Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review
Abstract
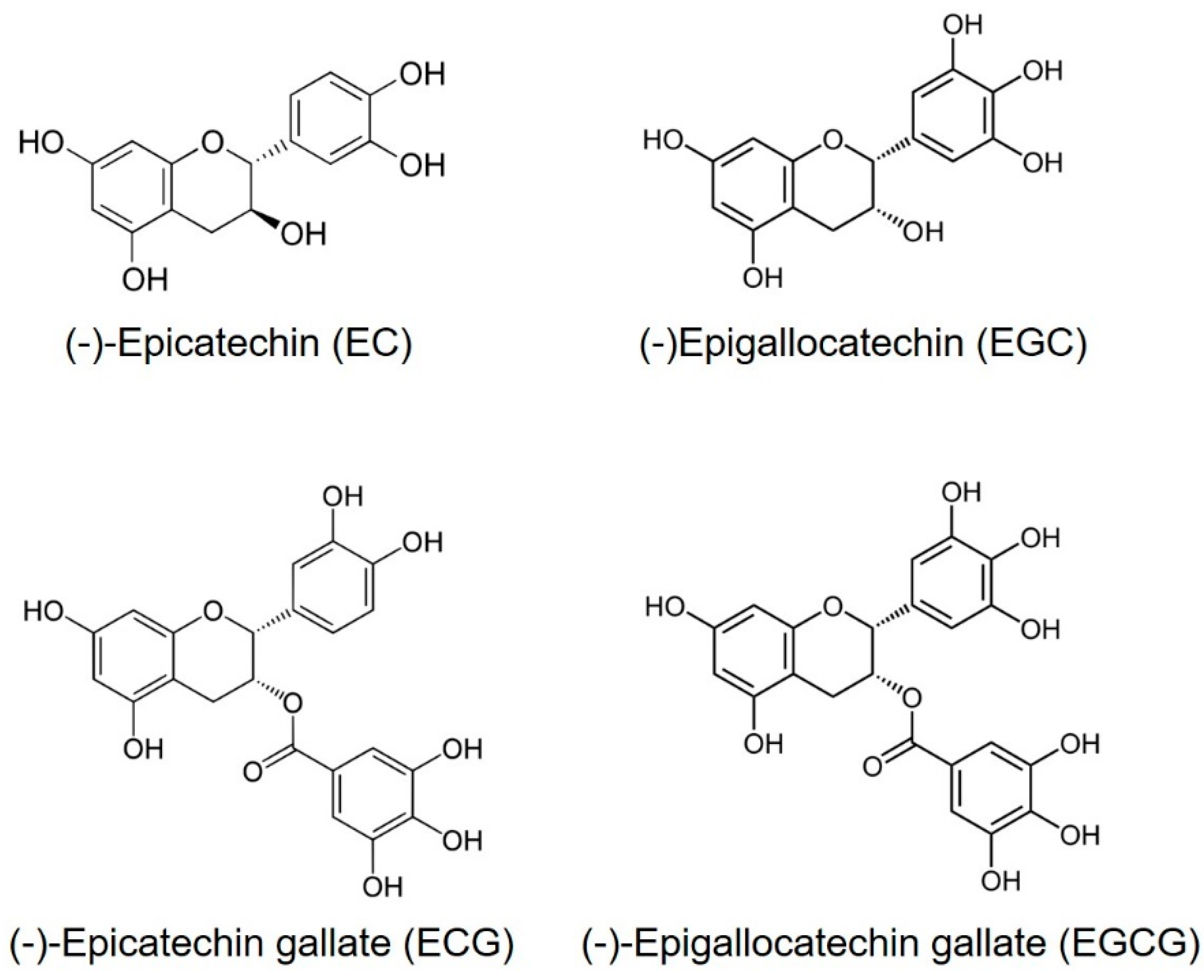
1. Introduction
2. Antimicrobial Activities of TP on Phytopathogens
2.1. Antifungal Activity of TP
2.2. Antibacterial Activity of TP
2.3. Antiviral Activity of TP
2.4. Synergistic Effect of TP Combination with Certain Bioagents
3. Mechanisms of Antimicrobial Activity of TP
3.1. Mechanism of Antifungal Activity of TP
3.2. Mechanism of Antibacterial Activity of TP
3.3. Mechanism of Antiviral Activity of TP
4. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kris-Etherton, P.M.; Keen, C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002, 13, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V. Epidemiological Evidence Linking Tea Consumption to Human Health: A Review. Crit. Rev. Food Sci. 2014, 54, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Gimenez, R.; Lopez, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Hara, Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res.-Rev. Mutat. Res. 1999, 436, 69–97. [Google Scholar] [CrossRef]
- Hamiltonmiller, J.M.T. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 1995, 39, 2375–2377. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Caruana, M.; Vassallo, N. Tea Polyphenols in Parkinson’s Disease. Adv. Exp. Med. Biol 2015, 863, 117–137. [Google Scholar] [PubMed]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lv, Z.; Chen, J.; Xu, H.; Zheng, X.; Chen, L.; Zhang, J.; Shentu, X. Review on research and development of botanical pesticides in China. Acta Agric. Zhejiangensis 2005, 17, 42–48. (In Chinese) [Google Scholar]
- Yang, Y.H.; Chen, Y.J.; Chen, F.J.; Yu, Y.; Bi, C.W. Tea polyphenol is a potential antifungal agent for the control of obligate biotrophic fungus in plants. J. Phytopathol. 2017, 165, 547–553. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, Y.; Chen, H.; Lu, H.; Lin, X.; Ma, L. Inhibitive Action of Tea-polyphenol on Some Plant Pathogenic Fungi. Nat. Prod. Res. Dev. 2008, 20, 690–694. (In Chinese) [Google Scholar]
- Wang, J.; Qiu, Y.; Fu, J.; Han, J.; Feng, W. Inhibitive Effect of Tea-polyphenols on Pyricularia oryzae and Its Mechanism. Nat. Prod. Res. Dev. 2011, 23, 918–922. (In Chinese) [Google Scholar]
- Zou, D.; Liao, W.; Huang, H.; Jiang, X. The Inhibition Effect of Tea Polyphenol on 8 kinds of Plant Pathogenic Fungi. Guangxi For. Sci. 2017, 46, 412–415. (In Chinese) [Google Scholar]
- Liu, H.M.; Guo, J.H.; Liu, P.; Cheng, Y.J.; Wang, B.Q.; Long, C.A.; Deng, B.X. Inhibitory activity of tea polyphenol and Candida ernobii against Diplodia natalensis infections. J. Appl. Microbiol. 2010, 108, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Guo, J.H.; Cheng, Y.J.; Liu, P.; Long, C.A.; Deng, B.X. Inhibitory activity of tea polyphenol and Hanseniaspora uvarum against Botrytis cinerea infections. Lett. Appl. Microbiol. 2010, 51, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Jiang, X.D.; Chen, J.J.; Zhang, S.S. Control of postharvest grey mould decay of nectarine by tea polyphenol combined with tea saponin. Lett. Appl. Microbiol. 2013, 57, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Fukai, K.; Ishigami, T.; Hara, Y. Antibacterial Activity of Tea Polyphenols against Phytopathogenic Bacteria. Agr. Biol. Chem. Tokyo 1991, 55, 1895–1897. [Google Scholar]
