Drug-1,3,4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation
Abstract
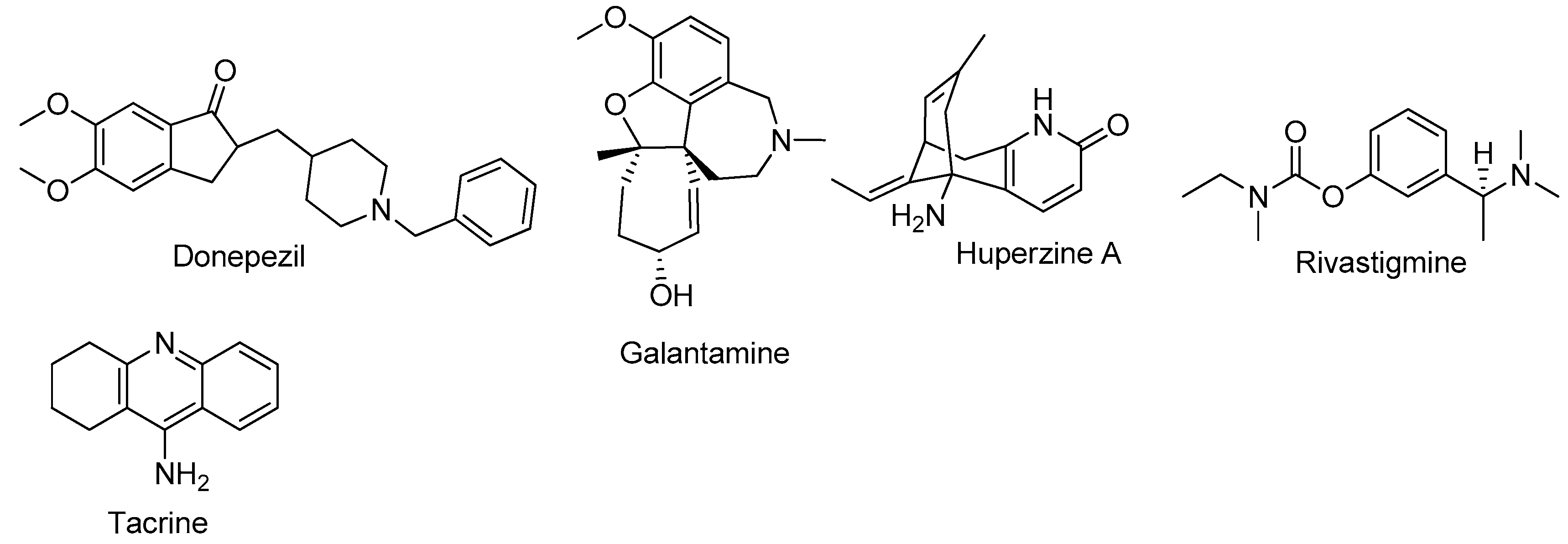
1. Introduction
2. Experimental
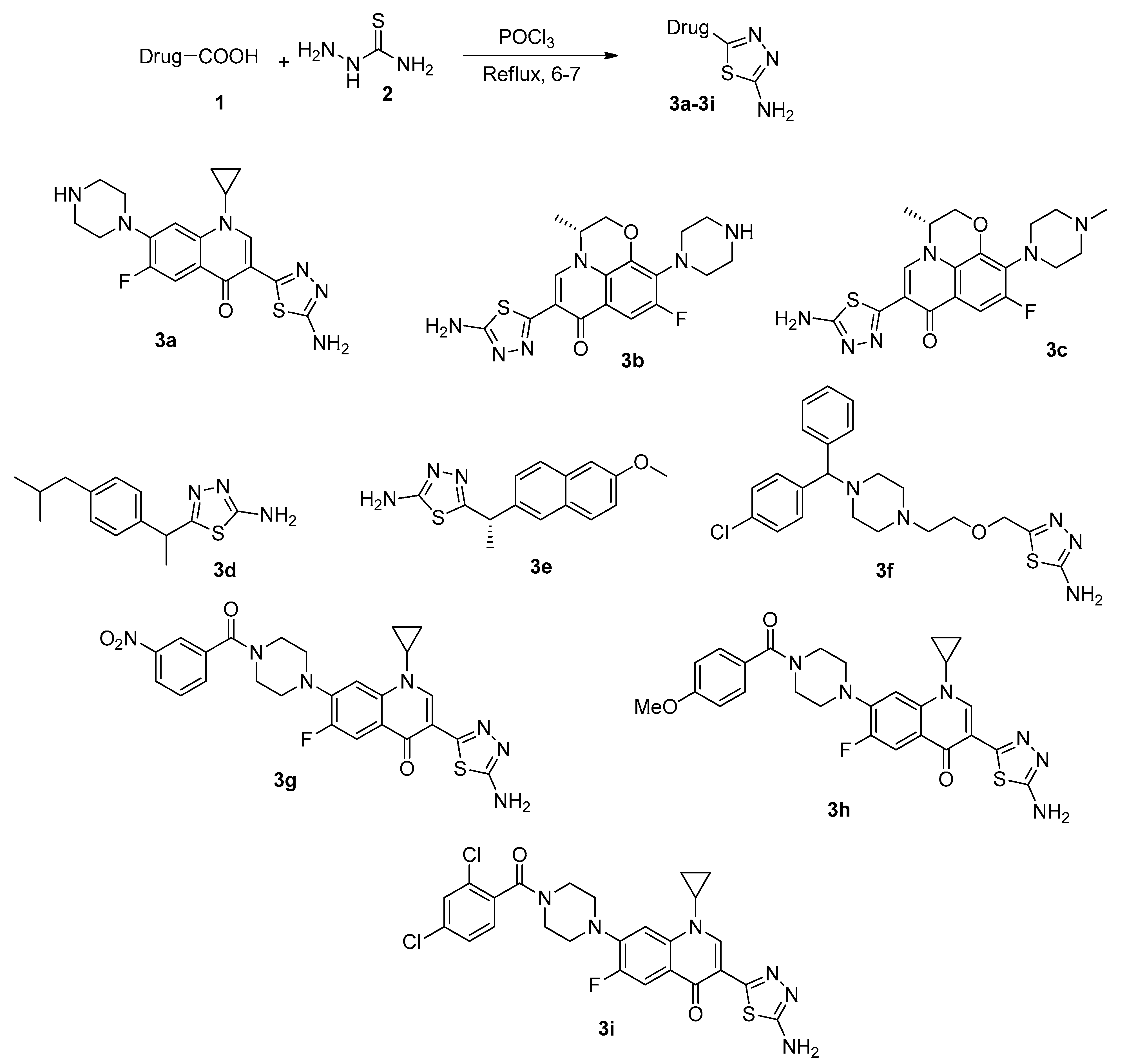
2.1. General Procedure for the Synthesis of Drug-Derivatives 1,3,4-Thiadiazole (3)
Acetylcholinesterase Inhibition Assay
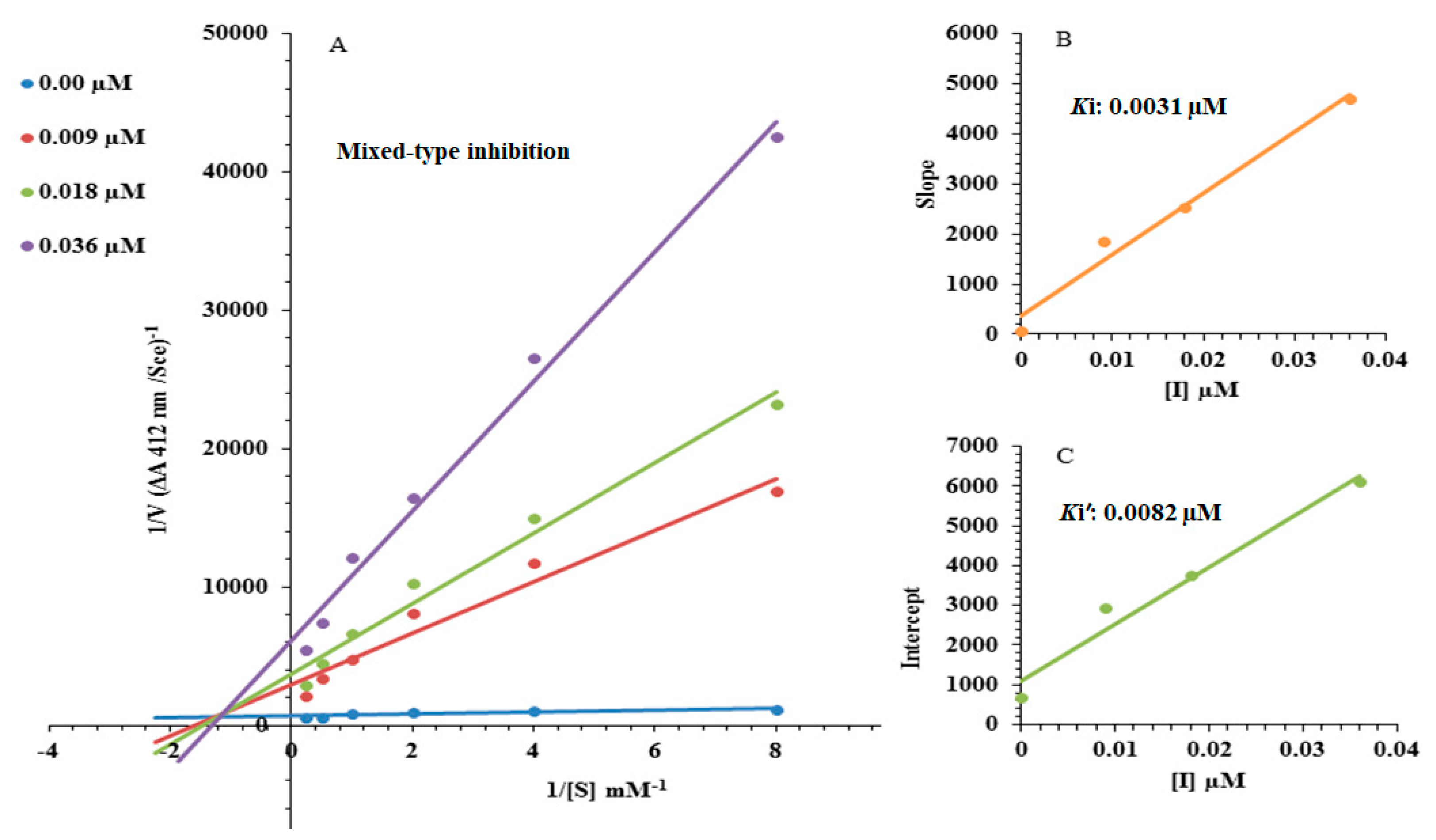
2.2. Kinetic Study
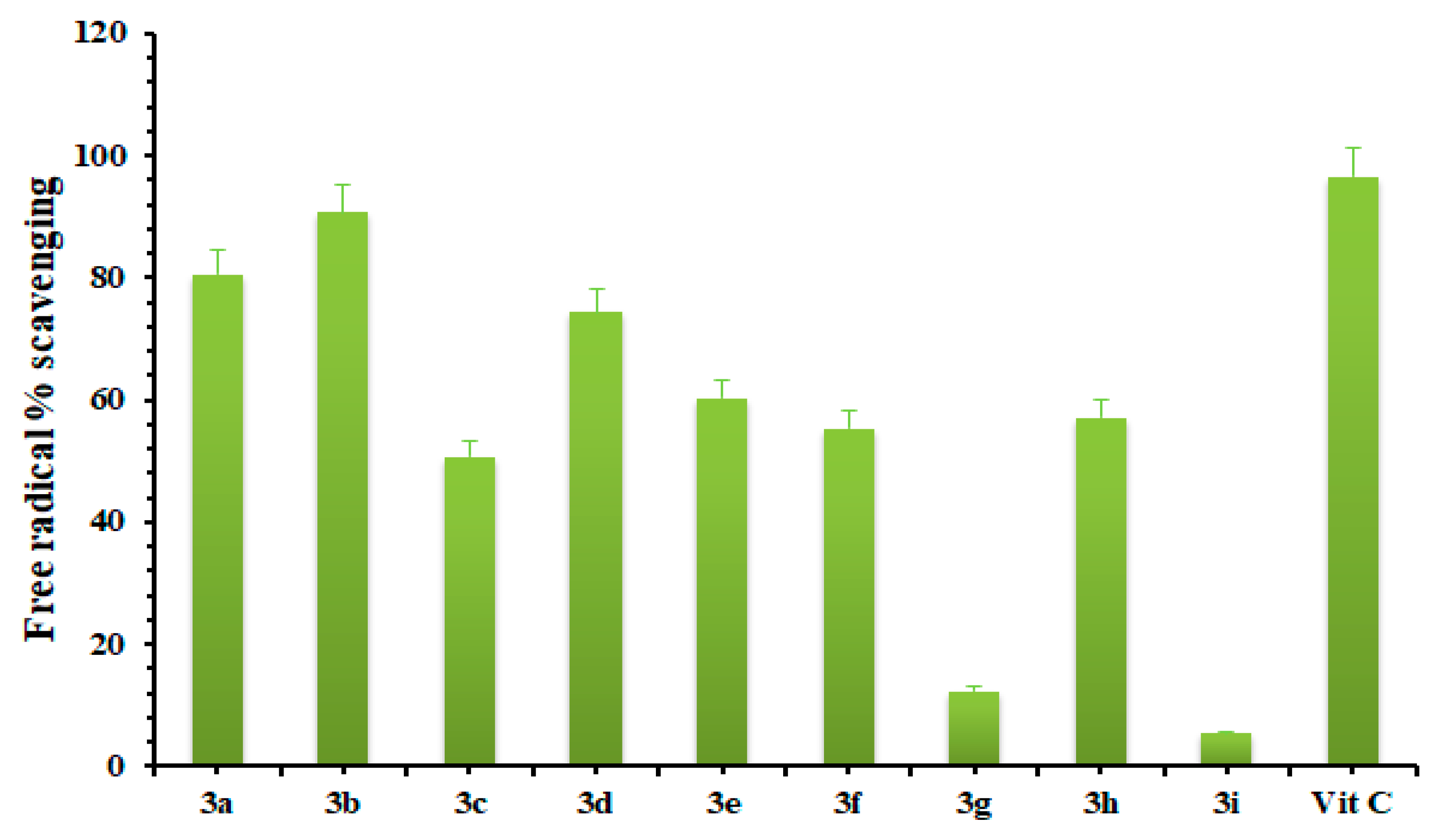
2.3. Free Radical Scavenging Assay
2.4. Computational Methodology
Retrieval of the Protein Structure from the PDB
2.5. Compound Structure
2.6. Molecular Docking
3. Results and Discussion
3.1. Acetyl Cholinesterase Inhibition Assay
3.2. Kinetic Mechanism
3.3. Free Radical Scavenging
3.4. Biochemical Properties and Lipinski’s Rule of Five (RO5) Validation
3.5. ADMET Assessment of Synthesized Compounds
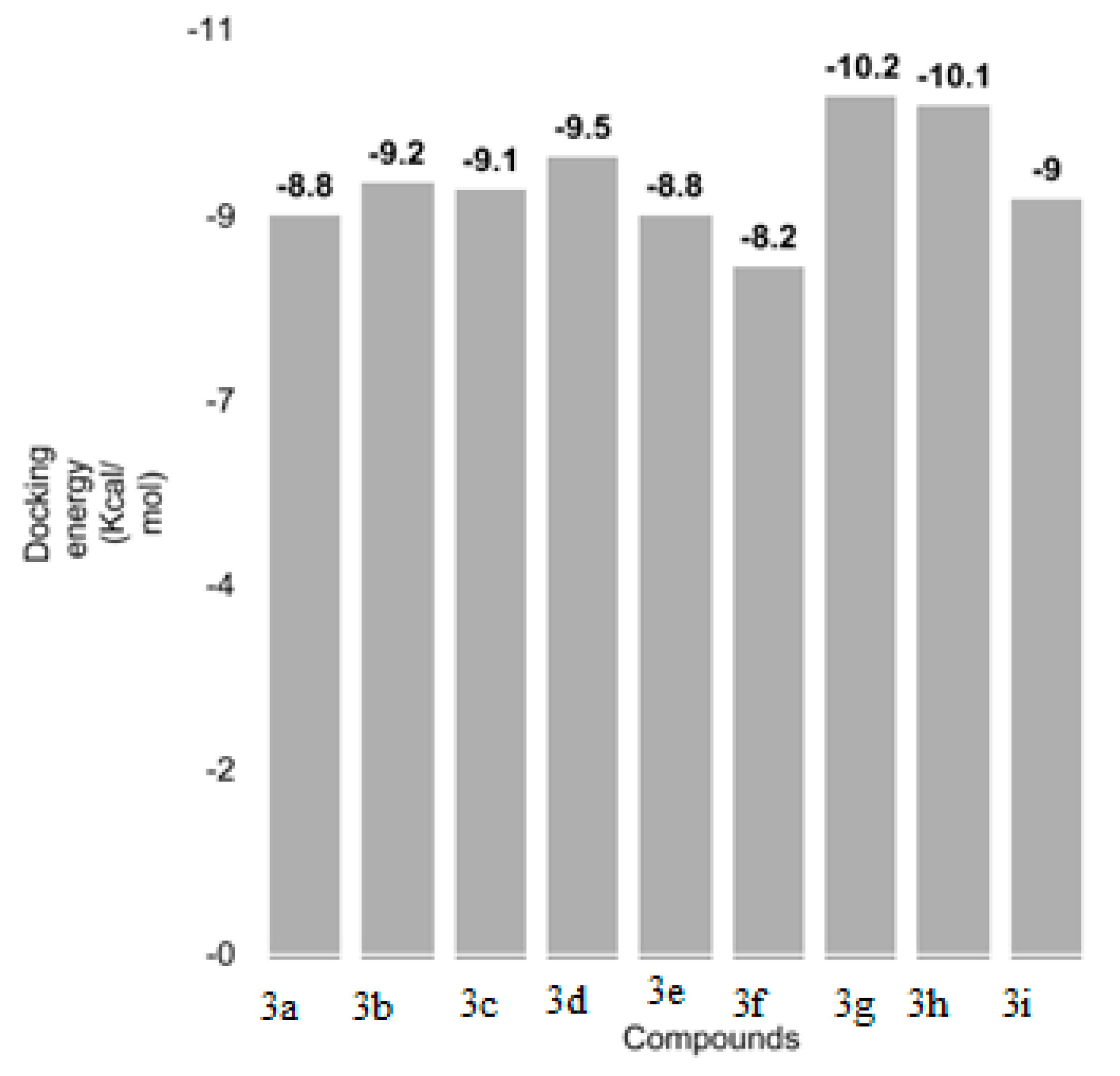
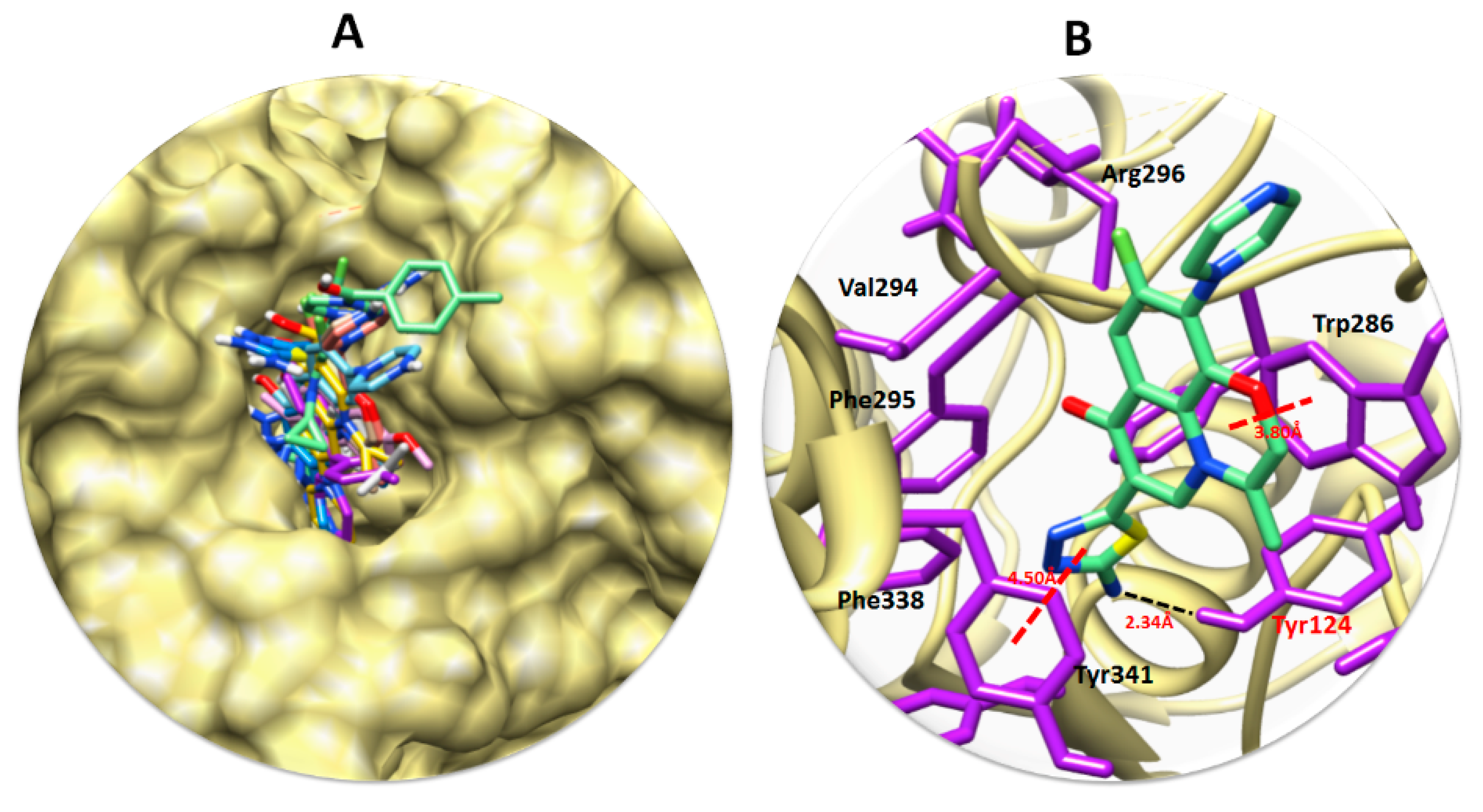
3.6. Molecular Docking Analyses
3.7. Structure–Activity Relationship (SAR) Analyses between 3b and Target Protein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; Cairnsi, N.J.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagustm, W.; Morris, J.C.; et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Dementia 2019, 15, 106–152. