Increased Selectivity of Novozym 435 in the Asymmetric Hydrolysis of a Substrate with High Hydrophobicity Through the Use of Deep Eutectic Solvents and High Substrate Concentrations
Abstract
1. Introduction
2. Results and Discussion
2.1. Asymmetric Hydrolysis of DMFG Catalyzed by Novozym 435
2.2. Effect of High Substrate Concentration on the Selectivity of Novozym 435
2.3. Solubility of R-MFG in ChCl:Urea 50% (v/v)
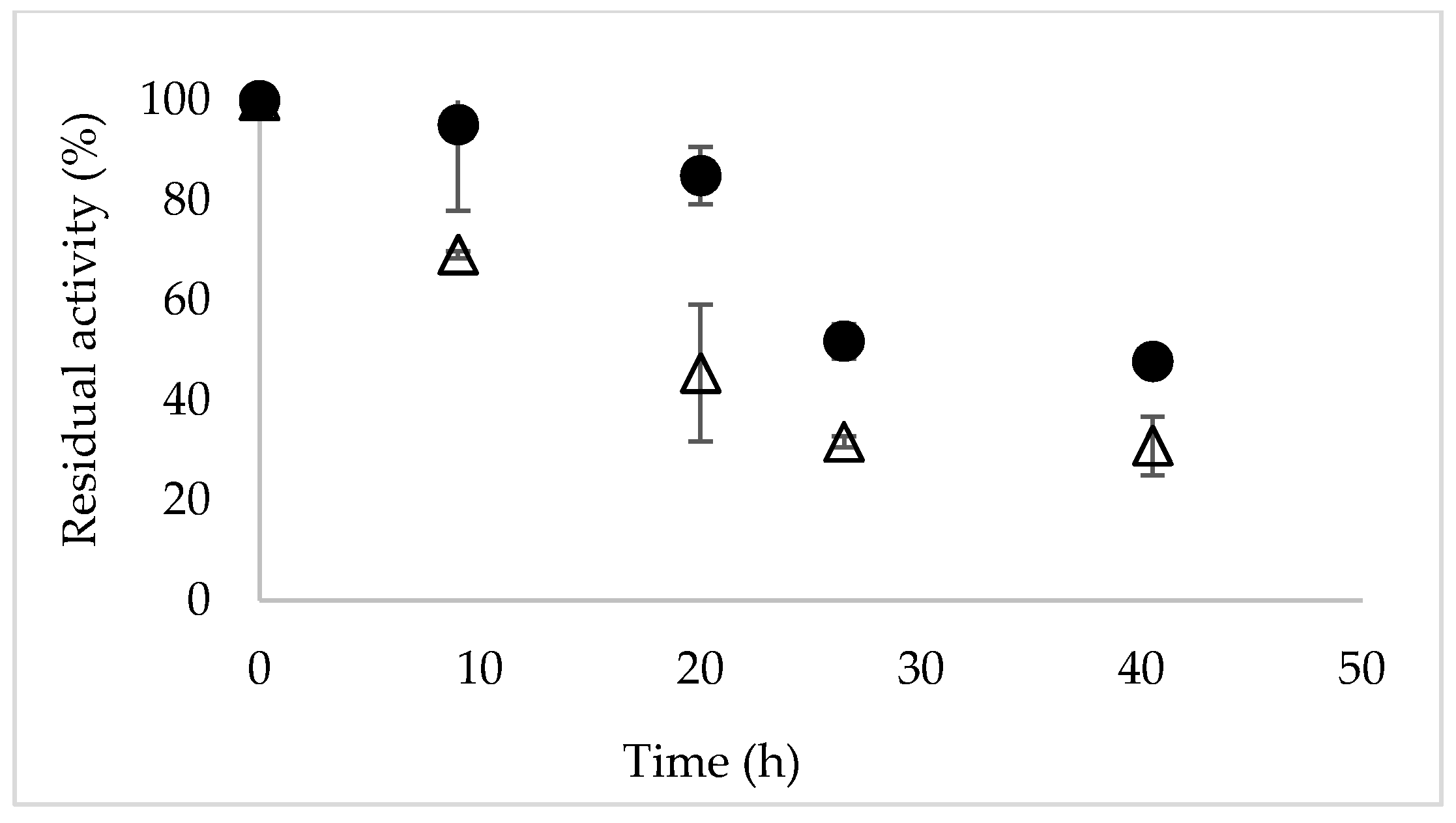
2.4. Stability of Novozym 435 in the Presence of Phosphate Buffer and ChCl:Urea 50% (v/v)
3. Materials and Methods
3.1. General
3.2. Lipase Activity Determination
3.3. Asymmetric Hydrolysis of DMFG Catalyzed by Novozym 435
3.4. Determination of Enantiomeric Excess
3.5. Stability of Novozym 435
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food & Drug Administration. FDA’s statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Federsel, H.J. Asymmetry on large scale: The roadmap to stereoselective processes. Nat. Rev. 2005, 4, 685–697. [Google Scholar]
- Guevara-Pulido, J.O.; Caicedo, J.; David, F.; Vela, M.; González, J. Catálisis asimétrica, una nueva era en la síntesis de fármacos: Historia y evolución. Revista Facultad de Ciencias Básicas 2017, 13, 105–116. [Google Scholar]
- García-Urdiales, E.; Alfonso, I.; Gotor, V. Update 1 of: Enantioselective enzymatic desymmetrizations in organic synthesis. Chem. Rev. 2011, 111, PR110–PR180. [Google Scholar]
- Palomo, J.M.; Cabrera, Z. Enzymatic desymmetrization of prochiral molecules. Curr. Org. Synth. 2012, 9, 791–805. [Google Scholar] [CrossRef]
- Doukyu, N.; Ogino, H. Organic solvent-tolerant enzymes. Biochem. Eng. J. 2010, 48, 270–282. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Mozhaev, V.V.; Belova, A.B.; Sergeeva, M.V.; Martinek, K. Denaturation capacity: A new quantitative criterion for selection of organic solvents as reaction media in biocatalysis. Eur. J. Biochem. 1991, 198, 31–41. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Illanes, A.; Altamirano, C.; Fuentes, M.; Zamorano, F.; Aguirre, C. Synthesis of cephalexin in organic medium at high substrate concentrations and low enzyme to substrate ratio. J. Mol. Catal. B: Enzym. 2005, 35, 45–51. [Google Scholar] [CrossRef]
