Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations
Abstract
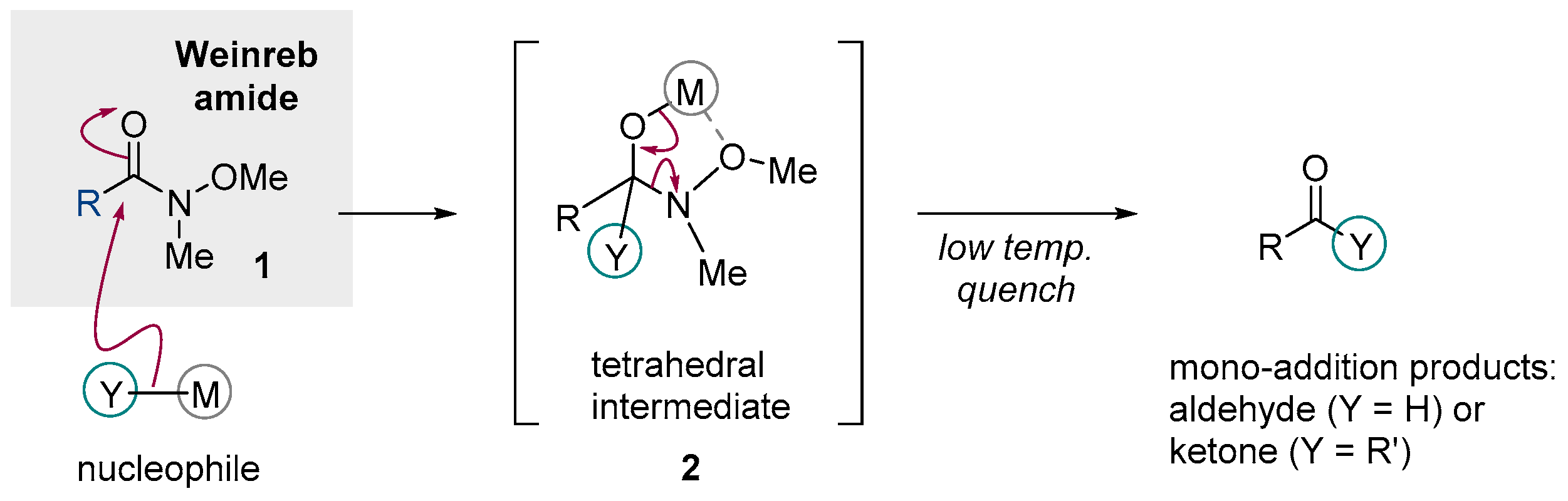
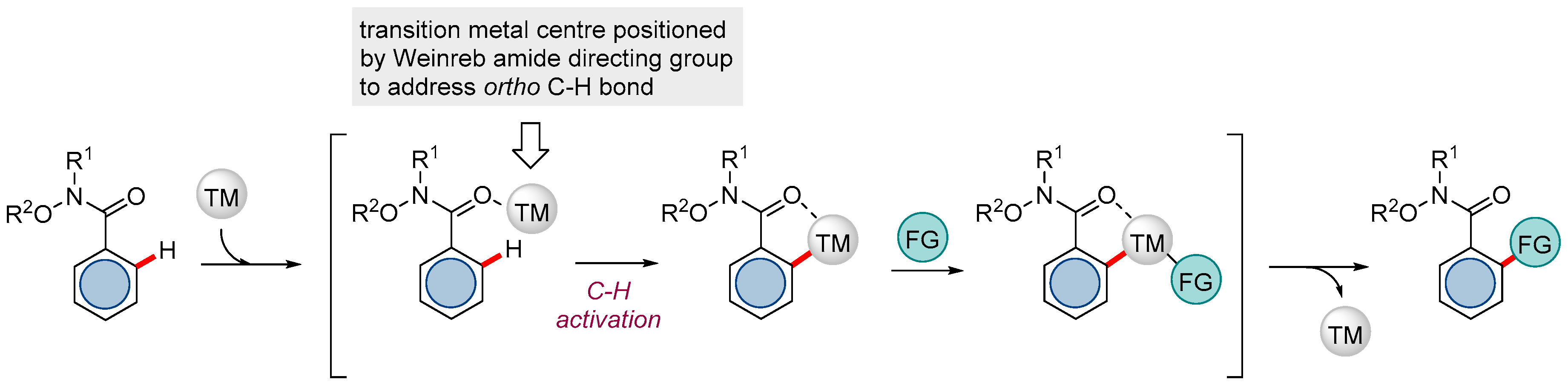
:1. Introduction
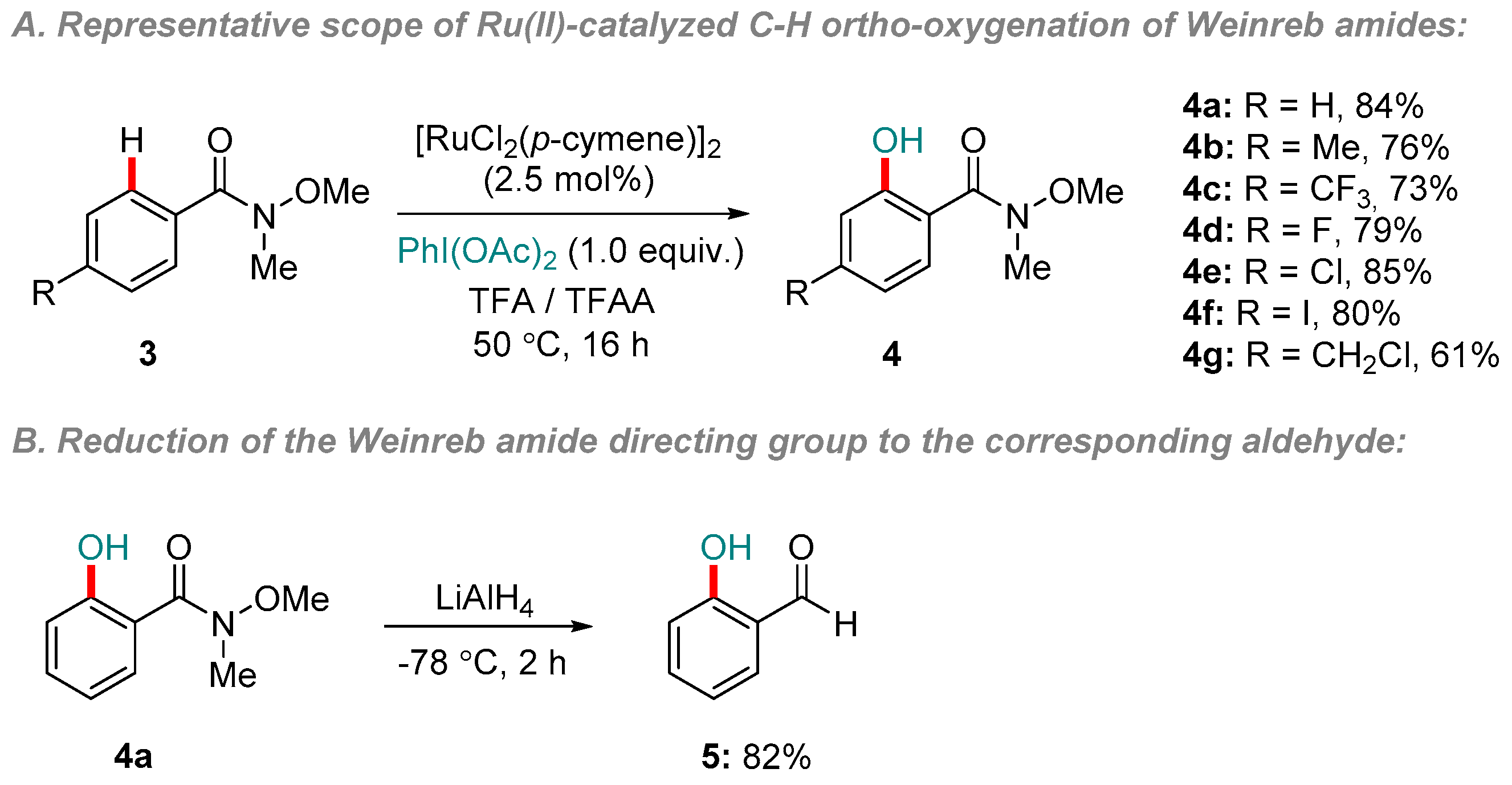
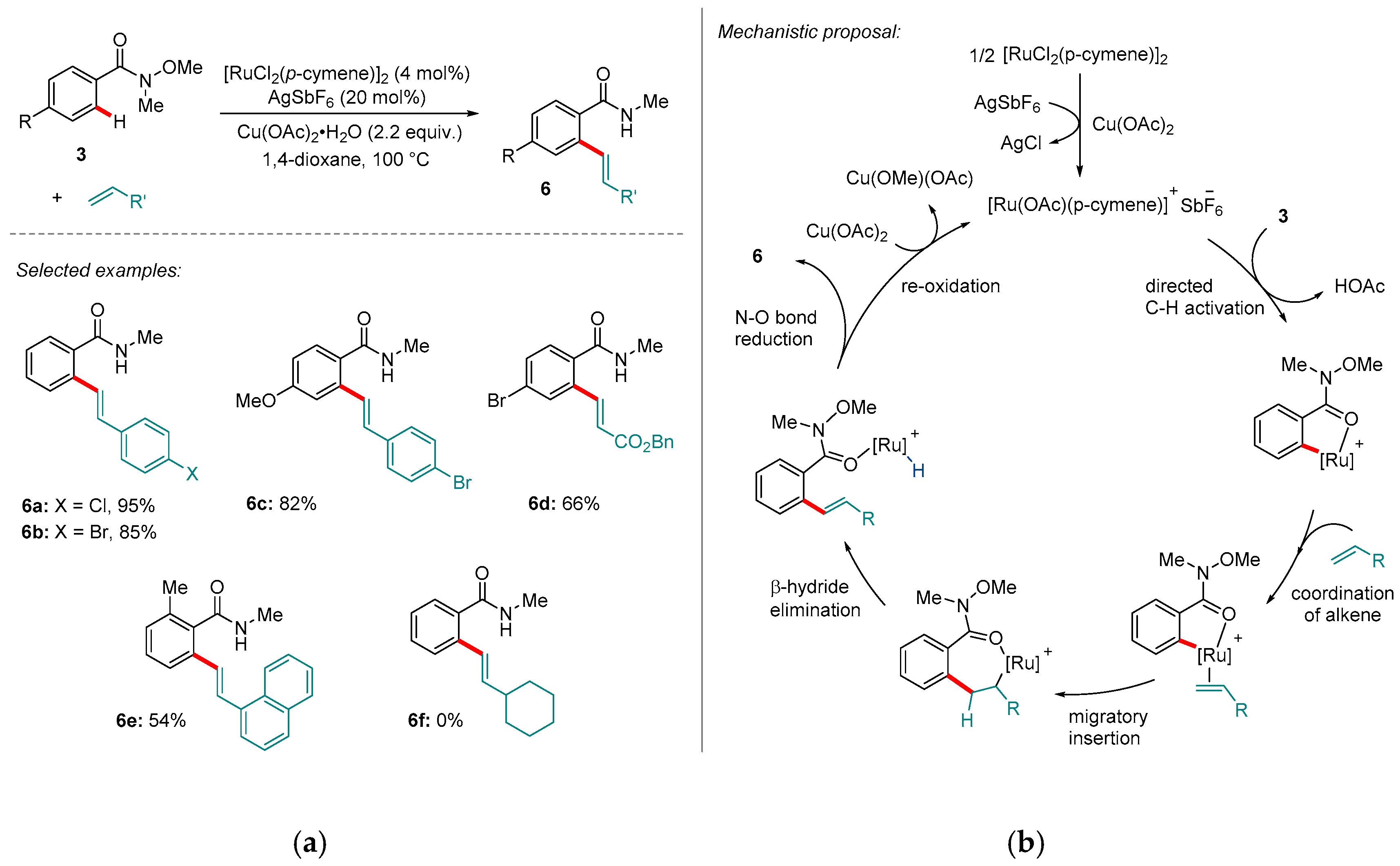
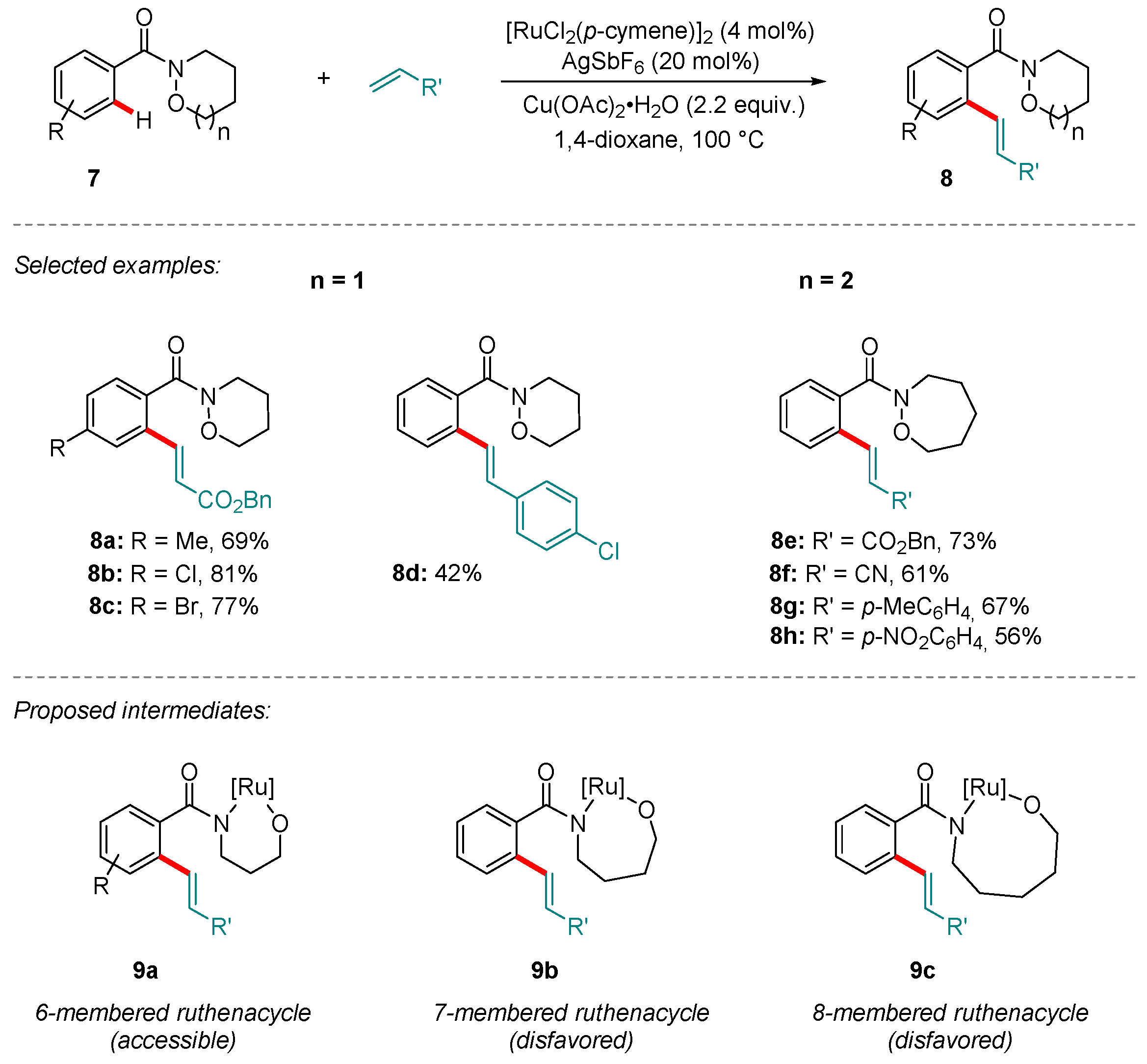
2. Ru-catalyzed Reactions
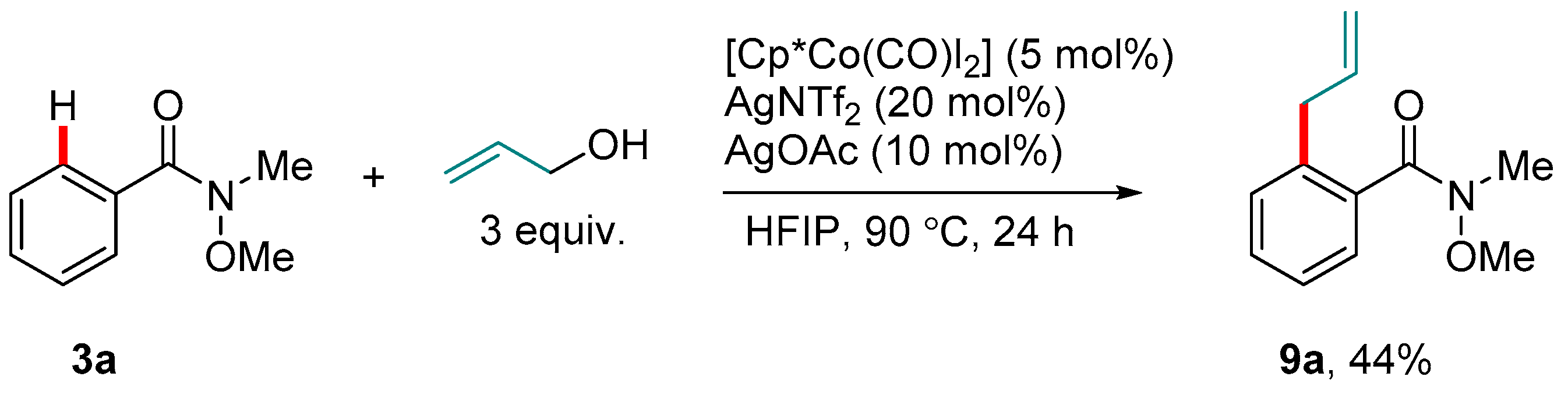
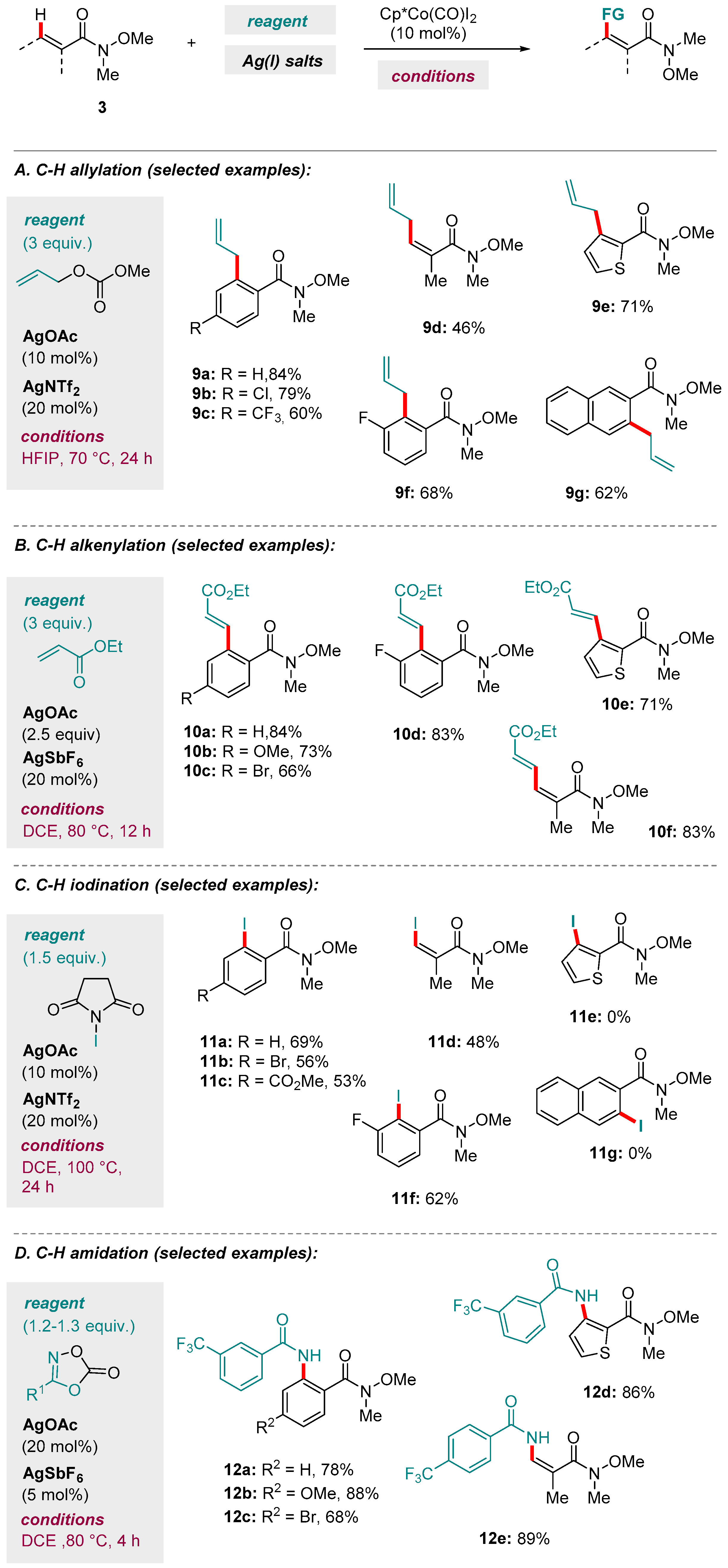
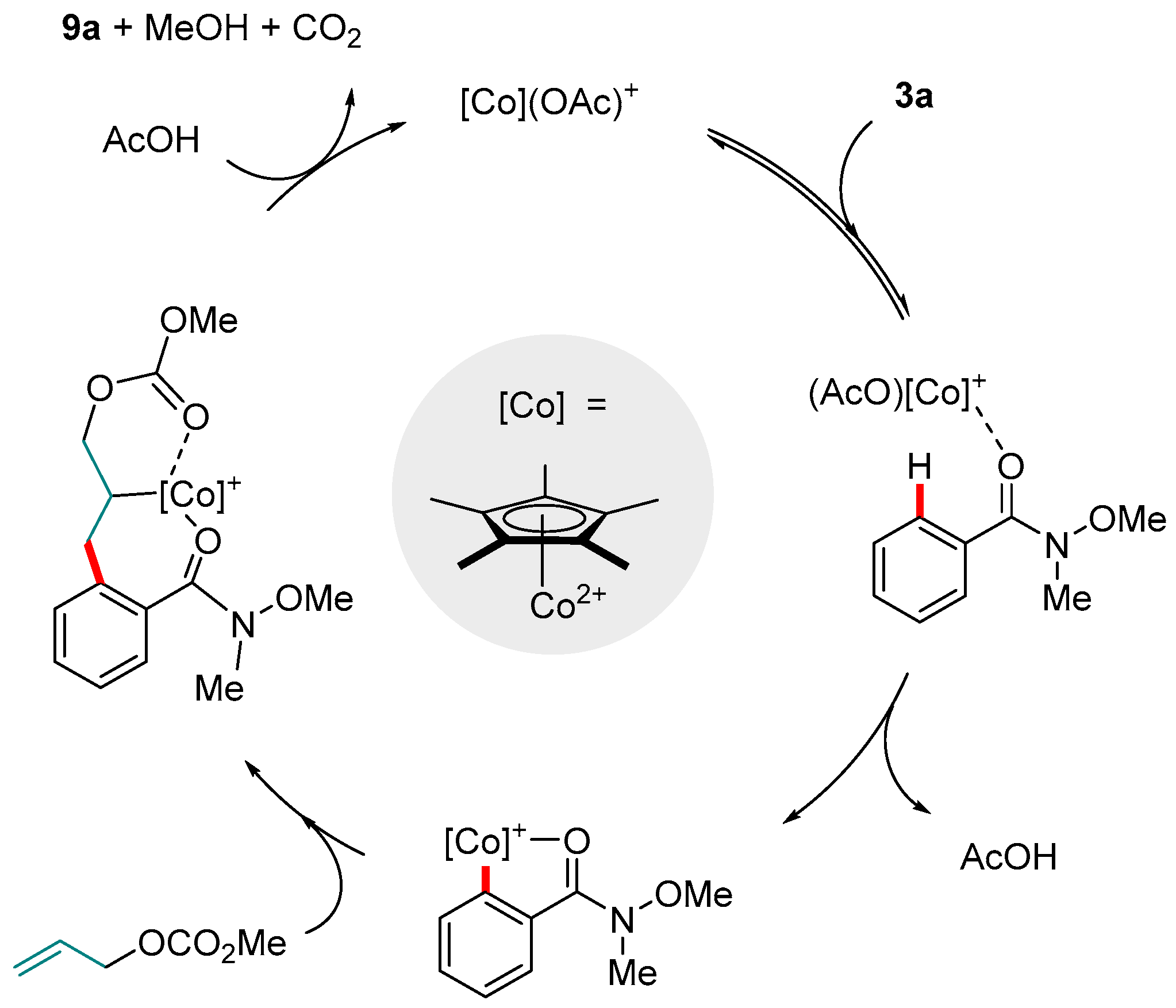
3. Co-catalyzed Reactions
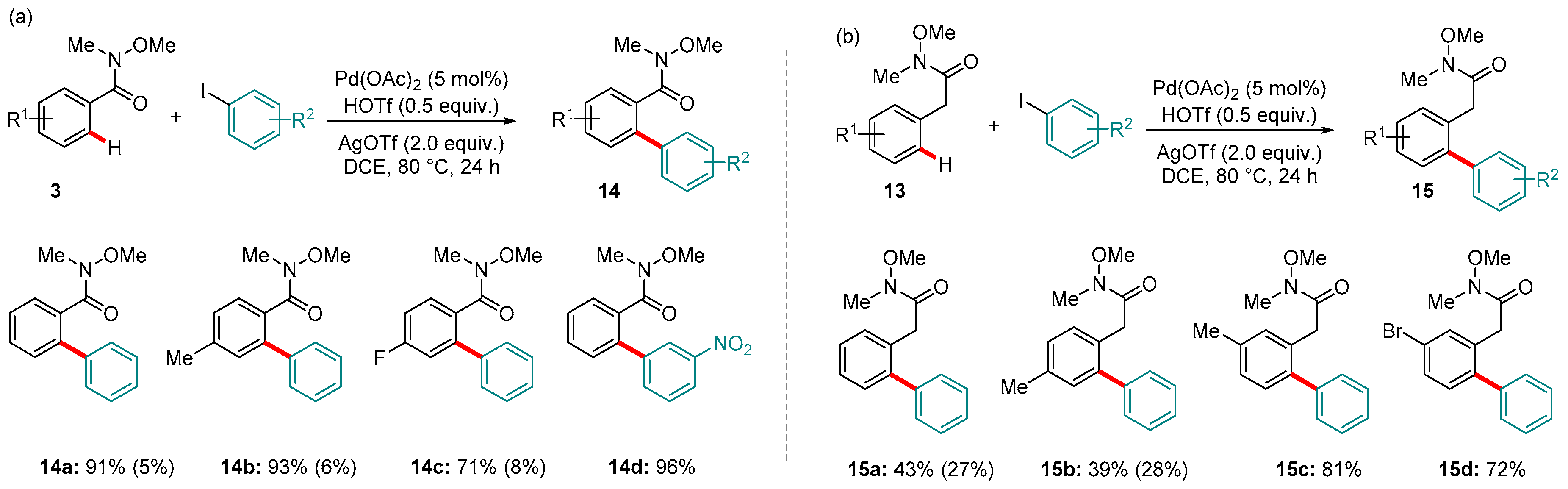
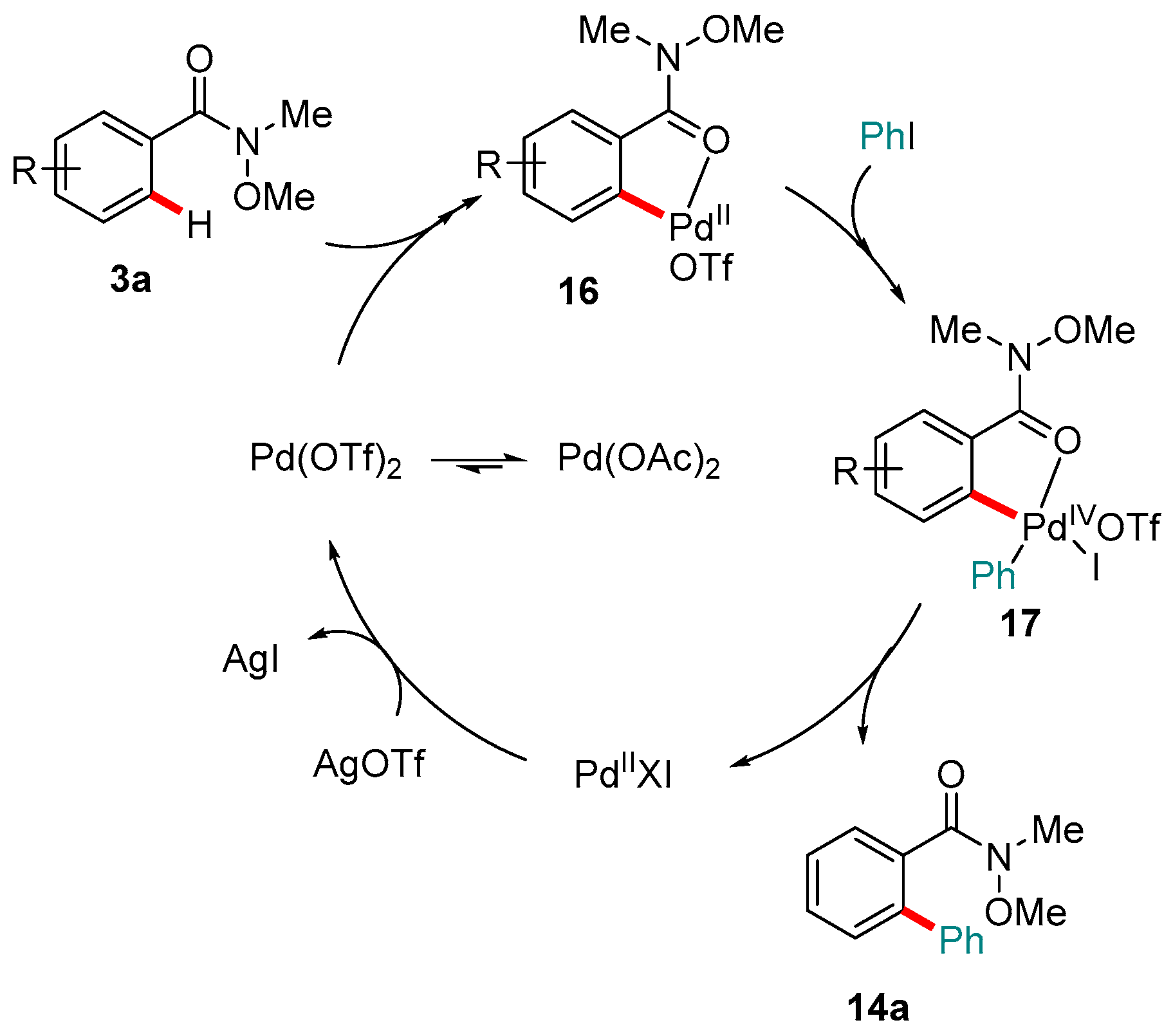
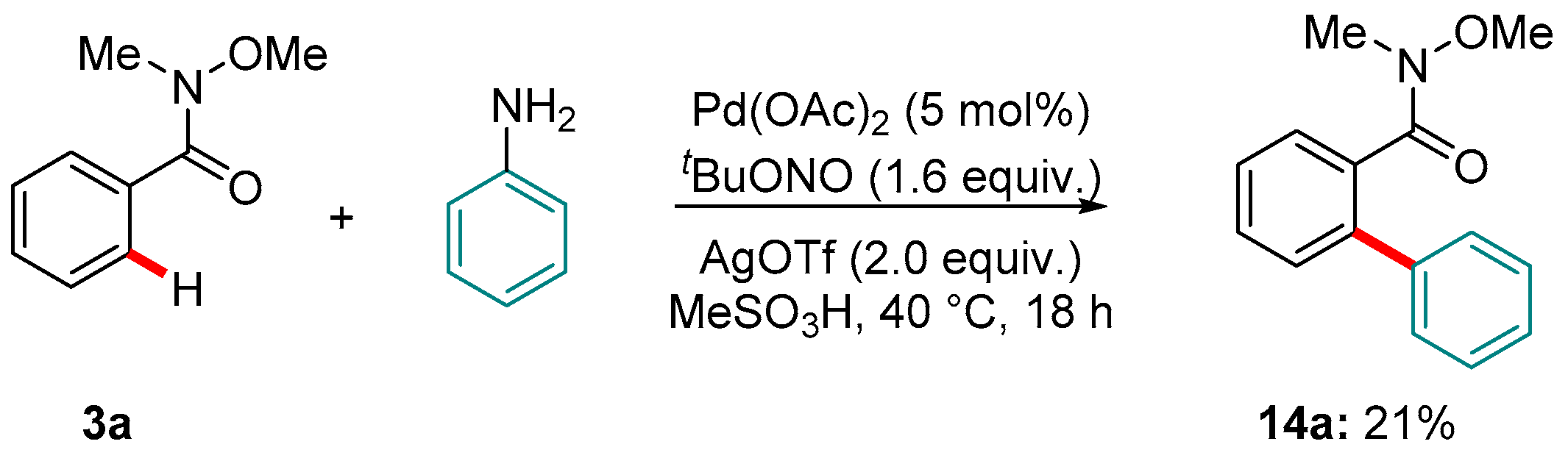
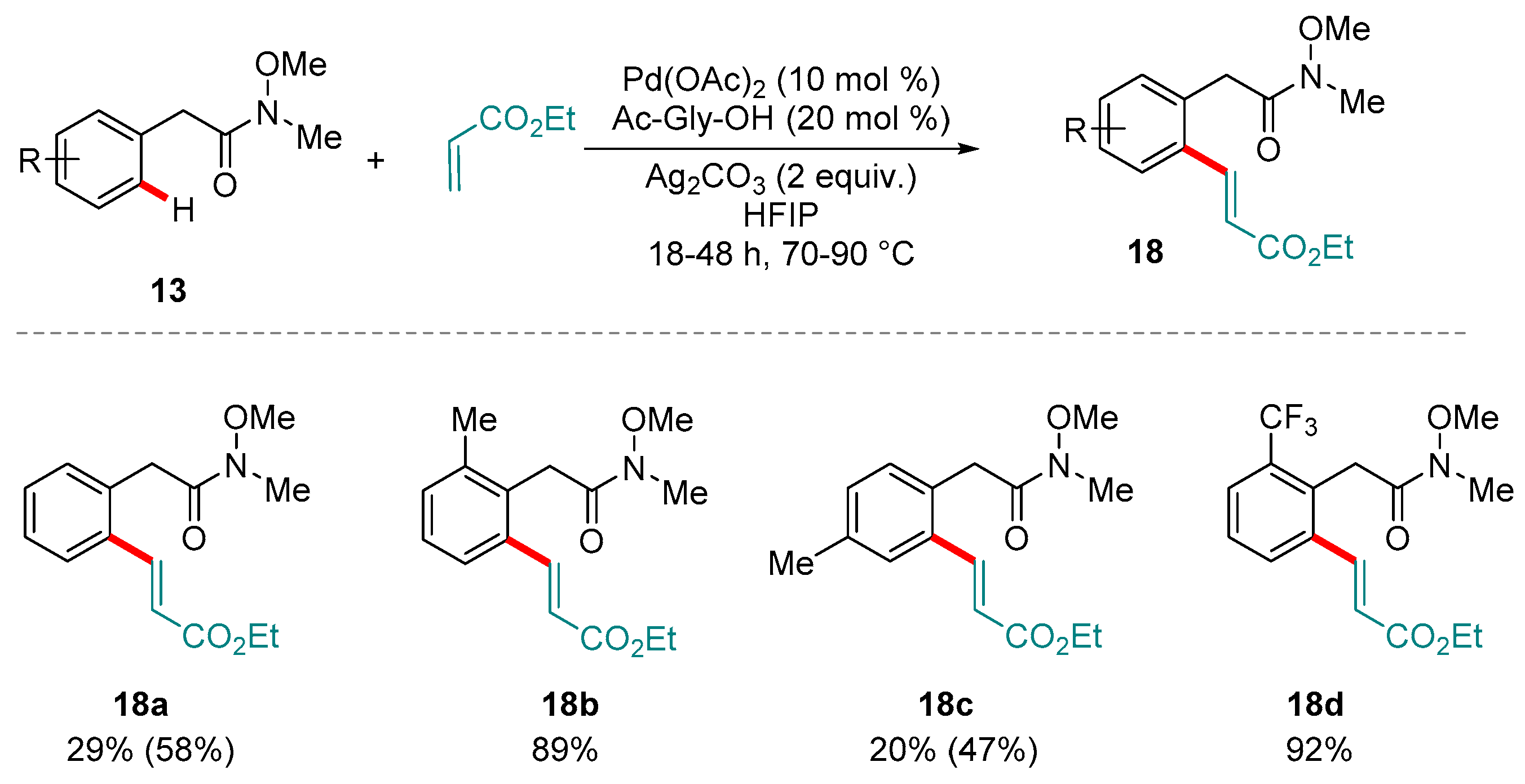
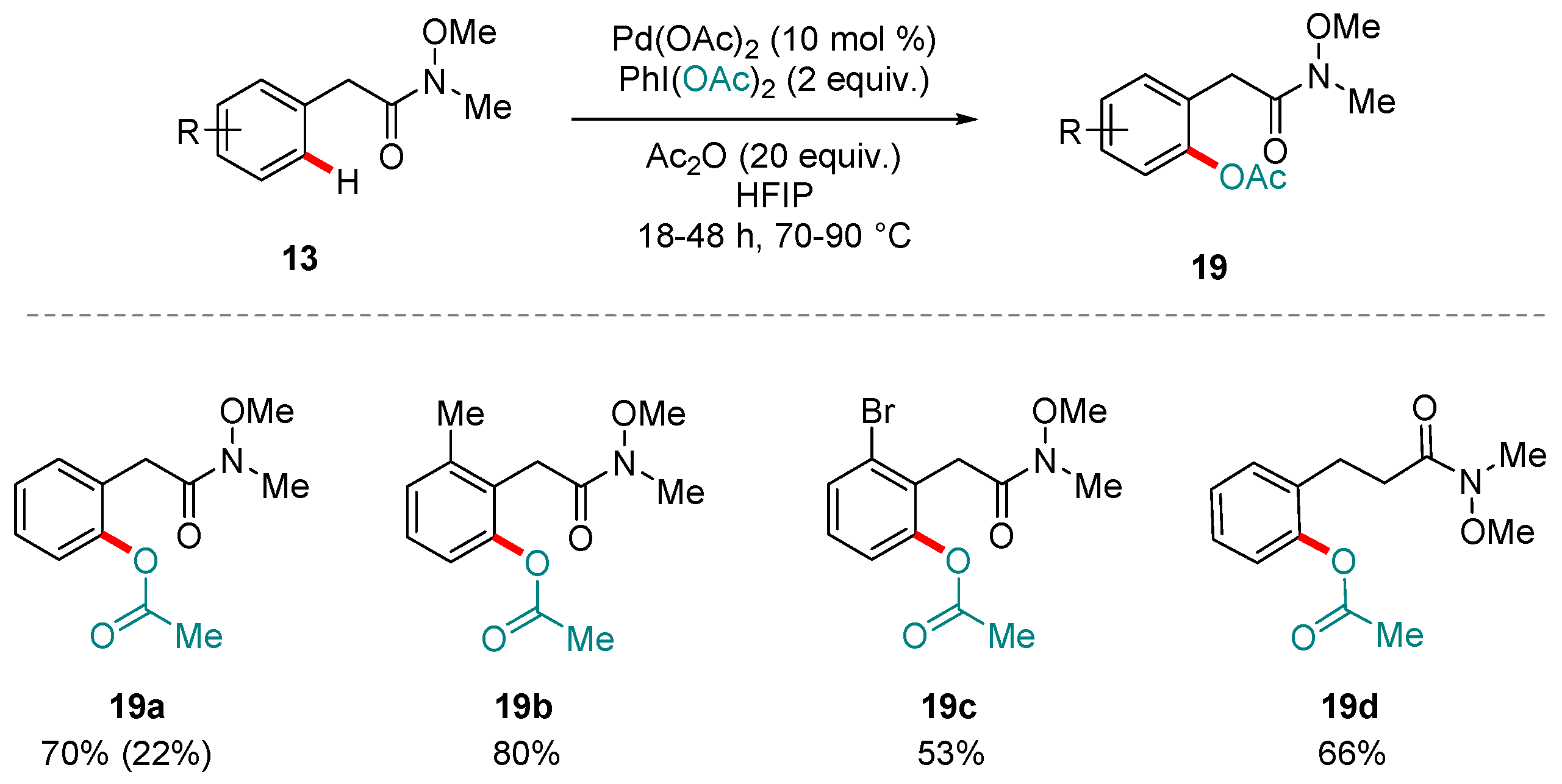
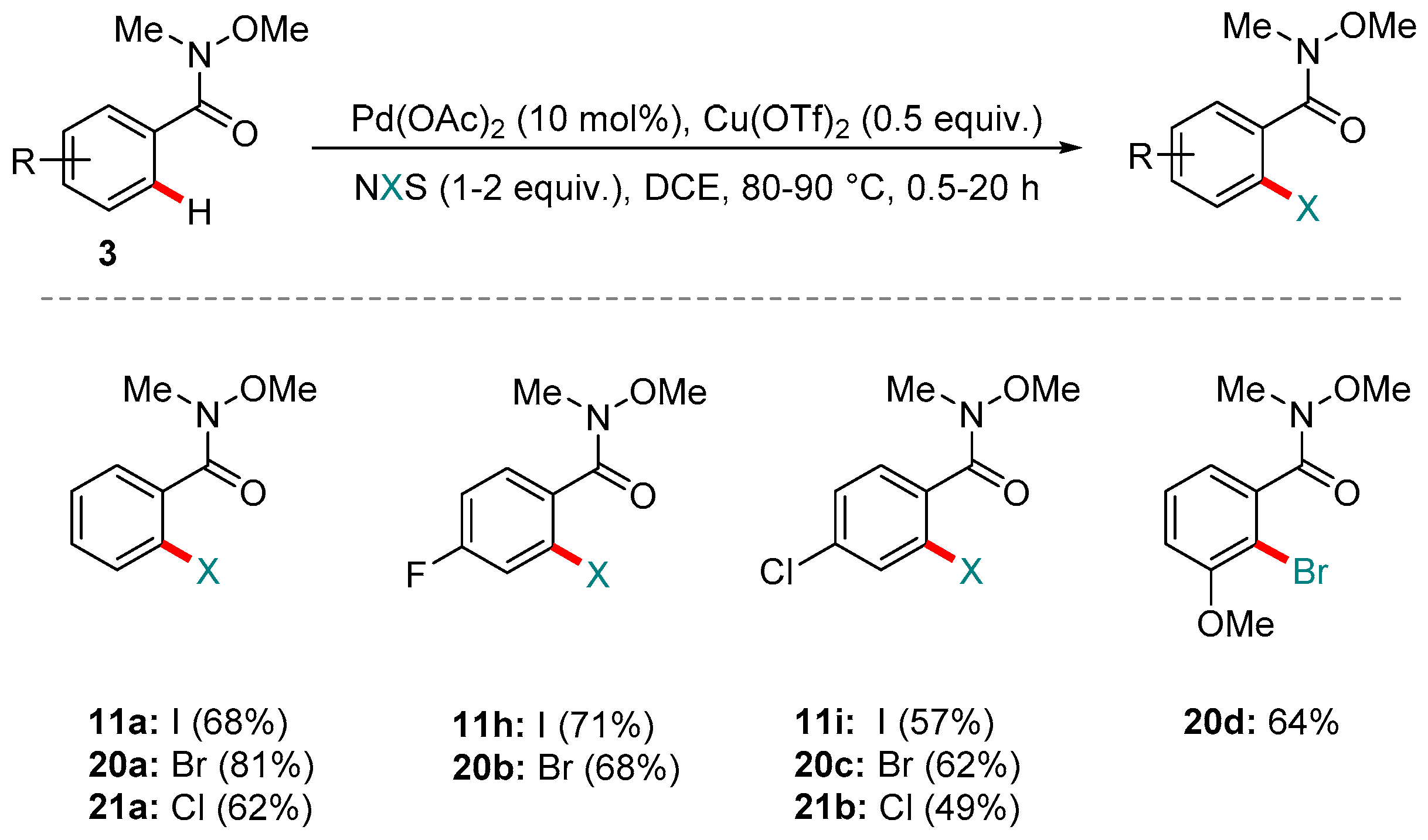
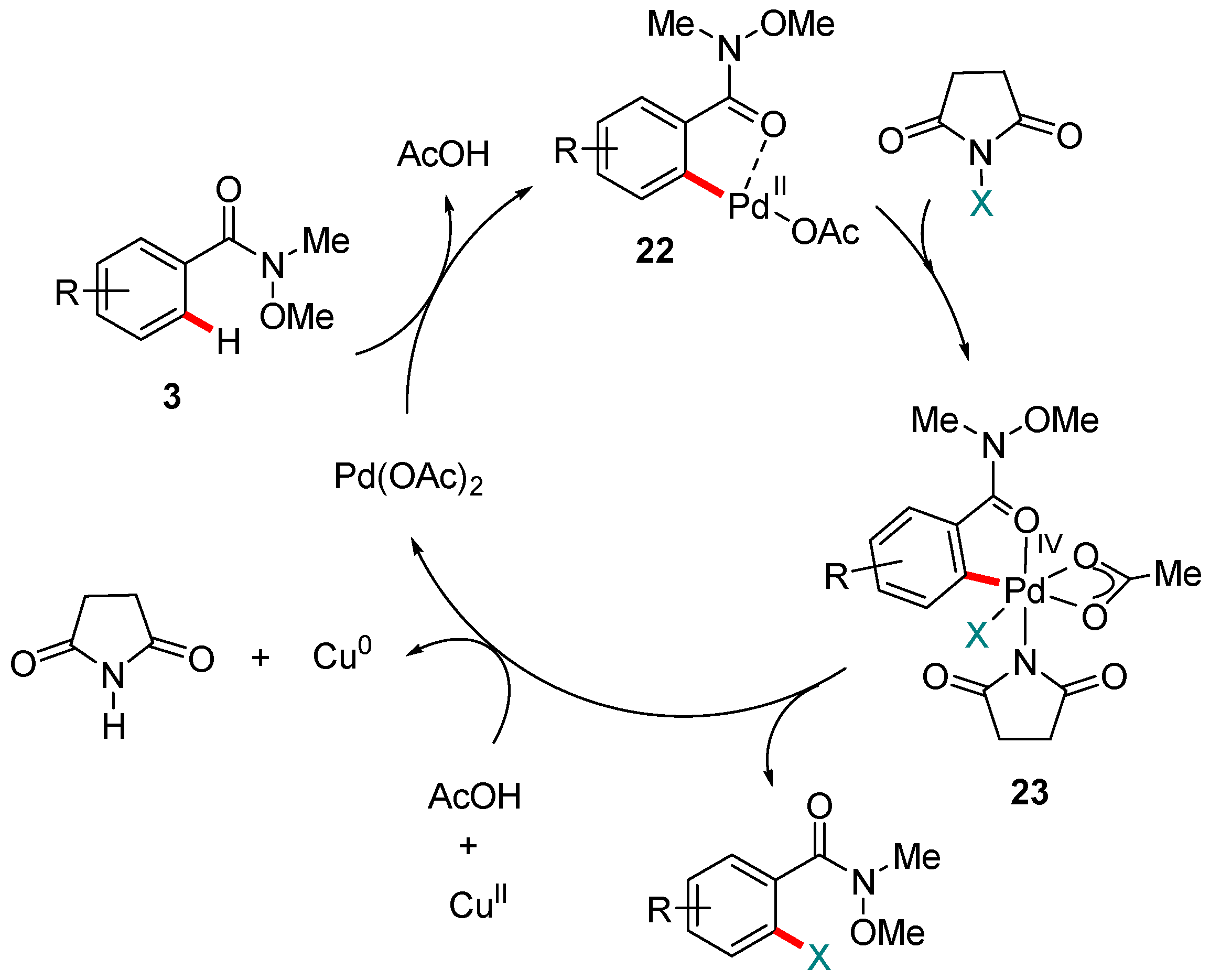
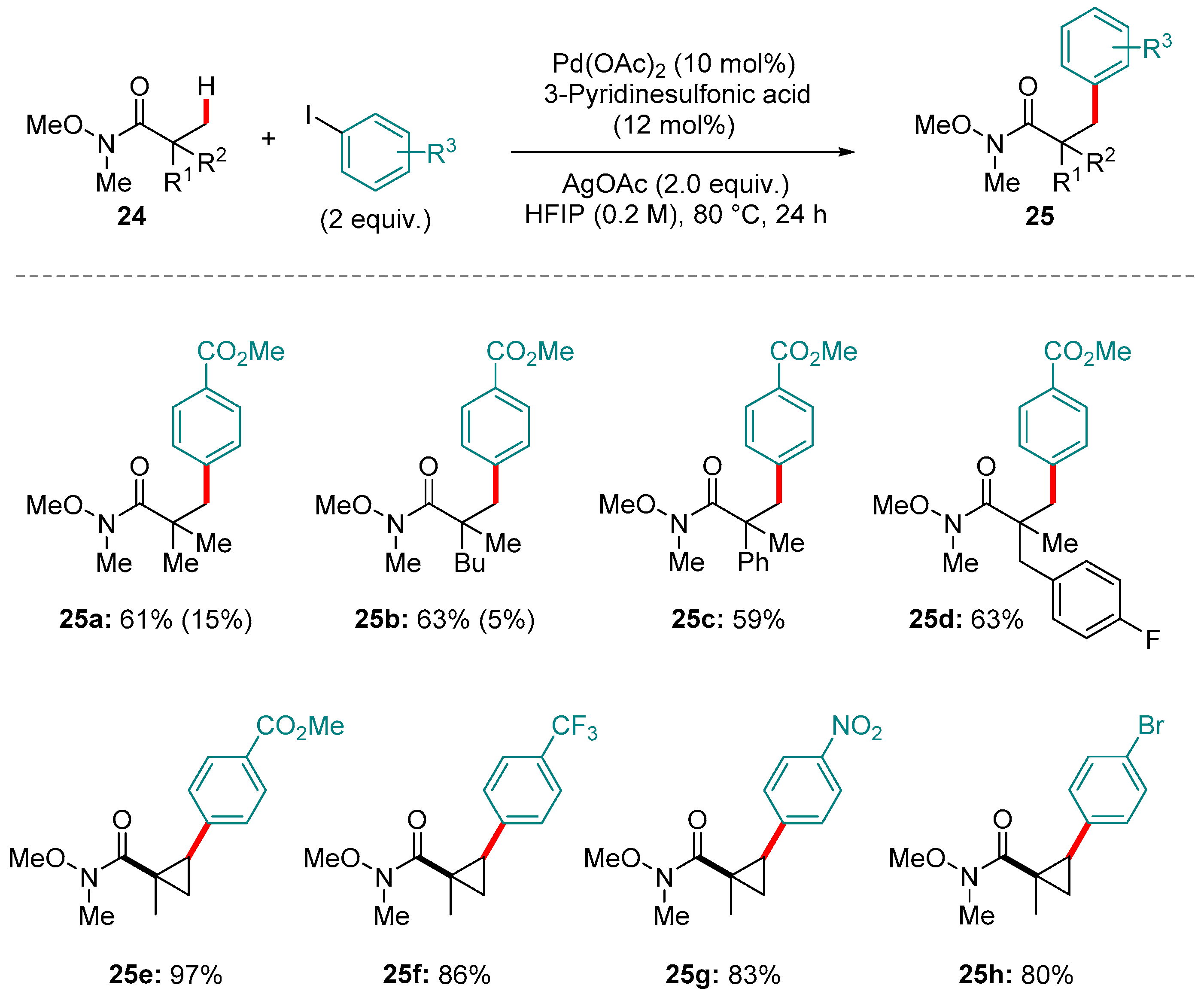
4. Pd-catalyzed Reactions
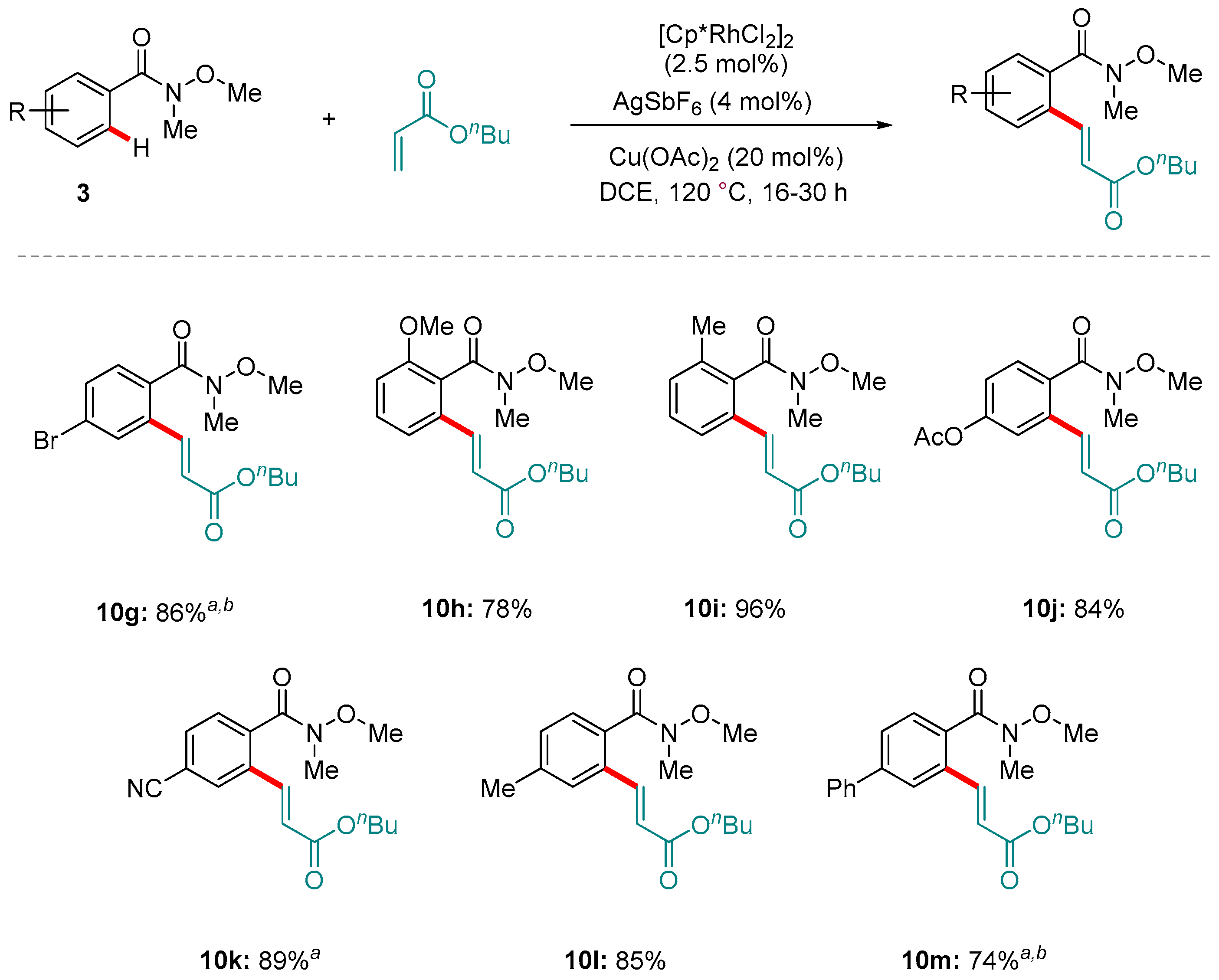
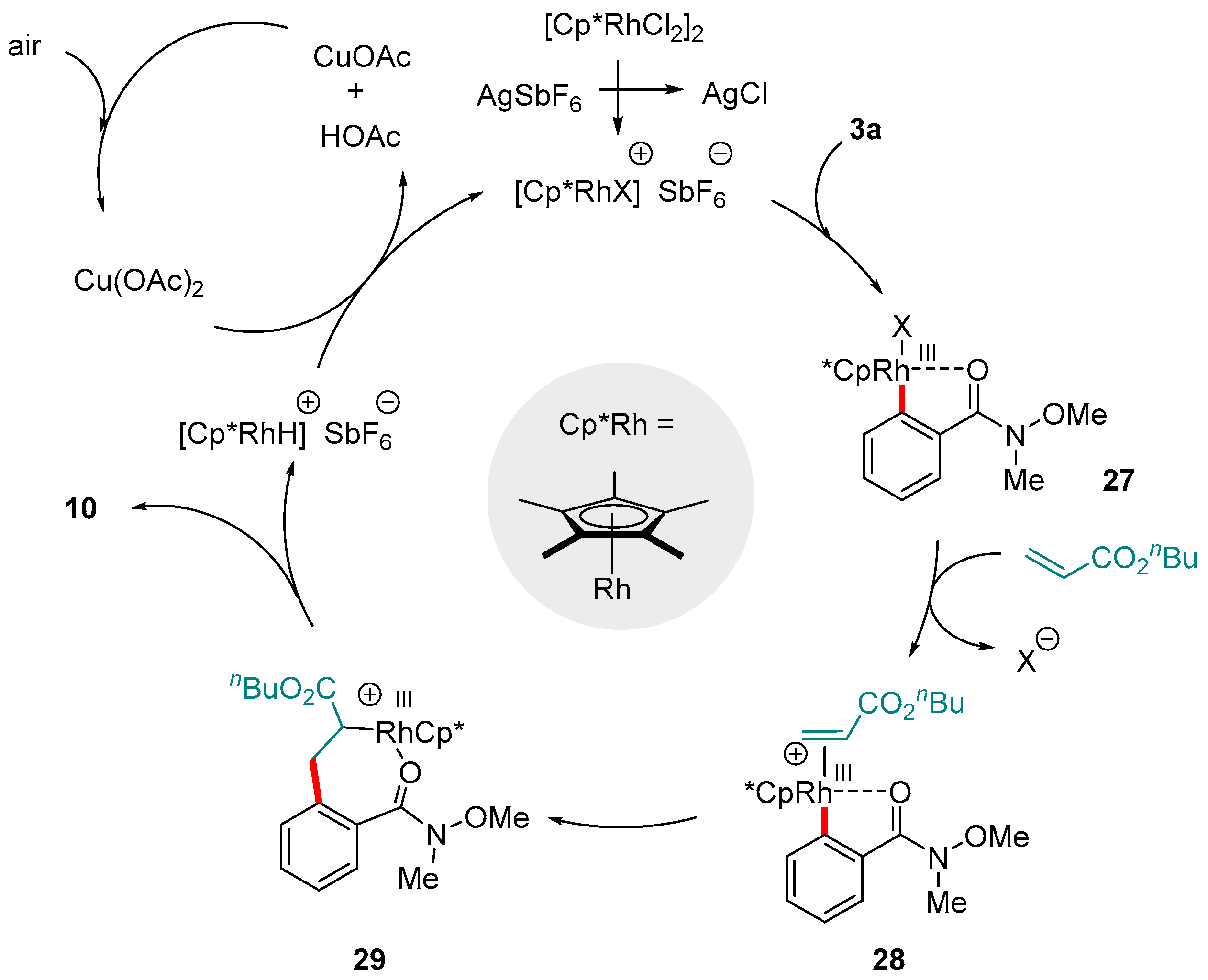
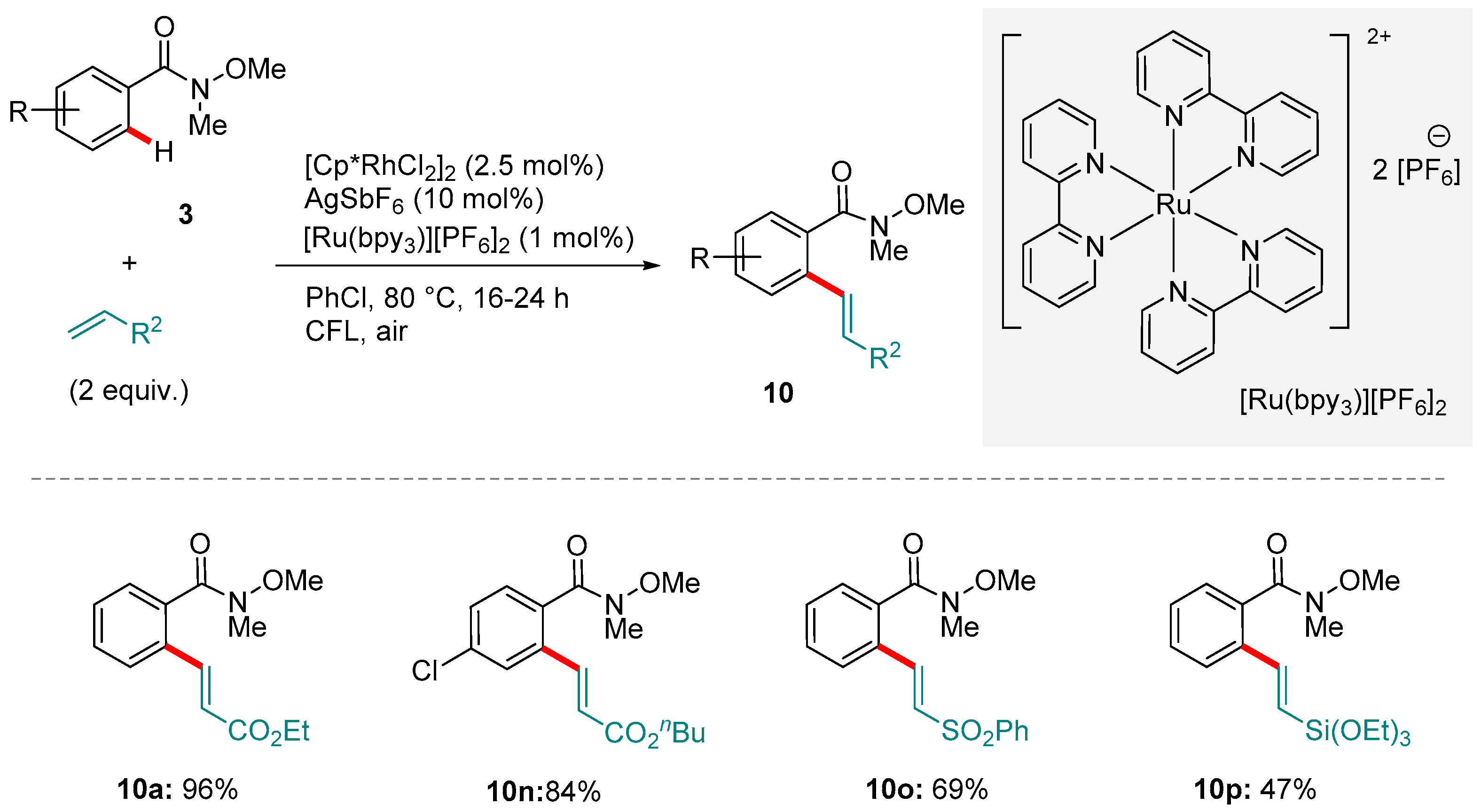
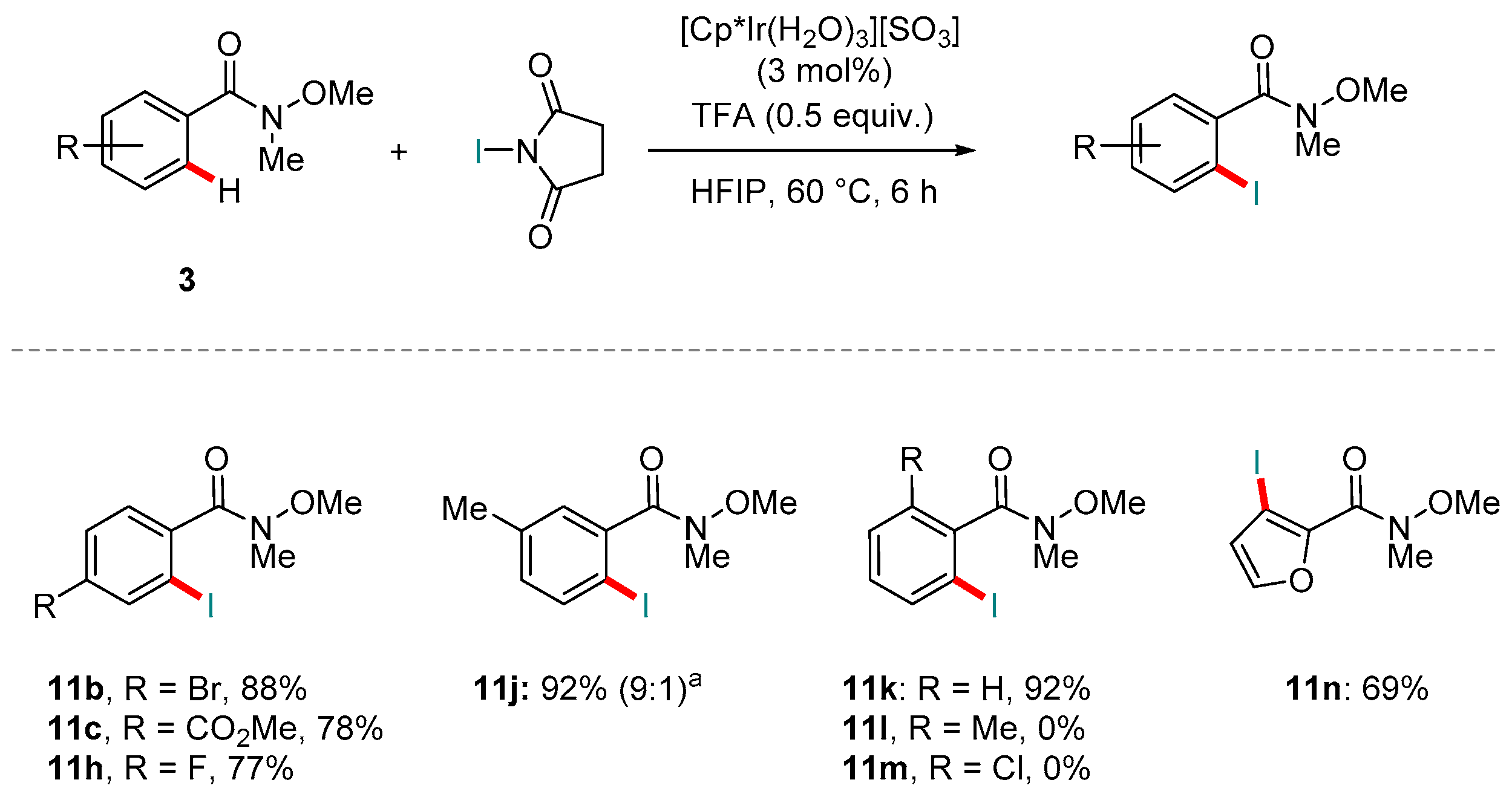
5. Rh-catalyzed Reactions
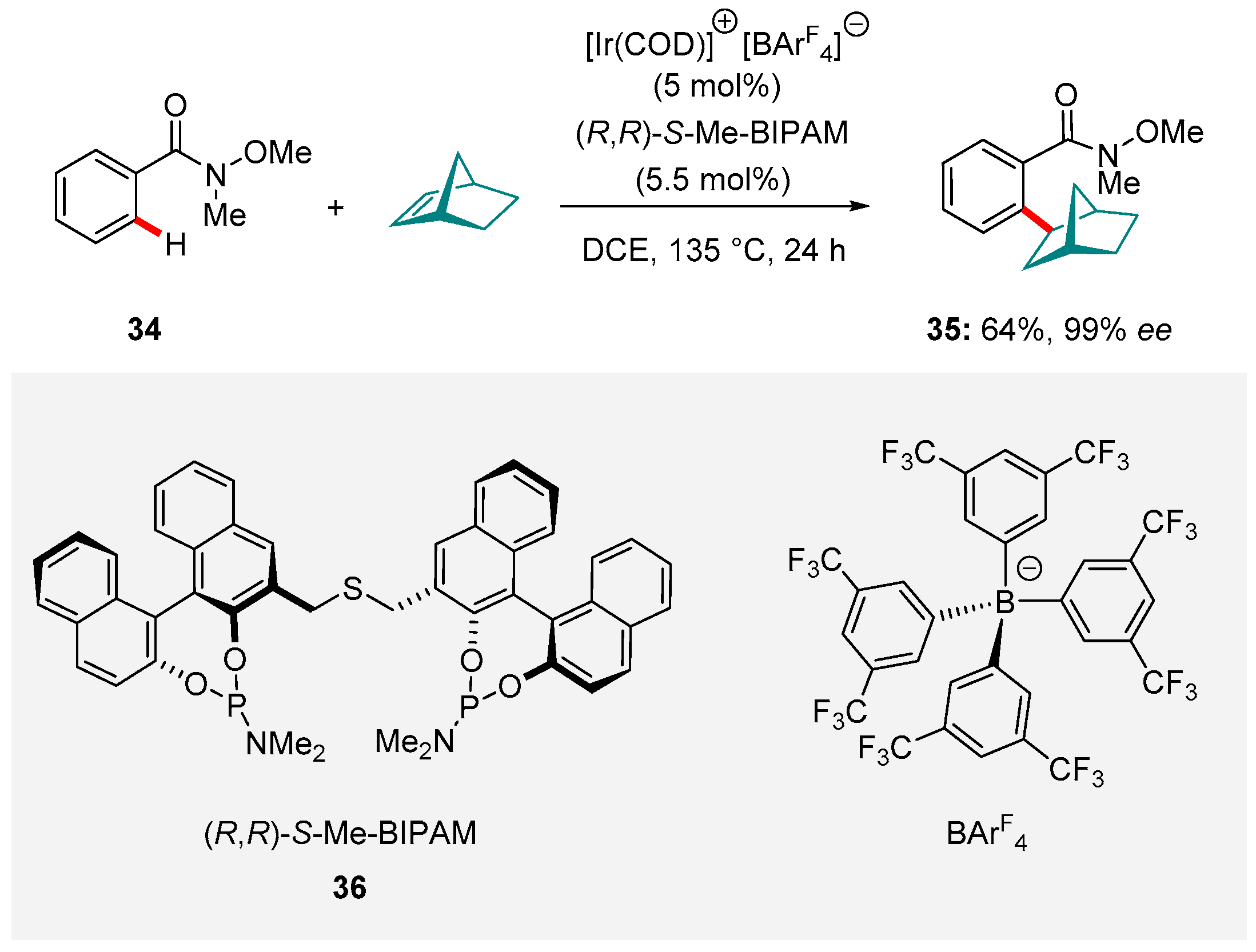
6. Ir-catalyzed Reactions
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Crabtree, R.H.; Lei, A. Introduction: CH Activation. Chem. Rev. 2017, 117, 8481–8482. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, Y.; Chang, S. Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yamada, S.; Kaneda, T.; Itami, K. C–H Functionalization of Azines. Chem. Rev. 2017, 117, 9302–9332. [Google Scholar] [CrossRef] [PubMed]
- Sandtorv, A.H. Transition Metal-Catalyzed C-H Activation of Indoles. ACS Catal. 2015, 357, 2403–2435. [Google Scholar] [CrossRef]
- Simmons, E.M.; Hartwig, J.F. On the Interpretation of Deuterium Kinetic Isotope Effects in C-H Bond Functionalizations by Transition-Metal Complexes. Angew. Chem. Int. Ed. Engl. 2012, 51, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Y.; Bai, R.; Lan, Y. Mechanism of Rhodium-Catalyzed C–H Functionalization: Advances in Theoretical Investigation. Acc. Chem. Res. 2017, 50, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.L.; Macgregor, S.A.; McMullin, C.L. Computational Studies of Carboxylate-Assisted C–H Activation and Functionalization at Group 8–10 Transition Metal Centers. Chem. Rev. 2017, 117, 8649–8709. [Google Scholar] [CrossRef] [PubMed]
- Balcells, D.; Clot, E.; Eisenstein, O. C–H Bond Activation in Transition Metal Species from a Computational Perspective. Chem. Rev. 2010, 110, 749–823. [Google Scholar] [CrossRef] [PubMed]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild metal-catalyzed C-H activation: Examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F.; Larsen, M.A. Undirected, Homogeneous C–H Bond Functionalization: Challenges and Opportunities. ACS Cent. Sci. 2016, 2, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Brückl, T.; Baxter, R.D.; Ishihara, Y.; Baran, P.S. Innate and Guided C–H Functionalization Logic. Acc. Chem. Res. 2012, 45, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Chung, D.S.; Bouffard, J.; Lee, S.G. Transition metal-catalyzed site- and regio-divergent C-H bond functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328. [Google Scholar] [CrossRef] [PubMed]