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Channar, K.P.A.; Shah, M.S.; Saeed, A.; Khan, S.; Larik, F.A.; Shabir, G.; Iqbal, J. Synthesis, Characterization and Cholinesterase Inhibition Studies of New Arylidene Aminothiazolylethanone Derivatives. Med. Chem. 2017, 13, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Cholinesterase inhibitors: A new class of psychotropic compounds. Am. J. Psychiatry 2000, 157, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Kavirajan, H.; Schneider, L.S. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: A meta-analysis of randomised controlled trials. Lancet Neurol. 2007, 6, 782–792. [Google Scholar] [CrossRef]
- Saeed, A.; Shah, M.S.; Larik, F.A.; Khan, S.U.; Channar, P.A.; Flörke, U.; Iqbal, J. Synthesis, computational studies and biological evaluation of new 1-acetyl-3-aryl thiourea derivatives as potent cholinesterase inhibitors. Med. Chem. Res. 2017, 26, 1635–1646. [Google Scholar] [CrossRef]
- Torre, P.D.L.; Saavedra, L.A.; Caballero, J.; Quiroga, J.; Alzate-Morales, J.H.; Cabrera, M.G.; Trilleras, J. A novel class of selective acetylcholinesterase inhibitors: Synthesis and evaluation of (E)-2-(benzo [d] thiazol-2-yl)-3-heteroarylacrylonitriles. Molecules 2012, 17, 12072–12085. