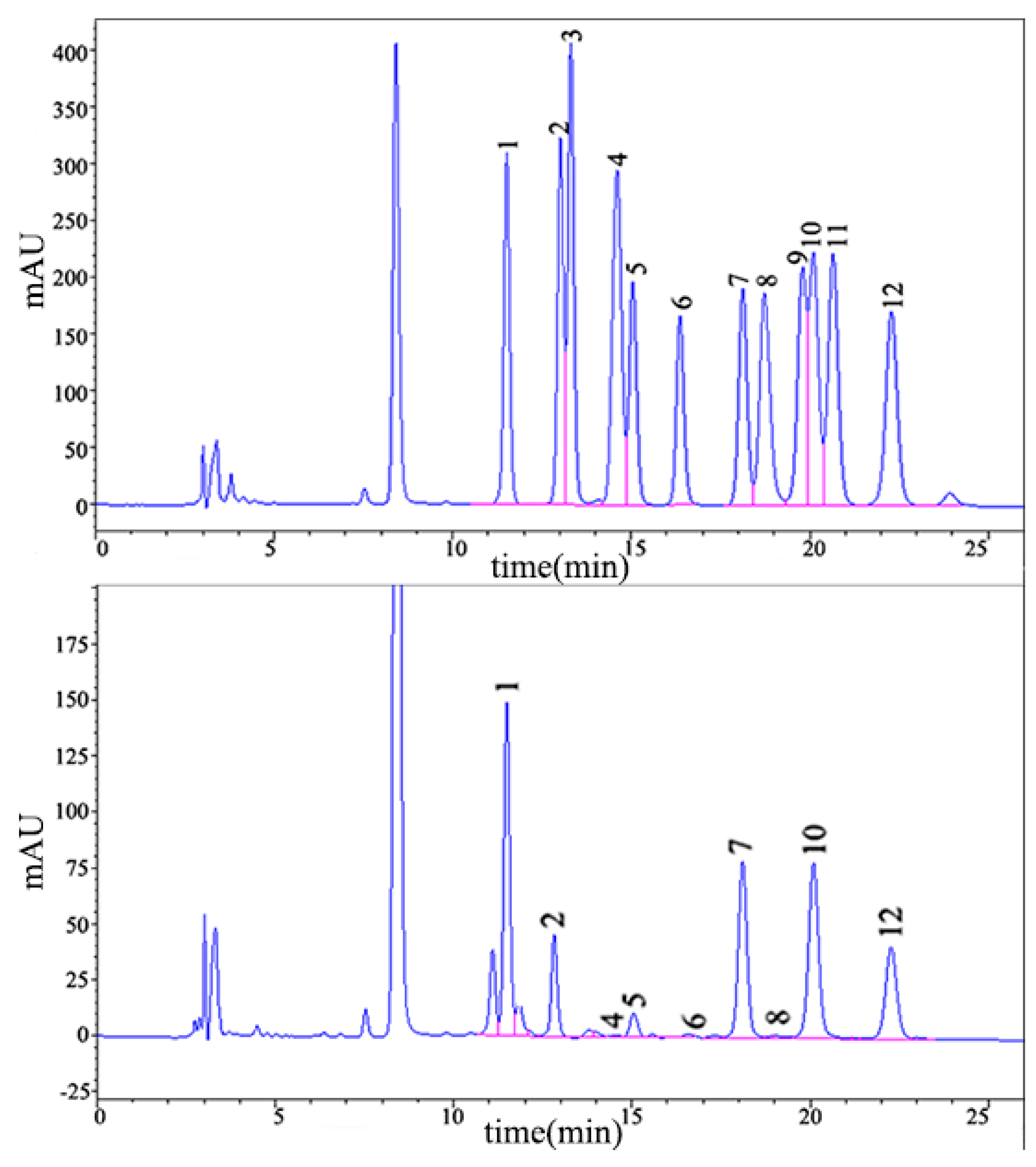
2.1.2. Monosaccharide Composition Analysis
2.1.3. Ultraviolet Spectra
2.1.4. Fourier-Transform Infrared Spectra
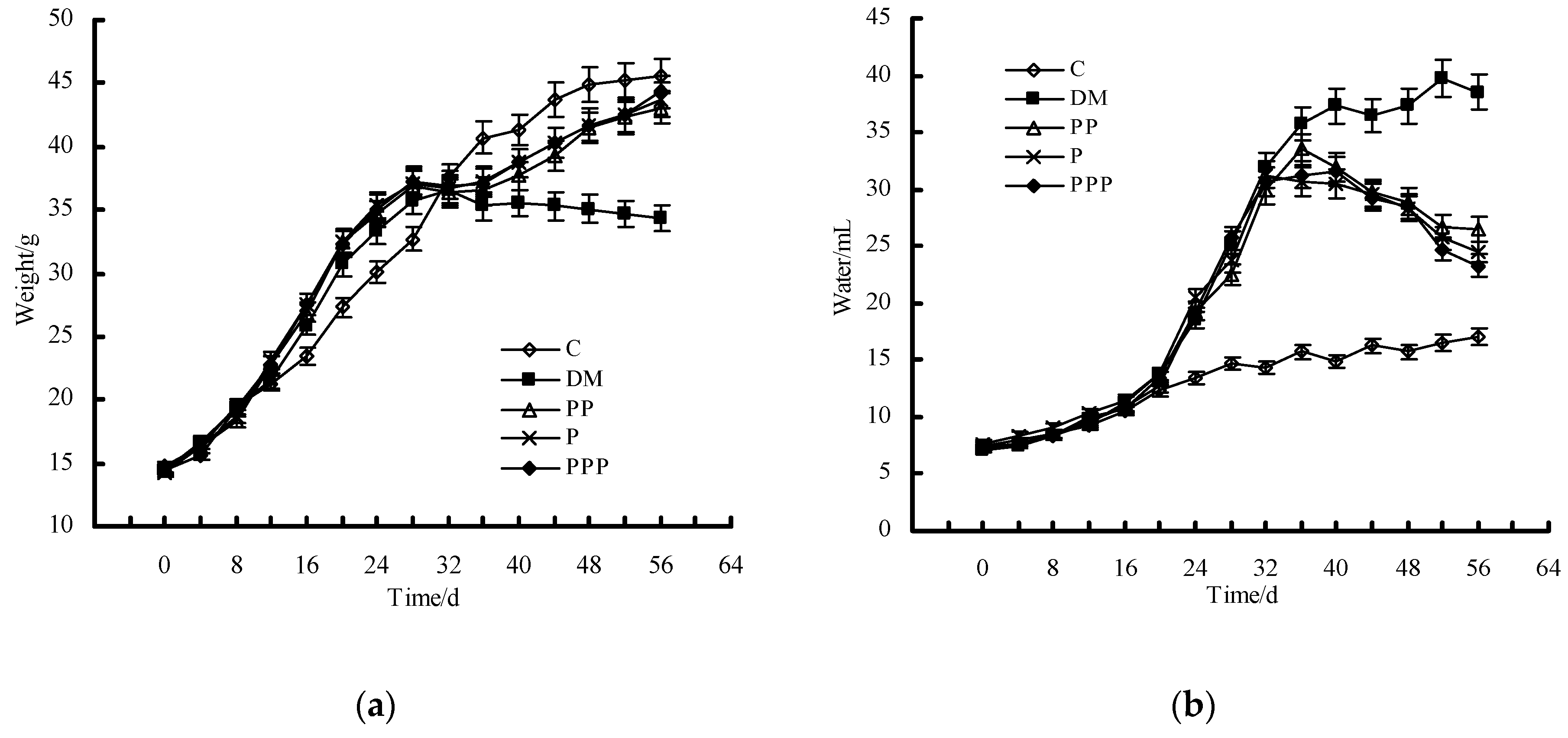
2.2. Effect on Body Weight and Water Intake
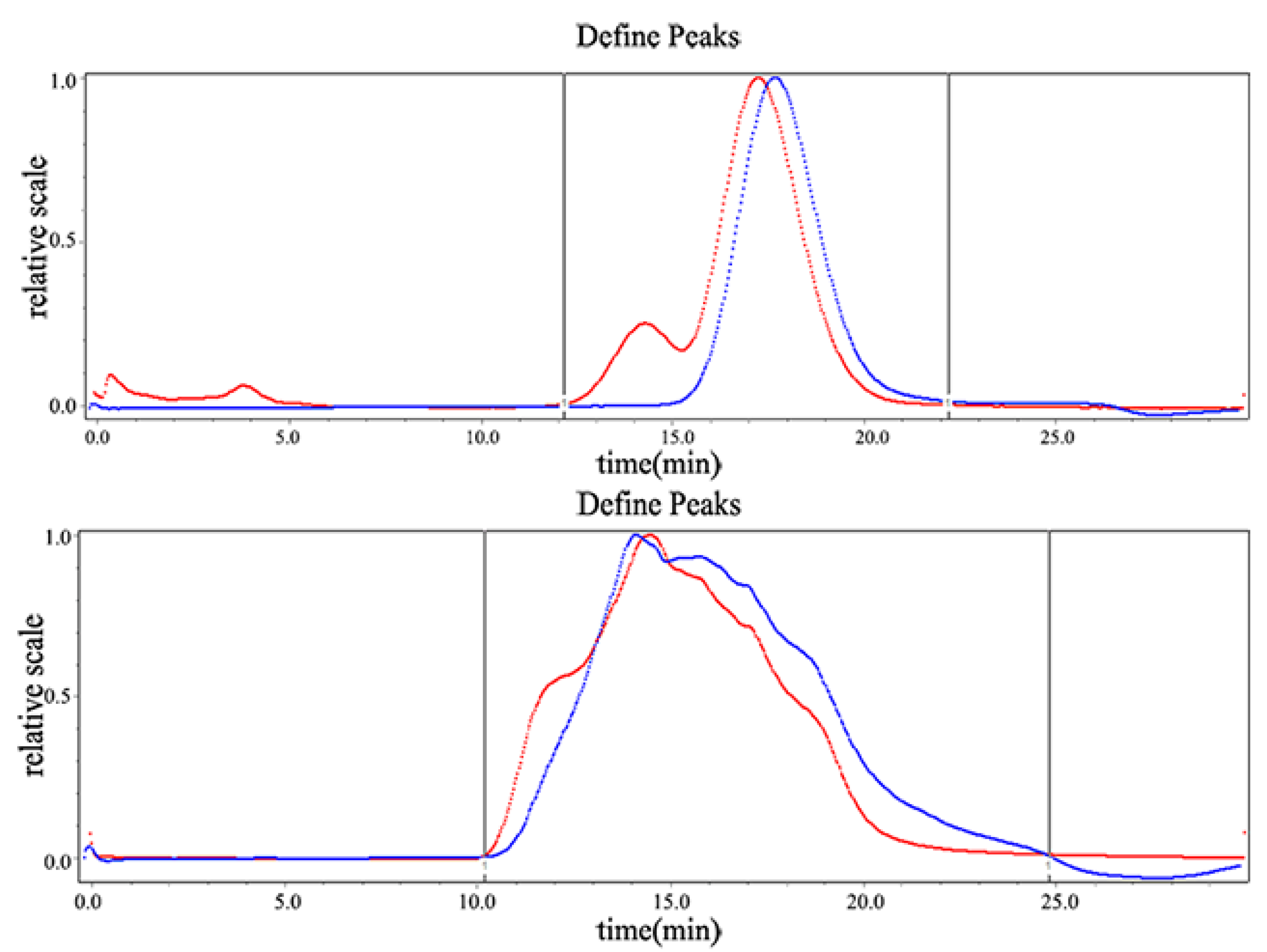
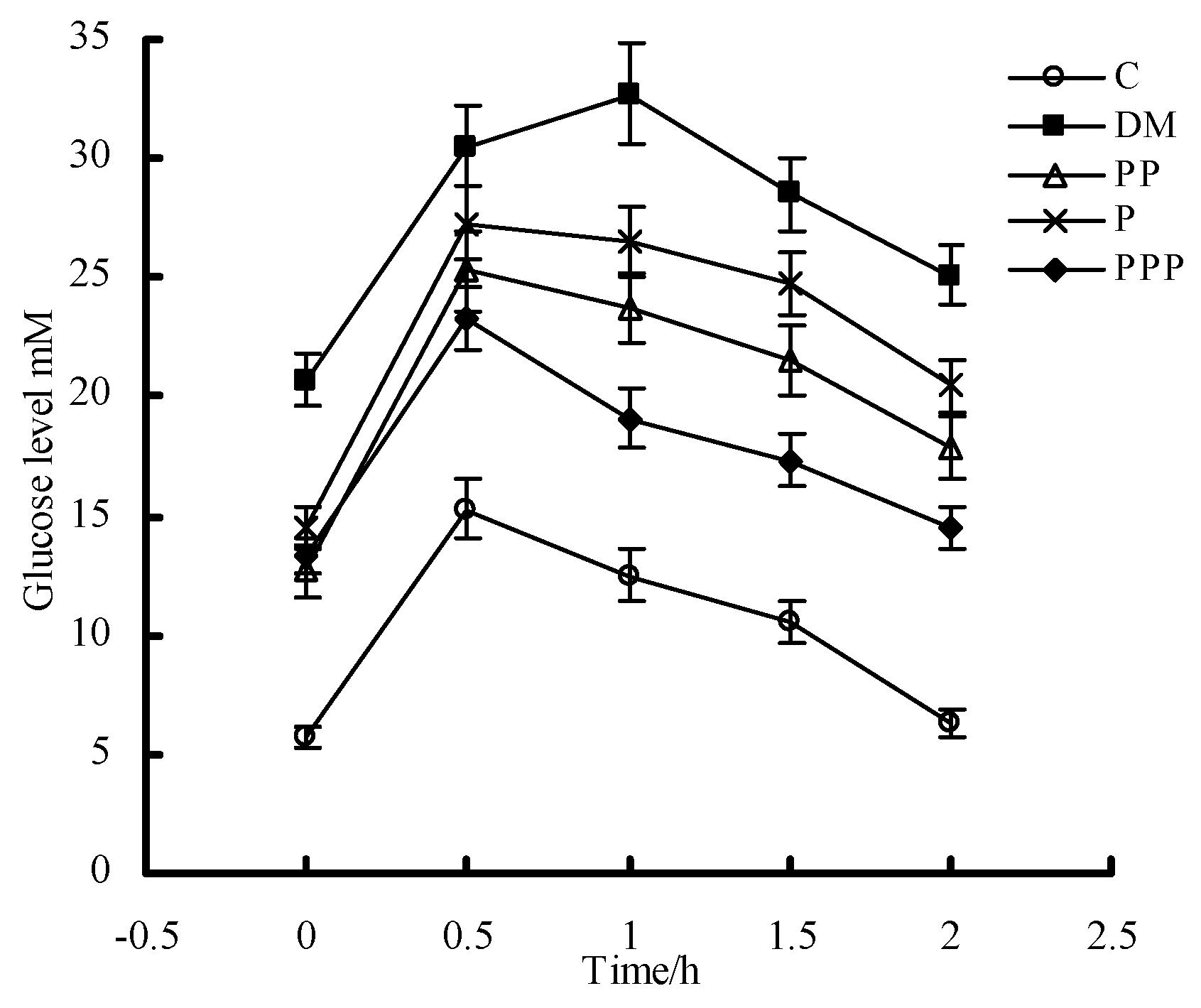
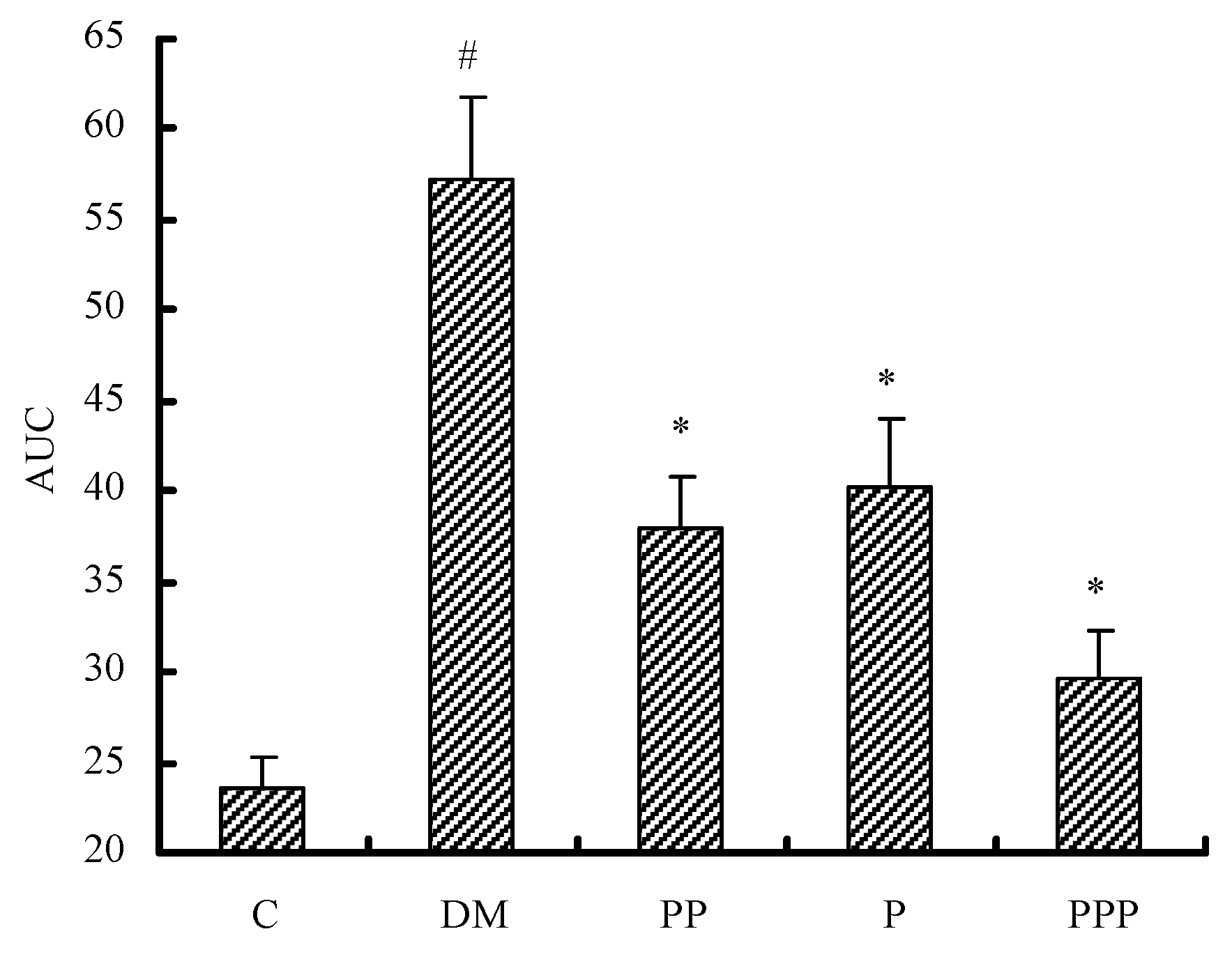
2.3. Oral Glucose Tolerance Test
2.4. Blood Glucose and Insulin Level
2.5. Effect on Serum Lipid Levels
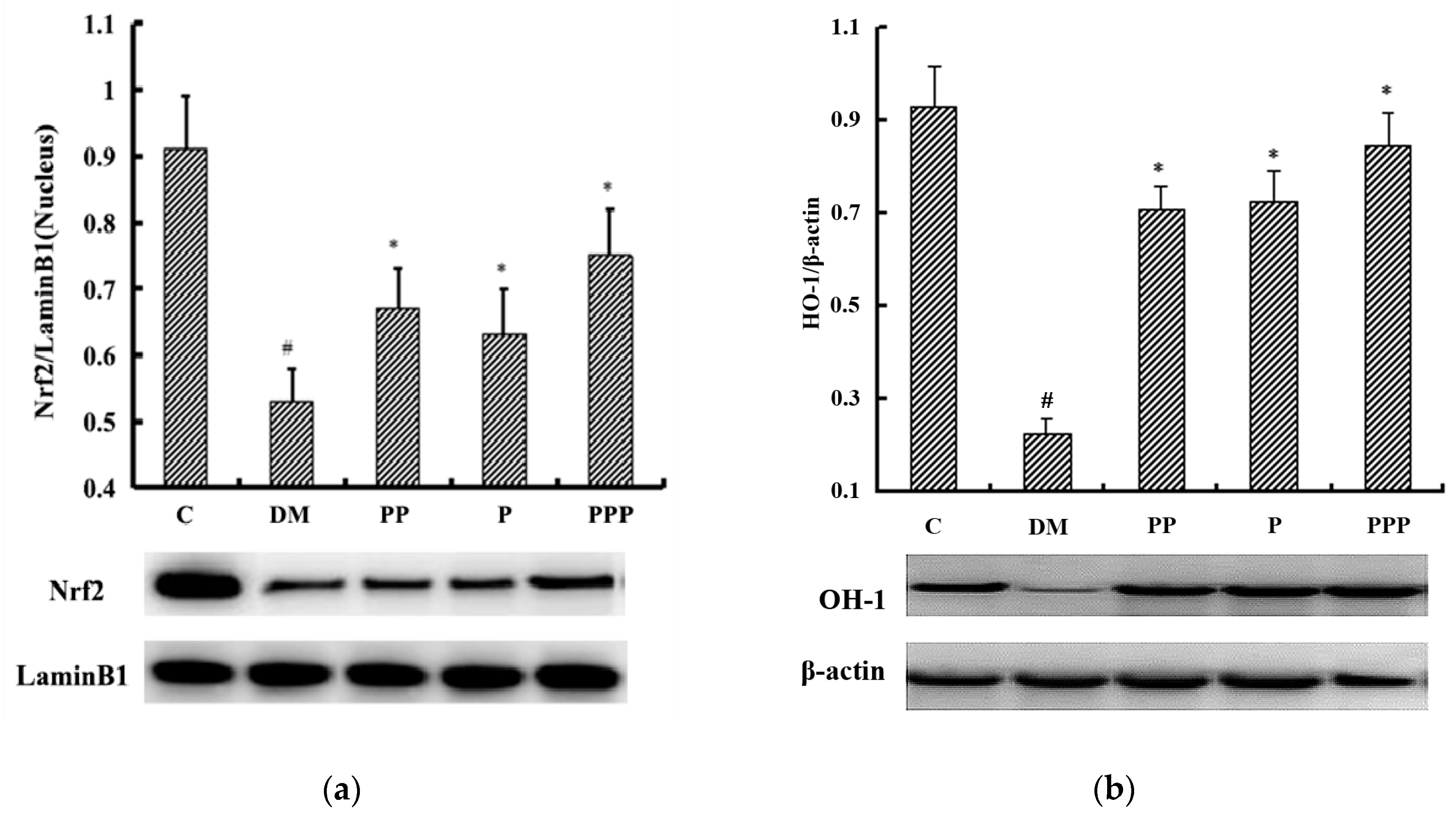
2.6. Effect on Oxidative Stress
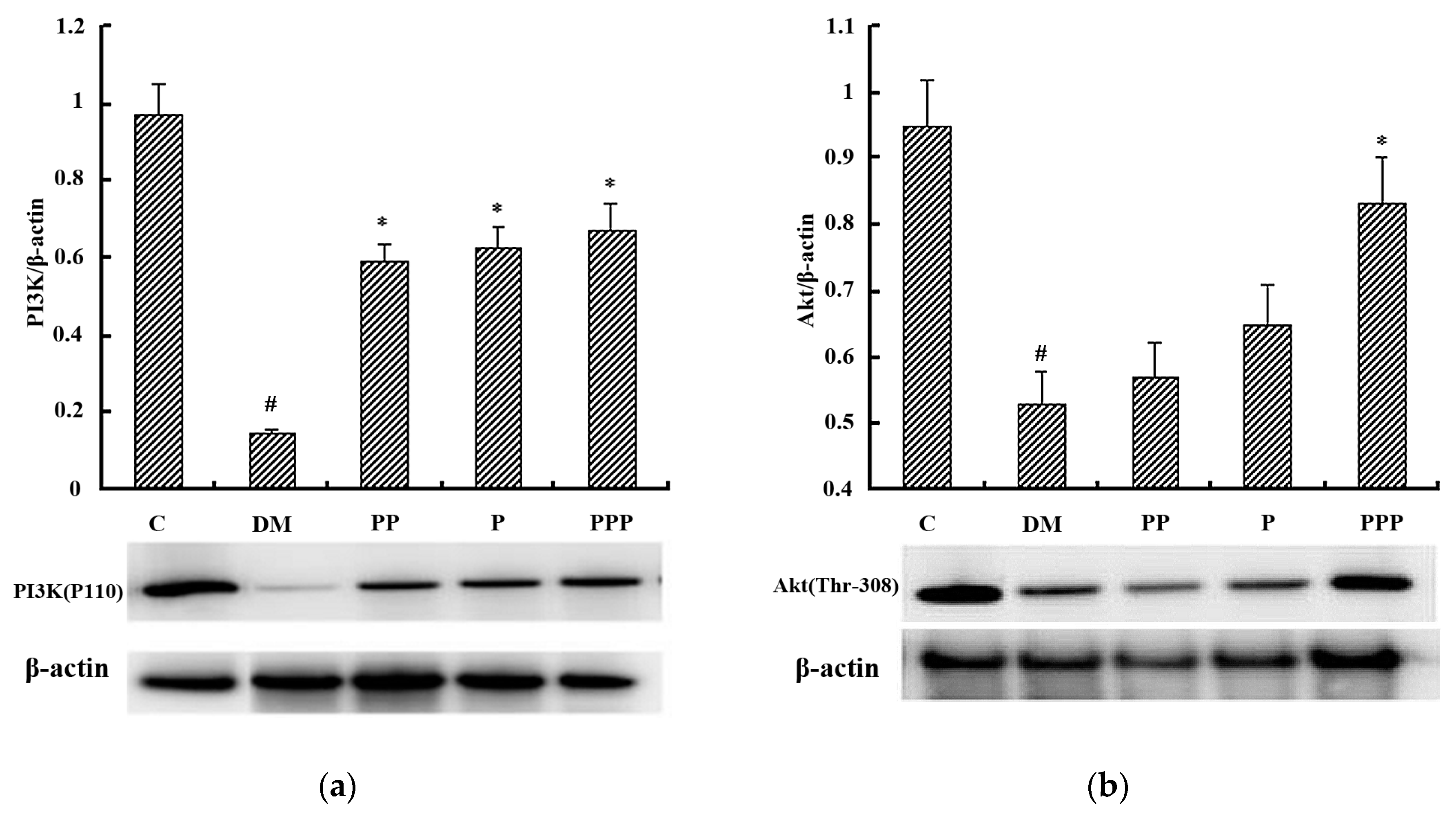
2.7. Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Separation and Identification of Pumpkin Polysaccharides
4.2.1. Separation of Pumpkin Polysaccharides
4.2.2. Molecular Weight Determination
4.2.3. Monosaccharide Composition Analysis
4.2.4. Ultraviolet Spectra
4.2.5. Fourier-Transform Infrared Spectra
4.3. Animals
4.4. Experimental Design
4.5. Oral Glucose Tolerance Test
4.6. Evaluation of Blood Lipid Metabolism
4.7. Oxidative Stress Assay
4.8. Western Blot Analysis
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARE | antioxidant response element |
| AUC | area under the curve |
| DM | diabetes mellitus |
| FFAs | free fatty acids |
| GSH | glutathione |
| HDL | high-density lipoprotein |
| HFD | high-fat diet |
| HPGPC | high-performance gel permeation chromatography |
| HO-1 | heme oxygenase-1 |
| IR | insulin resistance |
| Keap 1 | Kelch-like ECH-associated protein 1 |
| LDL | low-density lipoprotein |
| MDA | malondialdehyde |
| MW | molecular weight |
| Nrf2 | nuclear factor E2 related factor 2 |
| OGTT | oral glucose tolerance test |
| PI3K | phosphoinositide-3-kinase |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| TBST | tris-buffered saline tween |
| TC | total cholesterol |
| TG | triglycerides |