- Saint-Denis, T.G.; Zhu, R.-Y.; Chen, G.; Wu, Q.-F.; Yu, J.-Q. Enantioselective C(sp3)‒H bond activation by chiral transition metal catalysts. Science 2018, 359, eaao4798. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Agasti, S.; Maiti, D. Palladium catalysed meta-C–H functionalization reactions. Org. Biomol. Chem. 2016, 14, 5440–5453. [Google Scholar] [CrossRef] [PubMed]
- Leitch, J.A.; Frost, C.G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalisation. Chem. Soc. Rev. 2017, 46, 7145–7153. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Maity, S.; Maiti, D. Reaching the south: Metal-catalyzed transformation of the aromatic para-position. Chem. Commun. 2016, 52, 12398–12414. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.; Breit, B. Removable Directing Groups in Organic Synthesis and Catalysis. Angew. Chem. Int. Ed. 2011, 50, 2450–2494. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.R.; Rit, R.K.; Shankar, M.; Sahoo, A.K. Reusable and Removable Directing Groups for C(sp2)−H Bond Functionalization of Arenes. ASIAN J. Org. Chem. 2015, 4, 846–864. [Google Scholar] [CrossRef]
- Zhang, F.; Spring, D.R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 2014, 43, 6906–6919. [Google Scholar] [CrossRef] [PubMed]
- Font, M.; Quibell, J.M.; Perry, G.J.P.; Larrosa, I. The use of carboxylic acids as traceless directing groups for regioselective C–H bond functionalisation. Chem. Commun. 2017, 53, 5584–5597. [Google Scholar] [CrossRef] [PubMed]
- Gandeepan, P.; Ackermann, L. Transient Directing Groups for Transformative C–H Activation by Synergistic Metal Catalysis. Chem 2018, 4, 199–222. [Google Scholar] [CrossRef]
- Ihara, H.; Koyanagi, M.; Suginome, M. Anthranilamide: A Simple, Removable ortho-Directing Modifier for Arylboronic Acids Serving also as a Protecting Group in Cross-Coupling Reactions. Org. Lett. 2011, 13, 2662–2665. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Suginome, M. Easily Attachable and Detachable ortho-Directing Agent for Arylboronic Acids in Ruthenium-Catalyzed Aromatic C−H Silylation. J. Am. Chem. Soc. 2009, 131, 7502–7503. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Ueda, A.; Suginome, M. Ruthenium-catalyzed C-H Silylation of Methylboronic Acid Using a Removable α-Directing Modifier on the Boron Atom. Chem. Lett. 2011, 40, 916–918. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ishibashi, A.; Suginome, M. Regioselective Synthesis of o-Benzenediboronic Acids via Ir-Catalyzed o-C–H Borylation Directed by a Pyrazolylaniline-Modified Boronyl Group. Org. Lett. 2017, 19, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Poisson, T.; Pannecoucke, X.; Besset, T. The Transient Directing Group Strategy: A New Trend in Transition-Metal-Catalyzed C–H Bond Functionalization. Synthesis 2017, 49, 4808–4826. [Google Scholar] [CrossRef]
- Zhu, R.-Y.; Farmer, M.E.; Chen, Y.-Q.; Yu, J.-Q. A Simple and Versatile Amide Directing Group for C−H Functionalizations. Angew. Chem. Int. Ed. 2016, 55, 10578–10599. [Google Scholar] [CrossRef] [PubMed]
- Nahm, S.; Weinreb, S.M. N-methoxy-n-methylamides as effective acylating agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar] [CrossRef]
- Nowak, M. Weinreb Amides. Synlett 2015, 26, 561–562. [Google Scholar] [CrossRef]