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Ruiz, M.P.; Rubio, L.; García-Palomero, E.; Dorronsoro, I.; del Monte-Millán, M.; Valenzuela, R.; Usán, P.; de Austria, C.; Bartolini, M.; Andrisano, V.; et al. Design, synthesis, and biological evaluation of dual binding site acetylcholinesterase inhibitors: New disease-modifying agents for Alzheimer’s disease. J. Med. Chem. 2005, 48, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Terzioglu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2, 6-dimethylimidazo [2,1-b][1,3,4] thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Clerici, F.; Pocar, D.; Guido, M.; Loche, A.; Perlini, V.; Brufani, M. Synthesis of 2-amino-5-sulfanyl-1, 3, 4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001, 44, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Ranise, A.; Filippelli, W.; Rinaldi, B.; Capuano, A.; Falcone, G. New 1,3,4-thiadiazole derivatives endowed with analgesic and anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Oruç, E.E.; Rollas, S.; Kandemirli, F.; Shvets, N.; Dimoglo, A.S. 1,3,4-thiadiazole derivatives. Synthesis, structure elucidation, and structure− antituberculosis activity relationship investigation. J. Med. Chem. 2004, 47, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxicity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.Y.; Wang, X.M.; Yang, Y.H.; Zhu, H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Farina, R.; Catto, M.; Iacobazzi, R.M.; Nicolotti, O.; Cellamare, S.; Mangiatordi, G.F.; Denora, N.; Soto-Otero, R.; Siragusa, L.; et al. Exploring Basic Tail Modifications of Coumarin-Based Dual Acetylcholinesterase-Monoamine Oxidase B Inhibitors: Identification of Water-Soluble, Brain-Permeant Neuroprotective Multitarget. J. Med. Chem. 2016, 59, 6791–6806. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D., Jr.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Larik, F.A.; Saeed, A.; Channar, P.A.; Ismail, H.; Dilshad, E.; Mirza, B. New 1-octanoyl-3-aryl thiourea derivatives: Solvent-free synthesis, characterization and multi-target biological activities. Bangladesh J. Pharmacol. 2016, 11, 894–902. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Willard, L.; Ranjan, A.; Zhang, H.; Monzavi, H.; Boyko, R.F.; Sykes, B.D.; Wishart, D.S. VADAR: A web server for quantitative evaluation of protein structure quality. Nucleic. Acids. Res. 2003, 31, 3316–3319. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1996, 8, 127–134. [Google Scholar] [CrossRef]