| T2DM | type II diabetes mellitus |
References
- Saad, M.I.; Abdelkhalek, T.M.; Saleh, M.M.; Kamel, M.A.; Youssef, M.; Tawfik, S.H.; Dominguez, H. Insights into the molecular mechanisms of diabetes-induced endothelial dysfunction: Focus on oxidative stress and endothelial progenitor cells. Endocrine 2015, 50, 537–567. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Rahimi, R.; Farzaei, F.; Abdollahi, M. Traditional medicinal herbs for the management of diabetes and its complications: An evidence-based review. Int. J. Pharmacol. 2015, 11, 874–887. [Google Scholar]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-Related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Yun, J.W. Hypoglycemic effect of polysccharides produced by submerged mycelial culture of laetiporus sulphureus on streptozotocin-induced diabetic rats. Biotechnol. Bioprocess Eng. 2010, 15, 173–181. [Google Scholar] [CrossRef]
- Juárezrojop, I.E.; Díazzagoya, J.C.; Blecastillo, J.L.; Mirandaosorio, P.H.; Castellrodríguez, A.E.; Tovillazárate, C.A.; Rodríguezhernández, A.; Aguilarmariscal, H.; Ramónfrías, T.; Bermúdezocaña, D.Y. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 236. [Google Scholar]
- Park, M.H.; Kang, J.H.; Han, J.S. The hypoglycemic effect of fermented Pueraria thunbergiana extract in streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 2015, 24, 2199–2203. [Google Scholar] [CrossRef]
- Zou, Y.; Mao, G.; Zheng, M.; Zheng, D.; Feng, W.; Wu, X.; Yang, L. Isolation, purification and characterisation of selenium-containing polysaccharides and proteins in selenium-enriched Radix pueraria. J. Sci. Food Agric. 2014, 94, 349–358. [Google Scholar] [CrossRef]
- Sun, W.; Sang, Y.; Zhang, B.; Yu, X.; Xu, Q.; Xiu, Z.; Dong, Y. Synergistic effects of acarbose and an Oroxylum indicum seed extract in streptozotocin and high-fat-diet induced predibetic mice. Biomed. Pharmacother. 2017, 87, 160–170. [Google Scholar] [CrossRef]