- De Sarkar, S.; Liu, W.; Kozhushkov, S.I.; Ackermann, L. Weakly Coordinating Directing Groups for Ruthenium(II)- Catalyzed C—H Activation. Adv. Synth. Catal. 2014, 356, 1461–1479. [Google Scholar] [CrossRef]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-catalyzed C-H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Villuendas, P.; Urriolabeitia, E.P. Ru-catalysed C–H functionalisations as a tool for selective organic synthesis. Tetrahedron Lett. 2016, 57, 3413–3432. [Google Scholar] [CrossRef]
- Yang, F.; Ackermann, L. Ruthenium-Catalyzed C–H Oxygenation on Aryl Weinreb Amides. Org. Lett. 2013, 15, 718–720. [Google Scholar] [CrossRef] [PubMed]
- More, N.Y.; Padala, K.; Jeganmohan, M. Ruthenium-Catalyzed C–H Benzoxylation of tert-Benzamides with Aromatic Acids by Weak Coordination. J. Org. Chem. 2017, 82, 12691–12700. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kapur, M. Fujiwara-Moritani Reaction of Weinreb Amides using a Ruthenium-Catalyzed C-H Functionalization Reaction. Chem. Asian J. 2015, 10, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.M.; Zhang, H.; Stoltz, B.M. Oxidative Heck-Type Reactions (Fujiwara–Moritani Reactions). In The Mizoroki–Heck Reaction; Oestreich, M., Ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Ackermann, L. Carboxylate-Assisted Transition-Metal-Catalyzed C−H Bond Functionalizations: Mechanism and Scope. Chem. Rev. 2011, 111, 1315–1345. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Wang, L.; Liu, Y.; Cui, X. Transition-Metal-Catalyzed Direct C–H Functionalization under External-Oxidant-Free Conditions. Synthesis 2015, 47, 439–459. [Google Scholar] [CrossRef]
- Das, R.; Kapur, M. Product Control using Substrate Design: Ruthenium-Catalysed Oxidative C-H Olefinations of Cyclic Weinreb Amides. Chem. Eur. J. 2016, 22, 16986–16990. [Google Scholar] [CrossRef] [PubMed]
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moselage, M.; Li, J.; Ackermann, L. Cobalt-Catalyzed C–H Activation. ACS Catal. 2016, 6, 498–525. [Google Scholar] [CrossRef]
- Gao, K.; Yoshikai, N. Low-Valent Cobalt Catalysis: New Opportunities for C–H Functionalization. Acc. Chem. Res. 2014, 47, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Yoshikai, N. Development of Cobalt-Catalyzed C–H Bond Functionalization Reactions. Bull. Chem. Soc. Jpn. 2014, 87, 843–857. [Google Scholar] [CrossRef]
- Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. Cp*CoIII-Catalyzed C2-Selective Addition of Indoles to Imines. Chem. A Eur. J. 2013, 19, 9142–9146. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. A Cationic High-Valent Cp*CoIII Complex for the Catalytic Generation of Nucleophilic Organometallic Species: Directed C–H Bond Activation. Angew. Chem. Int. Ed. Engl. 2013, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Bunno, Y.; Murakami, N.; Suzuki, Y.; Kanai, M.; Yoshino, T.; Matsunaga, S. Cp*CoIII-Catalyzed Dehydrative C–H Allylation of 6-Arylpurines and Aromatic Amides Using Allyl Alcohols in Fluorinated Alcohols. Org. Lett. 2016, 18, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yoshino, T.; Matsunaga, S.; Kanai, M. Air-Stable Carbonyl(pentamethylcyclopentadienyl)cobalt Diiodide Complex as a Precursor for Cationic (Pentamethylcyclopentadienyl)cobalt(III) Catalysis: Application for Directed C-2 Selective C-H Amidation of Indoles. Adv. Synth. Catal. 2014, 356, 1491–1495. [Google Scholar] [CrossRef]