- Studio, D. Discovery.“Version 2.1.”; Accelrys: San Diego, CA, USA, 2008. [Google Scholar]
- Kadam, R.U.; Roy, N. Recent trends in drug-likeness prediction: A comprehensive review of in silico methods. Indian J. Pharm. Sci. 2007, 69, 609–615. [Google Scholar]
- Bakht, M.A.; Yar, M.S.; Abdel-Hamid, S.G.; Al Qasoumi, S.I.; Samad, A. Molecular properties prediction, synthesis and antimicrobial activity of some newer oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5862–5869. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug. Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wu, P.; Yang, R.; Gao, L.; Li, C.; Wang, D.; Wu, S.; Du, A.L.; Liu, G.H. Inhibition of acetylcholinesterase by two genistein derivatives: Kinetic analysis, molecular docking and molecular dynamics simulation. Acta Pharm. Sin. B 2014, 4, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Simeon, S.; Anuwongcharoen, N.; Shoombuatong, W.; Malik, A.A.; Prachayasittikul, V.; Wikberg, J.E.; Nantasenamat, C. Probing the origins of human acetylcholinesterase inhibition via QSAR modeling and molecular docking. PeerJ 2016, 4, 2322. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are available from the authors. |
| Compounds | Acetylcholine Esterase (from Human Erythrocytes) IC50 ± SEM (nM) |
|---|---|