- Liang, L.; Ao, L.; Ma, T.; Ni, Y.; Liao, X.; Hu, X.; Song, Y. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018, 106, 447–455. [Google Scholar] [CrossRef]
- Song, H.; Sun, Z. Hypolipidaemic and hypoglycaemic properties of pumpkin polysaccharides. 3 Biotech 2017, 7, 159. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Wang, A.; Yu, Z.; Xu, Y.; Yang, Y. Purification characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds. Food Hydrocoll. 2017, 66, 357–364. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, G.; Meng, G.; Liang, X. Characterization of pumpkin polysaccharides and protective effects on streptozotocin damaged islet cells. Chin. J. Nat. Med. 2015, 13, 199–207. [Google Scholar] [CrossRef]
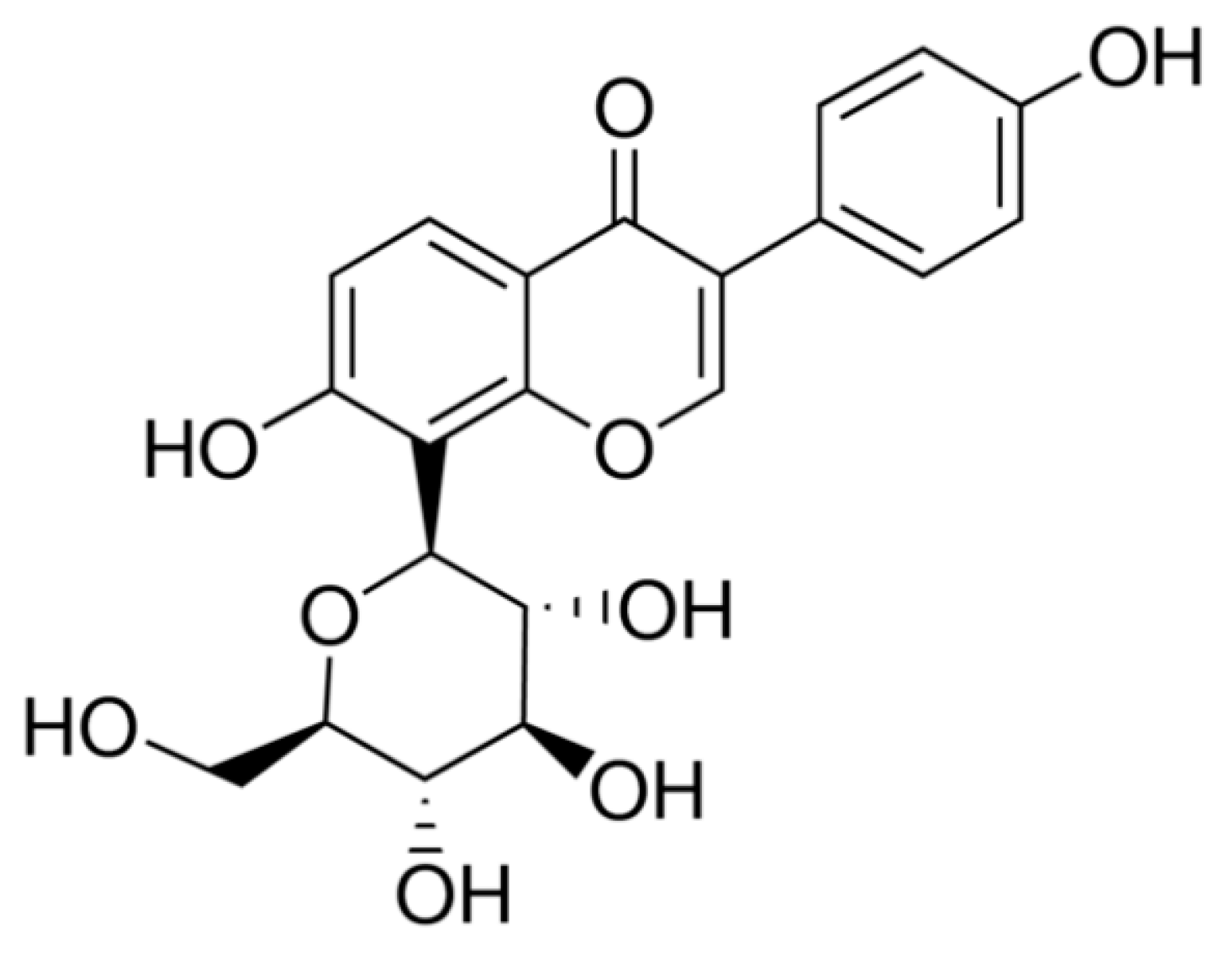
- Dong, S.; Zhang, M.; Niu, H.; Jiang, K.; Jiang, J.; Ma, Y.; Wang, X.; Meng, S. Upregulation of UDP-Glucuronosyltransferases 1a1 and 1a7 are involved in altered Puerarin Pharmacokinetics in type II diabetic rats. Molecules 2018, 23, 1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L.; Shen, M.; Xu, T.; Zhan, W.; Jin, H.; Zhang, Y.; Wang, W. Extraction and purification of pumpkin polysachharides and their hypoglycemic effect. Int. J. Biol. Macromol. 2017, 98, 182–187. [Google Scholar] [CrossRef]
- Ma, J.; Ding, J.; Xiao, Z.; Liu, C. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem. Toxicol. 2014, 71, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; Lee, K.; Liu, B.; Deng, Y.; Chen, Y.; Zhang, X.; He, J.; Zhong, Y. Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci. Rep. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ni, C.; Zhu, L.; Shen, Y.; Wang, L.; Chen, Y. Puerarin protects against high glucose-induced acute vascular dysfunction: Role of heme oxygenase-1 in rat thoracic aorta. Vasc. Pharmacol. 