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 0088. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Colobert, F. A remarkable solvent effect of fluorinated alcohols on transition metal catalysed C–H functionalizations. Org. Chem. Front. 2016, 3, 394–400. [Google Scholar] [CrossRef]
- Planas, O.; Chirila, P.G.; Whiteoak, C.J.; Ribas, X. Current Mechanistic Understanding of Cobalt-Catalyzed C–H Functionalization. Adv. Organomet. Chem. 2018, 69, 209–282. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the Concerted Metalation-Deprotonation Mechanism in Palladium-Catalyzed Direct Arylation Across a Broad Range of Aromatic Substrates. J. Am. Chem. Soc. 2008, 130, 10848–10849. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Bunno, Y.; Yoshino, T.; Matsunaga, S. Weinreb Amide Directed Versatile C–H Bond Functionalization under (eta(5)-Pentamethylcyclopentadienyl)cobalt(III) Catalysis. Chem. Eur. J. 2018, 24, 10231–10237. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Schröder, N.; Glorius, F. Rh(III)-Catalyzed Halogenation of Vinylic C–H Bonds: Rapid and General Access to Z-Halo Acrylamides. Org. Lett. 2013, 15, 3860–3863. [Google Scholar] [CrossRef] [PubMed]
- Chirila, P.G.; Adams, J.; Dirjal, A.; Hamilton, A.; Whiteoak, C.J. Cp*Co(III)-Catalyzed Coupling of Benzamides with α,β-Unsaturated Carbonyl Compounds: Preparation of Aliphatic Ketones and Azepinones. Chem. Eur. J. 2018, 24, 3584–3589. [Google Scholar] [CrossRef] [PubMed]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. Engl. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Weinstein, A.B.; White, P.B.; Stahl, S.S. Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev. 2018, 118, 2636–2679. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, P.; Taylor, R.J.K.; Fairlamb, I.J.S. Emergence of Palladium(IV) Chemistry in Synthesis and Catalysis. Chem. Rev. 2010, 110, 824–889. [Google Scholar] [CrossRef] [PubMed]
- Timsina, Y.N.; Gupton, B.F.; Ellis, K.C. Palladium-Catalyzed C–H Amination of C(sp2) and C(sp3)–H Bonds: Mechanism and Scope for N-Based Molecule Synthesis. ACS Catal. 2018, 8, 5732–5776. [Google Scholar] [CrossRef]
- Topczewski, J.J.; Sanford, M.S. Carbon–hydrogen (C–H) bond activation at PdIV: A Frontier in C–H functionalization catalysis. Chem. Sci. 2015, 6, 70–76. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.-Q. Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.J.; Schoenebeck, F. Experiment and computation: A combined approach to study the reactivity of palladium complexes in oxidation states 0 to iv. Chem. Soc. Rev. 2014, 43, 6609–6638. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. Engl. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 2017, 1, 0025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, K.; Lan, Q.; Wang, X.