- Alstrom, S. Antibacterial Activity of Tea and Coffee Wastes against Some Plant Pathogenic Pseudomonas-Syringae Strains. J. Phytopathol. 1992, 136, 329–334. [Google Scholar] [CrossRef]
- Thornberry, H.H. Effect of tannic acid on the infectivity of tobacco-mosaic virus. Phytopathology 1935, 25, 931–937. [Google Scholar]
- Okada, F.; Furuya, K. Inhibitory effect of tea catechins on some plant virus diseases. Chagyo Kenkyu Hokoku 1971, 1971, 69–76. (In Japanese) [Google Scholar] [CrossRef]
- Okada, F. Antiviral effects of tea catechins and black tea theaflavins on plant viruses. JARQ 1978, 12, 27–32. [Google Scholar]
- Hao, W.N.; Zhong, G.H.; Hu, M.Y.; Luo, J.J.; Weng, Q.F.; Rizwan-ul-Haq, M. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Tec. 2010, 56, 39–43. [Google Scholar] [CrossRef]
- Nas, M.N. In vitro studies on some natural beverages as botanical pesticides against Erwinia amylovora and Curtobacterium flaccumfaciensis subsp. Poinsettiae. Turkish J. Agric. For. 2004, 28, 57–61. [Google Scholar]
- Huang, J.; Chen, X.; Xu, H.; Wang, H. Studies on inhibitory activity of tea saponin against twelve plant pathogenic fungi. J. Huazhong Agric.Univ. 2013, 32, 50–53. (In Chinese) [Google Scholar]
- Chen, J.J.; Zhang, S.S.; Yang, X.P. Control of brown rot on nectarines by tea polyphenol combined with tea saponin. Crop. Prot. 2013, 45, 29–35. [Google Scholar] [CrossRef]
- Zou, D.; Liao, W.; Huang, H.; Jiang, X. Co-toxicities of tea saponin and tea polyphenol on 2 plant pathogens. J. West China For. Sci. 2018, 47, 41–44, 58. (In Chinese) [Google Scholar]
- Liu, H.; Guo, J.; Cheng, Y.; Luo, L.; Liu, P.; Wang, B.; Deng, B.; Long, C. Control of gray mold of grape by Hanseniaspora uvarum and its effects on postharvest quality parameters. Annals Microbiol. 2010, 60, 31–35. [Google Scholar] [CrossRef]
- Shadmani, N.; Ahmad, S.H.; Saari, N.; Ding, P.; Tajidin, N.E. Chilling injury incidence and antioxidant enzyme activities of Carica papaya L. ‘Frangi’ as influenced by postharvest hot water treatment and storage temperature. Postharvest Biol. Tec. 2015, 99, 114–119. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Yi, S.; Zhu, J.; Fu, L.; Li, J. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int. J. Food Microbiol. 2010, 144, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σE-dependent sRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Oh, Y.J.; Lim, J.; Youn, M.; Lee, I.; Pak, H.K.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.H.; Omura, T.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Letters 2011, 585, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.; Choi, H.; Sung, N.; Byun, E. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor on lipopolysaccharide-stimulated dendritic cells. Biochem. Biophys. Res. Commun. 2012, 426, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.H.; Fujimura, Y.; Yamada, K.; Tachibana, H. TLR4 Signaling Inhibitory Pathway Induced by Green Tea Polyphenol Epigallocatechin-3-Gallate through 67-kDa Laminin Receptor. J. Immunol. 2010, 185, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-Like Receptors in Innate Immunity. Int. Immunol. 2004, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Okada, F.; Takeo, T.; Okada, S.; Tamemasa, O. Antiviral Effect of Theaflavins on Tobacco Mosaic Virus. Agr. Biol. Chem. Tokyo 1977, 41, 791–794. [Google Scholar]