2009, 50, 110–115. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, N.; Huang, W.; Liang, T.; Kuang, H. Puerarin, isolated from Pueraria lobata (wild), protects aganst diabetic nephrophathy by attenuating oxidative stress. Gene 2016, 591, 411–416. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yu, L.; Chen, J. The puerarin improves renal function in STZ-induced diabetic rats by attenuating eNOS expression. Ren. Failure 2015, 37, 699–703. [Google Scholar] [CrossRef]
- Chan, K.; Han, X.D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616. [Google Scholar] [CrossRef]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Eggler, A.L.; Gay, K.A.; Mesecar, A.D. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2010, 52, S84–S94. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Mcnally, S.J.; Devey, L.; Garden, O.J.; Ross, J.A.; Wigmore, S.J. Insulin induces heme oxygenase-1 through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in renal cells. FEBS J. 2010, 273, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, T.; Han, P. The effect of tianmai xiaoke pian on insulin resistance through PI3-K/AKT signal pathway. J. Diabetes Res. 2016, 2016, 9261259. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; De Galarreta, C.M.; Cuadrado, A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef]
- Kalender, B.; Oztürk, M.; Tunçdemir, M.; Uysal, O.; Dagistanli, F.K.; Yegenaga, I.; Erek, E. Renoprotective effects of valsartan and enalapril in STZ-induced diabetes in rats. Acta Histochem. 2002, 104, 123–130. [Google Scholar] [CrossRef]