-S. Pd(II)-catalyzed C–H arylation of aryl and benzyl Weinreb amides. Org. Biomol. Chem. 2015, 13, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Mudarra, Á.L.; Martínez de Salinas, S.; Pérez-Temprano, M.H. Beyond the traditional roles of Ag in catalysis: The transmetalating ability of organosilver(i) species in Pd-catalysed reactions. Org. Biomol. Chem. 2018, 17, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Yedage, S.L.; Bhanage, B.M. Palladium-Catalyzed Deaminative Phenanthridinone Synthesis from Aniline via C–H Bond Activation. J. Org. Chem. 2016, 81, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wan, L.; Zhang, G.; Leow, D.; Spangler, J.; Yu, J.-Q. Pd(II)-Catalyzed C–H Functionalizations Directed by Distal Weakly Coordinating Functional Groups. J. Am. Chem. Soc. 2015, 137, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Ligand-Accelerated C−H Activation Reactions: Evidence for a Switch of Mechanism. J. Am. Chem. Soc. 2010, 132, 14137–14151. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kapur, M. Transition-Metal-Catalyzed Site-Selective C−H Halogenation Reactions. Asian J. Org. Chem. 2018, 7, 1524–1541. [Google Scholar] [CrossRef]
- Das, R.; Kapur, M. Palladium-Catalyzed, ortho-Selective C–H Halogenation of Benzyl Nitriles, Aryl Weinreb Amides, and Anilides. J. Org. Chem. 2017, 82, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Lee, E.; Ariafard, A.; Sanford, M.S.; Yates, B.F.; Canty, A.J.; Ritter, T. Connecting Binuclear Pd(III) and Mononuclear Pd(IV) Chemistry by Pd–Pd Bond Cleavage. J. Am. Chem. Soc. 2012, 134, 12002–12009. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Benitez, D.; Tkatchouk, E.; Goddard, W.A.; Ritter, T. Bimetallic Reductive Elimination from Dinuclear Pd(III) Complexes. J. Am. Chem. Soc. 2010, 132, 14092–14103. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Ritter, T. Bimetallic Pd(III) complexes in palladium-catalysed carbon–heteroatom bond formation. Nat. Chem. 2009, 1, 419. [Google Scholar] [CrossRef]
- Powers, D.C.; Geibel, M.A.L.; Klein, J.E.M.N.; Ritter, T. Bimetallic Palladium Catalysis: Direct Observation of Pd(III)−Pd(III) Intermediates. J. Am. Chem. Soc. 2009, 131, 17050–17051. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Chekshin, N.; Shen, P.-X.; Yu, J.-Q. Ligand-Enabled, Palladium-Catalyzed β-C(sp3)–H Arylation of Weinreb Amides. ACS Catal. 2018, 8, 9292–9297. [Google Scholar] [CrossRef]
- Willcox, D.; Chappell, B.G.N.; Hogg, K.F.; Calleja, J.; Smalley, A.P.; Gaunt, M.J. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 2016, 354, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.-Y.; Li, Z.-Q.; Park, H.S.; Senanayake, C.H.; Yu, J.-Q. Ligand-Enabled γ-C(sp3)–H Activation of Ketones. J. Am. Chem. Soc. 2018, 140, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.-Y.; He, J.; Wang, X.-C.; Yu, J.-Q. Ligand-Promoted Alkylation of C(sp3)–H and C(sp2)–H Bonds. J. Am. Chem. Soc. 2014, 136, 13194–13197. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, O. Ring Construction by Palladium(0)-Catalyzed C(sp3)–H Activation. Acc. Chem. Res. 2017, 50, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, O. Transition metal-catalyzed arylation of unactivated C(sp3)–H bonds. Chem. Soc. Rev. 2011, 40, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Chatani, N.; Rej, S. Rh-Catalyzed Removable Directing Group Assisted sp2 or sp3-C-H Bond Functionalization. Angew. Chem. Int. Ed. 2018. [Google Scholar] [CrossRef]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-Catalyzed C−C Bond Formation via Heteroatom-Directed C−H Bond Activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Wang, X.; Glorius, F. Plausible Rh(V) Intermediates in Catalytic C–H Activation Reactions. ACS Catal. 2018, 8, 242–257. [Google Scholar] [CrossRef]