- Liu, S.; Lu, H.; Zhao, Q.; He, Y.; Niu, J.; Debnath, A.K.; Wu, S.; Jiang, S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta 2005, 1723, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Ciesek, S.; Von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.H.L.; Sarkar, P. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018, 16, 59–70. [Google Scholar] [CrossRef]
- Lante, A.; Friso, D. Oxidative stability and rheological properties of nanoemulsions with ultrasonic extracted green tea infusion. Food Res. Int. 2013, 54, 269–276. [Google Scholar] [CrossRef]
- Kim, Y.J.; Houng, S.J.; Kim, J.H.; Kim, Y.; Ji, H.G.; Lee, S. Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J. Nutr. Biochem. 2012, 23, 186–191. [Google Scholar] [CrossRef] [PubMed]

| Pathogen | Host | Disease | Reference |
|---|---|---|---|
| Fungi | |||
| Bipolaris maydis | Maize | Leaf spot | [13] |
| Colletotrichum musae | Banana | Anthracnose | [13] |
| Fusarium oxysporum | Lotus/Banana | Corruption | [13,15] |
| Pyricularia oryzae | Rice | Blast | [14] |
| Phytophthora cryptogea | Gerbera jamesonii | Root rot | [15] |
| Pestalotiopsis apiculatus | Camellia oleifera | Leaf blight | [15] |
| C. fructicola | Camellia oleifera | Anthracnose | [15] |
| C. horii | Illicium verum | Anthracnose | [15,28] |
| Rhizoctorzia solani | Rice | Sheath blight | [15] |
| Lasiodiplodia theobromae | Eucalyptus spp. | Die-back | [15,28] |
| Sclerotinia sclerotiorum | Oilseed rape | Sclerotinia rot | [15] |
| Diplodia natalensis | Citrus fruit | Stem-end rot | [16] |
| Botrytis cinerea | Grape fruit/Nectarine fruit | Gray mold | [17,18] |
| Monilinia fructicola | Nectarine fruit | Brown rot | [27] |
| Puccinia striiformis f. sp. tritici | Wheat | Stripe rust | [12] |
| Bacteria | |||
| Erwinia carotovora | Lettuce/Tomato/Eggplant/Cabbage/Radish/Potato/Cauliflower | Soft rot | [19] |
| Clavibacter michiganensis | Tomato | Canker | [19] |
| Xanthomonas campestris | Lettuce | Spot | [19] |
| Agarobacterium tumefaciens | Grape | Crown gall | [19] |
| Pseudomonas cichorii | Lettuce/Eggplant | Black leg | [19] |
| Ps. marginalis | Lettuce/Onion/Cabbage | Spring rot | [19] |
| Ps. viridiflava | Lettuce/Tomato | Black rot/Leaf rot | [19] |
| Ps. syringae pv. pisi | Bean | Halo blight | [20] |
| Ps. s. pv. phaseolicola | Bean | Halo blight | [20] |
| Virus | |||
| Tobacco mosaic virus | Tobacco | Mosaic disease | [21,22,23] |
| Cucumber mosaic virus | Cucumber | Mosaic disease | [22,23] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules 2019, 24, 816. https://doi.org/10.3390/molecules24040816
Yang Y, Zhang T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules. 2019; 24(4):816. https://doi.org/10.3390/molecules24040816
Chicago/Turabian StyleYang, Yuheng, and Tong Zhang. 2019. "Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review" Molecules 24, no. 4: 816. https://doi.org/10.3390/molecules24040816
APA StyleYang, Y., & Zhang, T. (2019). Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules, 24(4), 816. https://doi.org/10.3390/molecules24040816