- Yamagishi, N.; Nakayama, K.; Wakatsuki, T.; Hatayama, T. Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci. 2001, 69, 2603–2609. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechnism of alloxan and streptozotocin action in β cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Sasase, T.; Yokoi, N.; Pezzolesi, M.G.; Shinohara, M. Animal models of diabetes and metabolic disease 2014. J. Diabetes Res. 2013, 3, 571809. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Wang, K.; Cao, P.; Shui, W.; Yang, Q.; Tang, Z.; Zhang, Y. Angelica sinensis polysaccharide regulates glucose and lipid metabolism disorder in prediabetic and streptozotocin-induced diabetic mice through the elevation of glycogen levels and reduction of inflammatory factors. Food Funct. 2015, 6, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Shi, H.; LI, Q. A review on pharmacological activities and utilization technologies of pumpkin. Plant Food. Hum. Nutr. 2006, 61, 73–80. [Google Scholar]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of ultrasound-assisted extraction and structural characterization of the polysaccharide from pumpkin (Cucurbita moschata) seeds. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohyd. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, O.; Cirillo, G.; Lemma, F.; Di Turi, G.; Jacchetti, E.; Curcio, M.; Barbuti, S.; Funel, N.; Parisi, O.I.; Puoci, F.; et al. Dextran-Catechin Conjugate: A potential treatment against the pancreatic ductal adenocarcinoma. Pharm. Res-Dordr. 2012, 29, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, W.; Ning, M.; Wang, Z.; Zou, L.; Zhang, H.; Wang, Q. Evaluation of antitumour activity of tea carbohydrate polymers in hepatocellular carcinorma animals. Int. J. Biol. Macromol. 2012, 50, 1103–1108. [Google Scholar] [CrossRef]
- Sarah, J.G.; Hui, C.; Rachel, T.; Rachel, T.M.; Jason, C.; Carol, A.P.; Muh, G.W.; Sonia, S. Mouse models of diabetes, obesity and related kidney disease. PLoS ONE 2016, 11, e0162131. [Google Scholar]