- Davies, D.L.; Ellul, C.E.; Macgregor, S.A.; McMullin, C.L.; Singh, K. Experimental and DFT Studies Explain Solvent Control of C–H Activation and Product Selectivity in the Rh(III)-Catalyzed Formation of Neutral and Cationic Heterocycles. J. Am. Chem. Soc. 2015, 137, 9659–9669. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, Z. DFT Studies on the Mechanism of the Rhodium(III)-Catalyzed C–H Activation of N-Phenoxyacetamide. J. Org. Chem. 2015, 80, 10686–10693. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Hyster, T.K.; Rovis, T. Rhodium(III)-catalyzed intramolecular hydroarylation, amidoarylation, and Heck-type reaction: Three distinct pathways determined by an amide directing group. Angew. Chem. Int. Ed. 2013, 52, 14181–14185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Li, Y.; Yin, F.; Wang, X.-S. Rhodium-Catalyzed C-H Olefination of Aryl Weinreb Amides. Adv. Synth. Catal. 2013, 355, 1724–1728. [Google Scholar] [CrossRef]
- Wang, S.-M.; Li, C.; Leng, J.; Bukhari, S.N.A.; Qin, H.-L. Rhodium(iii)-catalyzed Oxidative Coupling of N-Methoxybenzamides and Ethenesulfonyl fluoride: A C–H Bond Activation Strategy for the Preparation of 2-Aryl ethenesulfonyl fluorides and Sulfonyl fluoride Substituted γ-Lactams. Org. Chem. Front. 2018, 5, 1411–1415. [Google Scholar] [CrossRef]
- Wang, C.-S.; Dixneuf, P.H.; Soulé, J.-F. Photoredox Catalysis for Building C–C Bonds from C(sp2)–H Bonds. Chem. Rev. 2018, 118, 7532–7585. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Zhao, J.; Chen, X. Cooperative photoredox catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Fabry, D.C.; Rueping, M. Merging Visible Light Photoredox Catalysis with Metal Catalyzed C–H Activations: On the Role of Oxygen and Superoxide Ions as Oxidants. Acc. Chem. Res. 2016, 49, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Fabry, D.C.; Zoller, J.; Raja, S.; Rueping, M. Combining rhodium and photoredox catalysis for C-H functionalizations of arenes: Oxidative Heck reactions with visible light. Angew. Chem. Int. Ed. 2014, 53, 10228–10231. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.W.B.; Watson, A.J.B. Recent Developments in Organoboron Chemistry: Old Dogs, New Tricks. Chem 2017, 3, 31–55. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.; Li, P. Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev. 2015, 44, 8848–8858. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.W.; Blair, D.J.; Burke, M.D. Towards the generalized iterative synthesis of small molecules. Nat. Rev. Chem. 2018, 2, 0115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Grillo, A.S.; Burke, M.D. From Synthesis to Function via Iterative Assembly of N-Methyliminodiacetic Acid Boronate Building Blocks. Acc. Chem. Res. 2015, 48, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ballmer, S.G.; Gillis, E.P.; Fujii, S.; Schmidt, M.J.; Palazzolo, A.M.E.; Lehmann, J.W.; Morehouse, G.F.; Burke, M.D. Synthesis of many different types of organic small molecules using one automated process. Science 2015, 347, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Woerly, E.M.; Roy, J.; Burke, M.D. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nat. Chem. 2014, 6, 484. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Borylation and Silylation of C–H Bonds: A Platform for Diverse C–H Bond Functionalizations. Acc. Chem. Res. 2011, 45, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Mkhalid, I.A.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C-H activation for the construction of C-B bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Bisht, R.; Haldar, C.; Pandey, G.; Dannatt, J.E.; Ghaffari, B.; Maleczka, R.E.; Chattopadhyay, B. Achieving High Ortho Selectivity in Aniline C–H Borylations by Modifying Boron Substituents. ACS Catal. 2018, 8, 6216–6223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Kanai, M.; Kuninobu, Y. Iridium/Bipyridine-Catalyzed ortho-Selective C–H Borylation of Phenol and Aniline Derivatives. Org. Lett. 2017, 19, 5944–5947. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, B.; Dannatt, J.E.; Andujar-De Sanctis, I.L.; Gore, K.A.; Maleczka, R.E.; Singleton, D.A.; Smith, M.R. Ir-Catalyzed ortho-Borylation of Phenols Directed by Substrate–Ligand Electrostatic Interactions: A Combined Experimental/in Silico Strategy for Optimizing Weak Interactions. J. Am. Chem. Soc. 2017, 139, 7864–7871. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.V.A.; Emmerson, D.G.; Ling, E.F.; Roering, A.J.; Ringgold, M.A.; Clark, T.B. An ortho-directed C–H borylation/Suzuki coupling sequence in the formation of biphenylbenzylic amines. Org. Chem. Front. 2015, 2, 661–664. [Google Scholar] [CrossRef]
- Crawford, K.M.; Ramseyer, T.R.; Daley, C.J.A.; Clark, T.B. Phosphine-Directed C—H Borylation Reactions: Facile and Selective Access to Ambiphilic Phosphine Boronate Esters. Angew. Chem. 2014, 53, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Kawamorita, S.; Ohmiya, H.; Hara, K.; Fukuoka, A.; Sawamura, M. Directed Ortho Borylation of Functionalized Arenes Catalyzed by a Silica-Supported Compact Phosphine−Iridium System. J. Am. Chem. Soc. 2009, 131, 5058–5059. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Bisht, R.; Haldar, C.; Chattopadhyay, B. Noncovalent Interactions in Ir-Catalyzed C–H Activation: L-Shaped Ligand for Para-Selective Borylation of Aromatic Esters. J. Am. Chem. Soc. 2017, 139, 7745–7748. [Google Scholar] [CrossRef] [PubMed]
- Tajuddin, H.; Harrisson, P.; Bitterlich, B.; Collings, J.C.; Sim, N.; Batsanov, A.S.; Cheung, M.S.; Kawamorita, S.; Maxwell, A.C.; Shukla, L.; et al. Iridium-catalyzed C–H borylation of quinolines and unsymmetrical 1,2-disubstituted benzenes: Insights into steric and electronic effects on selectivity. Chem. Sci. 2012, 3, 3505–3515. [Google Scholar] [CrossRef]