| 3a | 126.3 ± 3.6 |
| 3b | 18.1 ± 0.9 |
| 3c | 576.3 ± 3.6 |
| 3d | 2241.7 ± 112.0 |
| 3e | 3806.4 ± 190.3 |
| 3f | 17274.8 ± 863.0 |
| 3g | 1182.19 ± 59.1 |
| 3h | 1710.7 ± 86.5 |
| 3i | 29228.0 ± 1461.4 |
| Neostigmine methyl sulfate | 2186.5 ± 98.0 |
| Concentration (µM) | Vmax (ΔA/Sec) | Km (mM) |
|---|---|---|
| 0.00 | 0.001856 | 0.07692 |
| 0.009 | 0.000467 | 0.51282 |
| 0.018 | 0.000343 | 0.55555 |
| 0.036 | 0.000183 | 0.6060 |
| Properties | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i |
|---|---|---|---|---|---|---|---|---|---|
| Mol. weight (g/mol) | 414 | 402 | 416 | 261 | 285 | 443 | 563 | 548 | 587 |
| No. HBA | 6 | 6 | 6 | 3 | 4 | 6 | 6 | 6 | 6 |
| No. HBD | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mol. Log P | 1.40 | 1.25 | 1.87 | 3.83 | 3.67 | 3.23 | 3.47 | 3.47 | 4.38 |
| No of stereo centers | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Mol. Vol (A3) | 401 | 373 | 394 | 546 | 256 | 408 | 525 | 525 | 537 |
| Drug likeness Score | 0.90 | 0.40 | 0.97 | 0.85 | 0.50 | 2.46 | 0.94 | 1.05 | 0.90 |
| ADMET Properties | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | |
|---|---|---|---|---|---|---|---|---|---|---|
| Absorption | WS (log mol/L) | −3.031 | −3.295 | −3.814 | −3.693 | −2.925 | −4.184 | −3.903 | −3.712 | −3.766 |