- Jens, J.H.; Sten, M.; Kirstine, N.B.; Maria, S.S.; Nils, B.J.; Carsten, D.; Christoffer, M. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg. Obes. Relat. Dis. 2018, 14, 708–714. [Google Scholar]
- Hawkins, M.; Tonelli, J.; Kishore, P.; Stein, D.; Ragucci, E.; Gitig, A.; Reddy, K. Contribution of elevated free fatty acid levels to the lack of glucose effectiveness in type 2 diabetes. Diabetes 2003, 52, 2748–2758. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Fang, C.; Zhang, L.; Li, Q.; Tang, Y.; Boden, G. Effects of free fatty acids on plasma resistin and insulin resistance in awake rats. Metabolism 2005, 54, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Takashi, M.; Yoshihiko, U. Fatty liver formation in fulminant type 1 diabetes. Endocrinology 2016, 1, 1–7. [Google Scholar]
- Tremblay, A.J.; Lamarche, B.; Deacon, C.F.; Weisnagel, S.J.; Couture, P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Osasenaga, M.I. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar]
- Wagner, H.; Efferth, T. Introduction: Novel hybrid combinations containing synthetic or antibiotic drugswith plant-derived phenolic or terpenoid compounds. Phytomedicine 2017, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic. Biol. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef]
- Tabassum, H.; Parvez, S.; Pasha, S.T.; Banerjee, B.D.; Raisuddin, S. Protective effect of lipoic acid against methotrexate-induced oxidative stress in liver mitochondria. Food Chem. Toxicol. 2010, 48, 1973–1979. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- So, H.S.; Kim, H.J.; Lee, J.H.; Park, S.Y.; Park, C.; Kim, Y.H.; Kim, J.K.; Lee, K.M.; Kim, K.S.; Chung, S.Y.; et al. Flunarizine induces Nrf2-mediated transcriptional activation of heme oxygenase-1 in protection of auditory cells from cisplatin. Cell Death Differ. 