- Jacobsen, E.E.; Hoff, B.H.; Moen, A.R.; Anthonsen, T. Enantioselective enzymatic preparation of chiral glutaricmonocarboxylic acids and amides. J. Mol. Catal. B: Enzym. 2003, 21, 55–58. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.-W.; Zong, M.-H.; Li, N.; Lou, W.-Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Lecomte, J.; Baréa, B.; Villeneuve, P. Towards a better understanding of how to improve lipase-catalyzed reactions using deep eutectic solvents based on choline chloride. Eur. J. Lipid. Sci. Technol. 2014, 116, 16–23. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.; Yu, H.; Kim, J.H.; Kim, H.J.; Yang, Y.-H.; Lee, S.H. Effect of deep eutectic solvent mixtures on lipase activity and stability. J. Mol. Catal. B: Enzym. 2016, 128, 65–72. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Hashim, M.A.; Hayyan, A. Pure and aqueous deep eutectic solvents for a lipase-catalysed hydrolysis reaction. Biochem. Eng. J. 2017, 117, 129–138. [Google Scholar] [CrossRef]
- Brenna, D.; Massolo, E.; Puglisi, A.; Rossi, R.; Celentano, G.; Benaglia, M.; Capriati, V. Towards the development of continuous, organocatalytic, and stereoselective reactions in deep eutectic solvents. Beilstein J. Org. Chem. 2016, 12, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, Z.; Domínguez de María, P. Benzaldehyde lyase (BAL)-catalyzed enantioselective CC bond formation in deep-eutectic-solvents-buffer mixtures. J. Mol. Catal. B: Enzym. 2014, 107, 120–123. [Google Scholar] [CrossRef]
- Homann, M.; Vail, R.; Morgan, B.; Sabesan, V.; Levy, C.; Dodds, D.; Zaks, A. Enzymatic hydrolysis of a prochiral 3-substituted glutarate ester, an intermediate in the synthesis of an NK1/NK2 dual antagonist. Adv. Synth. Catal. 2001, 343, 744–749. [Google Scholar] [CrossRef]
- Yu, M.S.; Lantos, I.; Peng, Z.Q.; Lu, J.; Cacchio, T. Asymmetric synthesis of (−)-paroxetine using PLE hydrolysis. Tetrahedron 2000, 41, 5647–5651. [Google Scholar] [CrossRef]
- Lee, T.L.; Hong, P.C.; Huang, H.L.; Chen, S.F.; Wang, C.L.; Wen, Y.S. Asymmetric syntheses of trans-3,4-disubstituted 2-piperidinones and piperidines. Tetrahedron Asymmetry 2001, 12, 419–426. [Google Scholar]