- Larsen, M.A.; Hartwig, J.F. Iridium-Catalyzed C–H Borylation of Heteroarenes: Scope, Regioselectivity, Application to Late-Stage Functionalization, and Mechanism. J. Am. Chem. Soc. 2014, 136, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 2011, 40, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, B.; Preshlock, S.M.; Plattner, D.L.; Staples, R.J.; Maligres, P.E.; Krska, S.W.; Maleczka, R.E.; Smith, M.R. Silyl Phosphorus and Nitrogen Donor Chelates for Homogeneous Ortho Borylation Catalysis. J. Am. Chem. Soc. 2014, 136, 14345–14348. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.G.; Wang, S.-G.; Oliveira, C.C.; Cramer, N. Catalytic Enantioselective Transformations Involving C–H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Yamamoto, Y. Cationic Iridium/S-Me-BIPAM-Catalyzed Direct Asymmetric Intermolecular Hydroarylation of Bicycloalkenes. Angew. Chem. Int. Ed. 2015, 54, 9894–9897. [Google Scholar] [CrossRef] [PubMed]
- Erbing, E. Development of New Efficient Iridium-Catalyzed Methods for the Construction of Carbon-Heteroatom Bonds. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2018. [Google Scholar]
- Collins, K.D.; Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 2013, 5, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Gensch, T.; Teders, M.; Glorius, F. Approach to Comparing the Functional Group Tolerance of Reactions. J. Org. Chem. 2017, 82, 9154–9159. [Google Scholar] [CrossRef] [PubMed]
- Erbing, E.; Sanz-Marco, A.; Vázquez-Romero, A.; Malmberg, J.; Johansson, M.J.; Gómez-Bengoa, E.; Martín-Matute, B. Base- and Additive-Free Ir-Catalyzed ortho-Iodination of Benzoic Acids: Scope and Mechanistic Investigations. ACS Catal. 2018, 8, 920–925. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Which Metals are Green for Catalysis? Comparison of the Toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au Salts. Angew. Chem. Int. Ed. 2016, 55, 12150–12162. [Google Scholar] [CrossRef] [PubMed]
- Hayler, J.D.; Leahy, D.K.; Simmons, E.M. A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38, 36–46. [Google Scholar] [CrossRef]
- Sherwood, J. European Restrictions on 1,2-Dichloroethane: C-H Activation Research and Development Should Be Liberated and not Limited. Angew. Chem. Int. Ed. 2018, 57, 14286–14290. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Murray, J.; Sneddon, H.F.; Wheelhouse, K.M.P.; Watson, A.J.B. Connecting the Dots: Method Development Using Sustainable Solvents. Chem 2017, 3, 365–368. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471. [Google Scholar] [CrossRef] [PubMed]
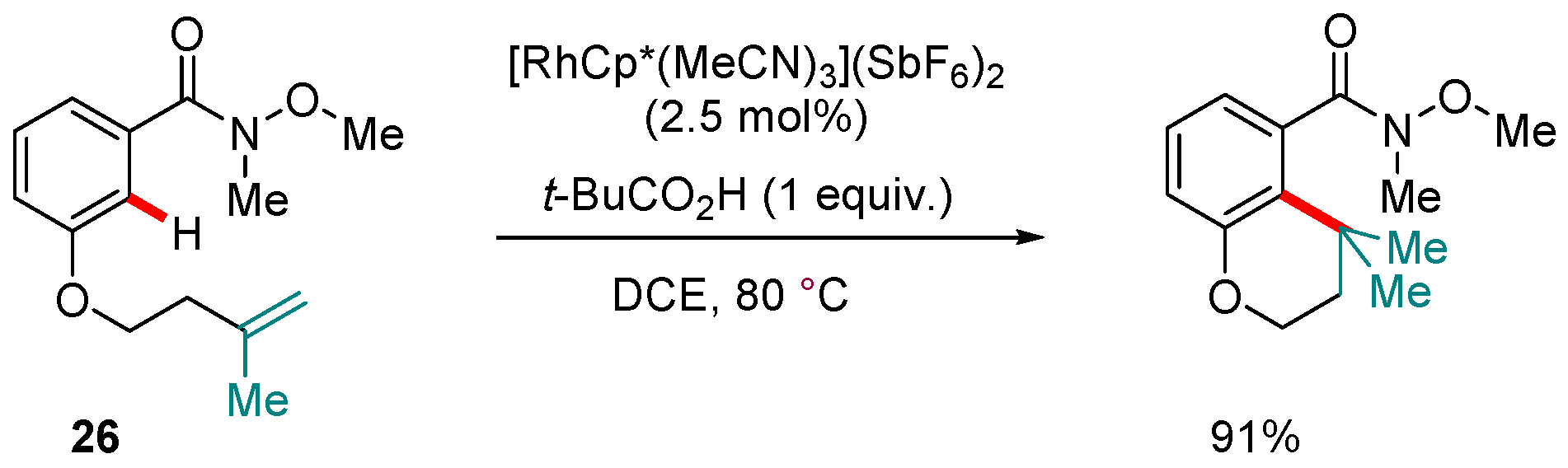

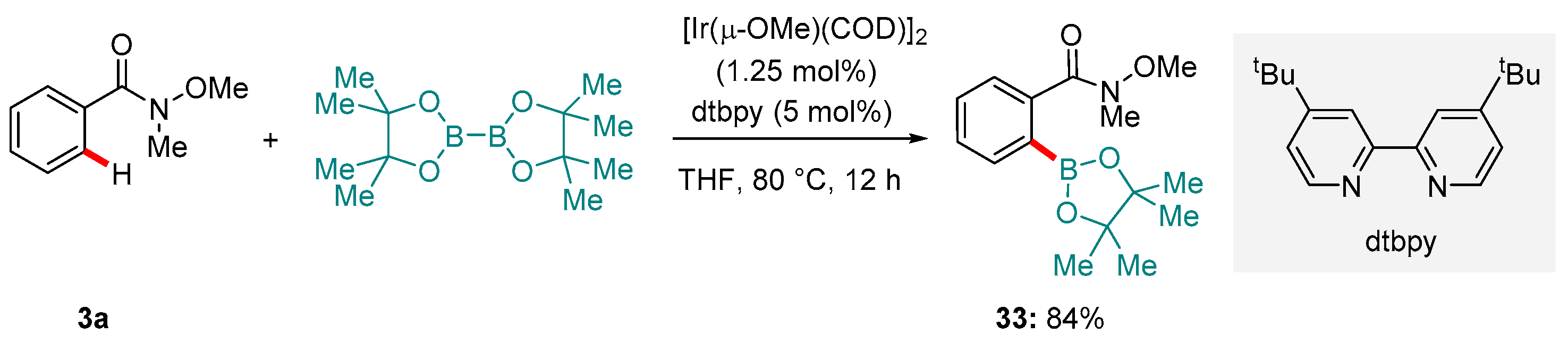
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalepu, J.; Pilarski, L.T. Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations. Molecules 2019, 24, 830. https://doi.org/10.3390/molecules24050830
Kalepu J, Pilarski LT. Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations. Molecules. 2019; 24(5):830. https://doi.org/10.3390/molecules24050830
Chicago/Turabian StyleKalepu, Jagadeesh, and Lukasz T. Pilarski. 2019. "Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations" Molecules 24, no. 5: 830. https://doi.org/10.3390/molecules24050830
APA StyleKalepu, J., & Pilarski, L. T. (2019). Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations. Molecules, 24(5), 830. https://doi.org/10.3390/molecules24050830