2006, 13, 1763–1775. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Kileen, E.; Green, C.J. Curcumin activates the haemoxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Hamdulay, S.S.; Wang, B.; Birdsey, G.M.; Ali, F.; Dumont, O.; Evans, P.C.; Haskard, D.O.; Wheeler-Jones, C.P.; Mason, J.C. Celecoxib activates PI-3K/Akt and mitochondrial redox signaling to enhance heme oxygenase-1-mediated anti-inflammatory activity in vascular endothelium. Free Radic. Biol. Med. 2010, 48, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.E.; Lee, I.T.; Lin, C.C.; Kou, Y.R.; Yang, C.M. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: Role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radic. Biol. Med. 2010, 48, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.G.; Westwick, J.; Harris, S. Sat-Nav for T cells: Role of PI3K isoforms and lipid phosphatases in migration of T lymphocytes. Immunol. Lett. 2011, 138, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, H.; Rotman, T.; Rubinstein, S.; Etkovitz, N. Role and regulation of PI3K in sperm capacitation and the acrosome reaction. Mol. Cell. Endocrinol. 2010, 314, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, Y.; Ding, X.; Hou, W.; Song, B.; Wang, T.; Li, J.; Zeng, Y. Structure elucidation and antioxidant effect of a polysaccharide from Lactarius camphoratum (Bull). Fr. Int. J. Biol. Macromol. 2013, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Group | Fasting Blood Glucose (mM) | Insulin (mU/L) | IR |
|---|---|---|---|
| C | 3.03 ± 0.27 | 6.78 ± 0.59 | 1.11 ± 0.09 |
| DM | 17.85 ± 1.53 # | 13.05 ± 0.95 # | 10.77 ± 1.05 # |
| PP | 9.87 ± 1.07 * | 8.37 ± 0.75 * | 3.75 ± 0.35 * |
| P | 11.23 ± 1.28 * | 8.03 ± 0.66 * | 4.05 ± 0.47 * |
| PPP | 6.35 ± 0.53 * | 7.05± 0.57 * | 2.53 ± 0.23 * |