- Chen, L.-Y.; Zaks, A.; Chackalamannil, S.; Dugar, S. Asymmetric synthesis of substituted 2-azaspiro-[3.5]-nonan-1-ones: An enantioselective synthesis of the cholesterol absorption inhibitor (+)-SCH 54016. J. Org. Chem. 1996, 61, 8341–8343. [Google Scholar]
- Cabrera, Z.; Palomo, J.M. Enantioselective desymmetrization of prochiral diesters catalyzed by immobilized Rhizopus oryzae lipase. Tetrahedron Asymmetry 2011, 22, 2080–2084. [Google Scholar] [CrossRef]
- Fryszkowska, A.; Komar, M.; Koszelewski, D.; Ostaszewski, R. Enzymatic desymmetrization of 3-arylglutaric acid anhydrides. Tetrahedron Asymmetry 2005, 16, 2475–2485. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Enhancement of Novozym-435 catalytic properties by physical or chemical modification. Process Biochem. 2009, 226–231. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Dubreucq, E.; Lortie, R.; Villeneuve, P. Evaluation of deep eutectic solvent-water binary mixtures for lipase-catalyzed lipophilization of phenolic acids. Green Chem. 2013, 15, 2275–2282. [Google Scholar] [CrossRef]
- Wu, B.P.; Wen, Q.; Xu, H.; Yang, Z. Insights into the impact of deep eutectic solvents on horseradish peroxidase: Activity, stability and structure. J. Mol. Catal. B Enzym. 2014, 101, 101–107. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Palomo, J.M.; Guisán, J.M. Novozym 435 displays very different selectivity compared to lipase from Candida antarctica B adsorbed on other hydrophobic supports. J. Mol. Catal. B: Enzym. 2009, 57, 171–176. [Google Scholar] [CrossRef]
- Vitale, P.; Abbinante, V.M.; Perna, F.M.; Salomone, A.; Cardellicchio, C.; Capriati, V. Unveiling the Hidden Performance of Whole Cells in the Asymmetric Bioreduction of Aryl-containing Ketones in Aqueous Deep Eutectic Solvents. Adv. Synth. Catal. 2017, 359, 1049–1057. [Google Scholar] [CrossRef]
- Turner, N. Controlling chirality. Curr. Opin. Biotechnol. 2003, 401–406. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Asymmetric hydrolysis of dimethyl-3-phenylglutaratecatalyzed by Lecitase Ultra® Effect of the immobilization protocol on its catalytic properties. Enzyme Microb. Technol. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Gloria Fernandez-Lorente, G.; Palomo, J.M.; Cabrera, Z.; Guisan, J.M.; Fernandez-Lafuente, R. Specificity enhancement towards hydrophobic substrates by immobilization of lipases by interfacial activation on hydrophobic supports. Enzyme Microb. Technol. 2007, 41, 565–569. [Google Scholar] [CrossRef]