| IS (%abs) | 96.491 | 83.841 | 95.331 | 93.288 | 83.648 | 93.375 | 95.172 | 93.294 | 89.651 | |
| SP (log Kp) | −2.743 | −2.815 | −2.785 | −2.741 | −2.743 | −2.895 | −2.74 | −2.741 | −2.731 | |
| Distribution | BBBP (Log BB) | −1.267 | −1.167 | 0.143 | −0.926 | −1.287 | 0.234 | −1.261 | −0.92 | −1.114 |
| CNSP (Log PS) | −3.105 | −3.134 | −2.108 | −3.346 | −3.131 | −2.087 | −3.277 | −3.346 | −2.449 | |
| VDss (log L/kg) | 0.693 | 0.964 | 0.389 | 0.508 | 0.631 | 0.573 | 0.42 | 0.463 | 1.47 | |
| Metabolism | CYP3A4 inhibitor | No | No | No | Yes | No | No | Yes | Yes | Yes |
| CYP1A2 inhibitor | No | No | Yes | No | Yes | Yes | No | No | No | |
| CYP2C19 inhibitor | No | No | Yes | Yes | No | Yes | Yes | Yes | No | |
| CYP2C9 inhibitor | No | No | Yes | Yes | No | No | Yes | Yes | No | |
| Excretion | TC (log mL/min/kg) | 0.508 | 0.871 | 0.097 | 0.009 | 0.585 | −0.03 | 0.126 | 0.011 | 0.842 |
| Toxicity | AMES toxicity | No | No | Yes | No | No | No | No | No | No |
| Max. tolerat. dose | −0.245 | −0.377 | 0.143 | −0.07 | −0.237 | 0.864 | −0.057 | −0.092 | 0.244 | |
| ORAT(LD50) | 2.528 | 2.924 | 2.914 | 2.605 | 2.483 | 2.669 | 2.593 | 2.606 | 2.698 | |
| HT | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | |
| SS | No | No | No | No | No | No | No | No | No | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ujan, R.; Saeed, A.; Channar, P.A.; Larik, F.A.; Abbas, Q.; Alajmi, M.F.; El-Seedi, H.R.; Rind, M.A.; Hassan, M.; Raza, H.; et al. Drug-1,3,4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation. Molecules 2019, 24, 860. https://doi.org/10.3390/molecules24050860
Ujan R, Saeed A, Channar PA, Larik FA, Abbas Q, Alajmi MF, El-Seedi HR, Rind MA, Hassan M, Raza H, et al. Drug-1,3,4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation. Molecules. 2019; 24(5):860. https://doi.org/10.3390/molecules24050860
Chicago/Turabian StyleUjan, Rabail, Aamer Saeed, Pervaiz Ali Channar, Fayaz Ali Larik, Qamar Abbas, Mohamed F. Alajmi, Hesham R. El-Seedi, Mahboob Ali Rind, Mubashir Hassan, Hussain Raza, and et al. 2019. "Drug-1,3,4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation" Molecules 24, no. 5: 860. https://doi.org/10.3390/molecules24050860
APA StyleUjan, R., Saeed, A., Channar, P. A., Larik, F. A., Abbas, Q., Alajmi, M. F., El-Seedi, H. R., Rind, M. A., Hassan, M., Raza, H., & Seo, S.-Y. (2019). Drug-1,3,4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation. Molecules, 24(5), 860. https://doi.org/10.3390/molecules24050860