| Group | TG (mM) | TC (mM) | HDL (mM) | LDL (mM) | FFAs (µM) |
|---|---|---|---|---|---|
| C | 0.87 ± 0.09 | 2.85 ± 0.23 | 1.75 ± 0.13 | 0.57 ± 0.05 | 0.37 ± 0.03 |
| DM | 2.75 ± 0.23 # | 5.28 ± 0.49 # | 0.83 ± 0.07 # | 2.79 ± 0.23 # | 0.95 ± 0.09 # |
| PP | 1.73 ± 0.15 * | 4.02 ± 0.37 * | 1.33 ± 0.11 * | 1.73 ± 0.15 * | 0.53 ± 0.05 * |
| P | 1.57 ± 0.13 * | 3.79 ± 0.35 * | 1.41 ± 0.13 * | 1.57 ± 0.13 * | 0.47 ± 0.05 * |
| PPP | 1.18 ± 0.11 * | 3.18 ± 0.31 * | 1.68 ± 0.13 * | 1.22 ± 0.11 * | 0.39 ± 0.03 * |
| Group | SOD (U/mg) | GSH (U/mg prot) | MDA (nmol/L) | ROS (IU/L) |
|---|---|---|---|---|
| C | 217.95 ± 19.09 | 27.57 ± 3.09 | 6.07 ± 0.47 | 509.3 ± 21.93 |
| DM | 133.59 ± 10.65 # | 15.77 ± 1.31 # | 11.59 ± 0.95 # | 597.1 ± 25.01 # |
| PP | 175.47 ± 11.05 * | 21.85 ± 1.75 * | 7.31 ± 0.66 * | 537.5 ± 20.87 * |
| P | 191.33 ± 13.03 * | 21.37 ± 1.91 * | 7.07 ± 0.59 * | 531.5 ± 23.07 * |
| PPP | 209.11 ± 17.85 * | 25.07 ± 2.53 * | 6.28 ± 0.53 * | 517.3 ± 21.55 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Qian, L.; Wang, B.; Zhang, Z.; Liu, H.; Zhang, Y.; Liu, J. Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice. Molecules 2019, 24, 955. https://doi.org/10.3390/molecules24050955
Chen X, Qian L, Wang B, Zhang Z, Liu H, Zhang Y, Liu J. Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice. Molecules. 2019; 24(5):955. https://doi.org/10.3390/molecules24050955
Chicago/Turabian StyleChen, Xue, Lei Qian, Bujiang Wang, Zhijun Zhang, Han Liu, Yeni Zhang, and Jinfu Liu. 2019. "Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice" Molecules 24, no. 5: 955. https://doi.org/10.3390/molecules24050955
APA StyleChen, X., Qian, L., Wang, B., Zhang, Z., Liu, H., Zhang, Y., & Liu, J. (2019). Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice. Molecules, 24(5), 955. https://doi.org/10.3390/molecules24050955