Sample Availability: Substrate samples are available from the authors. |
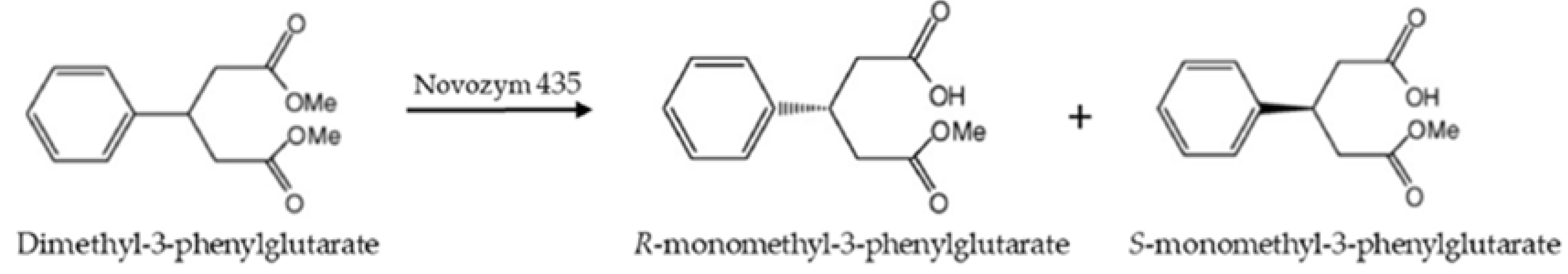
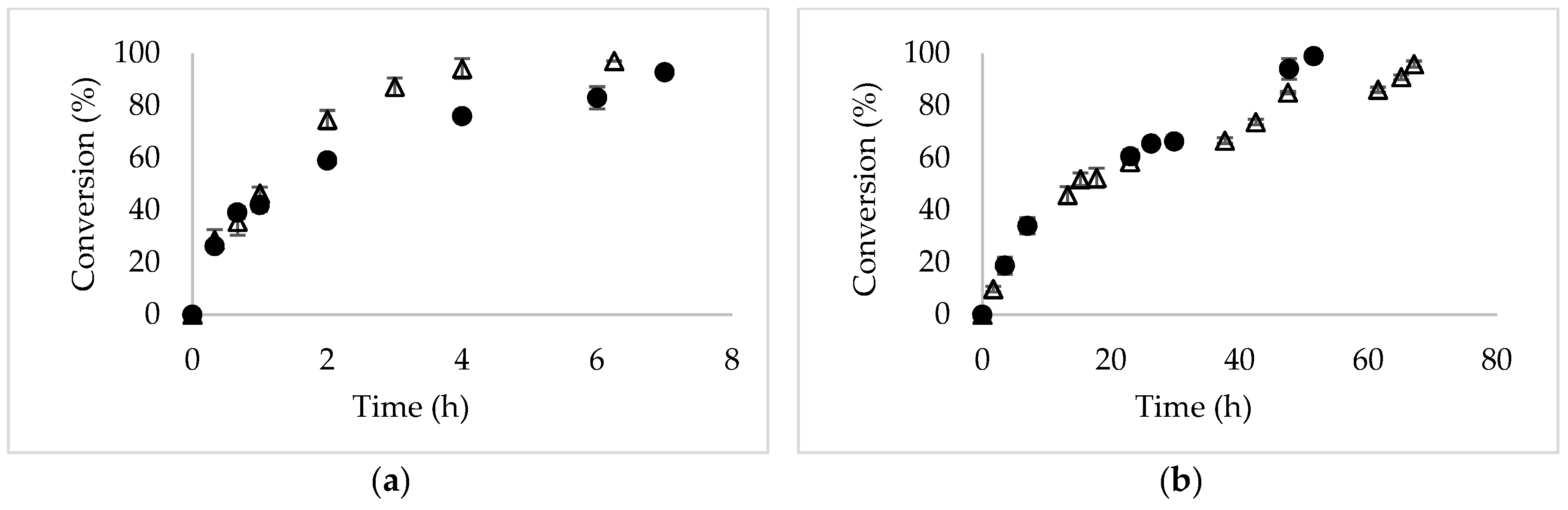

| Reaction Medium | DMFG (mM) | Reaction System | Specific Initial Reaction Rate (μmol Product/g Biocatalyst/min) | e.e1 (%) | Specific Productivity1 (g Product/g Biocatalyst/d) |
|---|---|---|---|---|---|
| Phosphate Buffer 100% (v/v) | 0.45 | homogeneous | 2.2 ± 0.3 | 76 | 0.15 ± 0.01 |
| 18 | heterogeneous | 6.0 ± 0.3 | 88 | 0.39 ± 0.02 | |
| ChCl:urea 50% (v/v) | 0.45 | homogeneous | 2.1 ± 0.1 | 87 | 0.1 ± 0.01 |
| 18 | heterogeneous | 6.2 ± 0.3 | 94 | 0.48 ± 0.01 |
| DMFG (mM) | Specific Initial Reaction Rate (μmoles Product/g Biocatalyst/min) | e.e1 (%) | Specific Productivity 1 (g Product/g Biocatalyst/d) |
|---|---|---|---|
| 18 | 6.2 ± 0.3 | 94 | 0.48 ± 0.01 |
| 36 | 5.6 ± 0.3 | 98 | 0.46 ± 0.03 |
| 54 | 6.5 ± 0.3 | 99 | 0.68 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredes, Y.; Chamorro, L.; Cabrera, Z. Increased Selectivity of Novozym 435 in the Asymmetric Hydrolysis of a Substrate with High Hydrophobicity Through the Use of Deep Eutectic Solvents and High Substrate Concentrations. Molecules 2019, 24, 792. https://doi.org/10.3390/molecules24040792
Fredes Y, Chamorro L, Cabrera Z. Increased Selectivity of Novozym 435 in the Asymmetric Hydrolysis of a Substrate with High Hydrophobicity Through the Use of Deep Eutectic Solvents and High Substrate Concentrations. Molecules. 2019; 24(4):792. https://doi.org/10.3390/molecules24040792
Chicago/Turabian StyleFredes, Yerko, Lesly Chamorro, and Zaida Cabrera. 2019. "Increased Selectivity of Novozym 435 in the Asymmetric Hydrolysis of a Substrate with High Hydrophobicity Through the Use of Deep Eutectic Solvents and High Substrate Concentrations" Molecules 24, no. 4: 792. https://doi.org/10.3390/molecules24040792
APA StyleFredes, Y., Chamorro, L., & Cabrera, Z. (2019). Increased Selectivity of Novozym 435 in the Asymmetric Hydrolysis of a Substrate with High Hydrophobicity Through the Use of Deep Eutectic Solvents and High Substrate Concentrations. Molecules, 24(4), 792. https://doi.org/10.3390/molecules24040792






