Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material
Abstract
1. Introduction
2. Results and Discussion
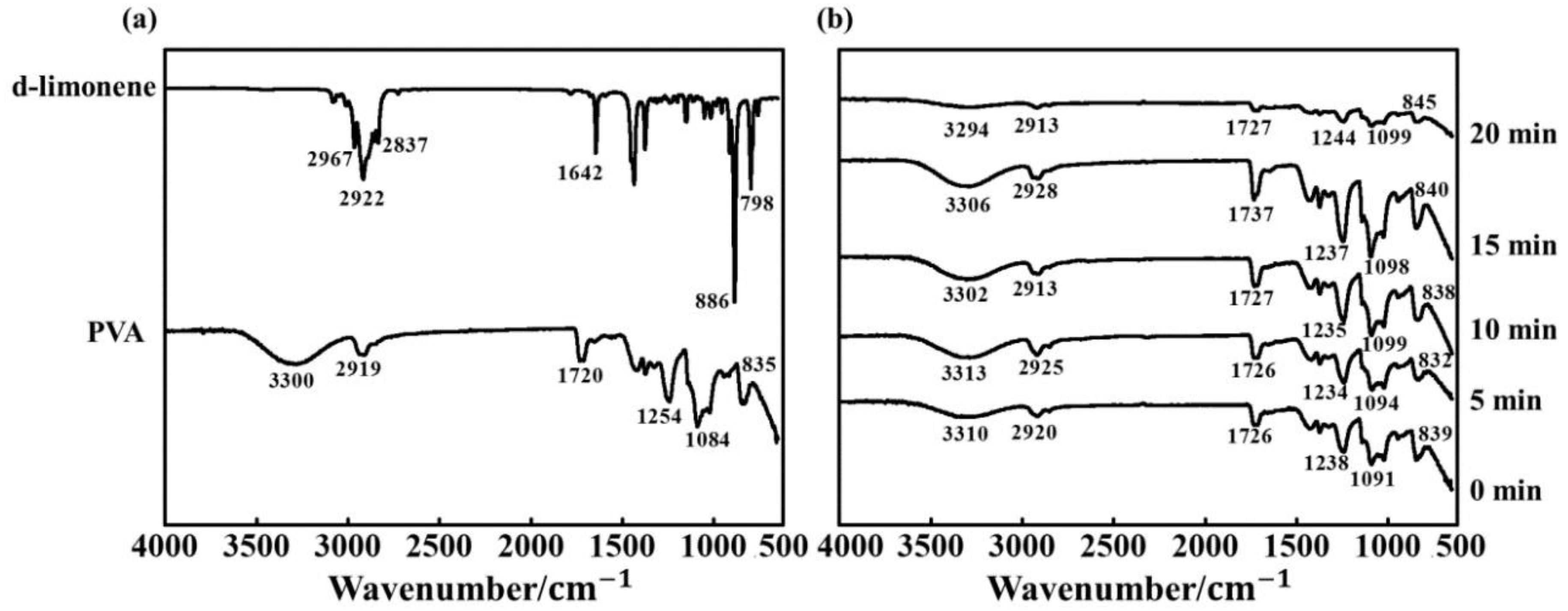
2.1. Chemical Structure
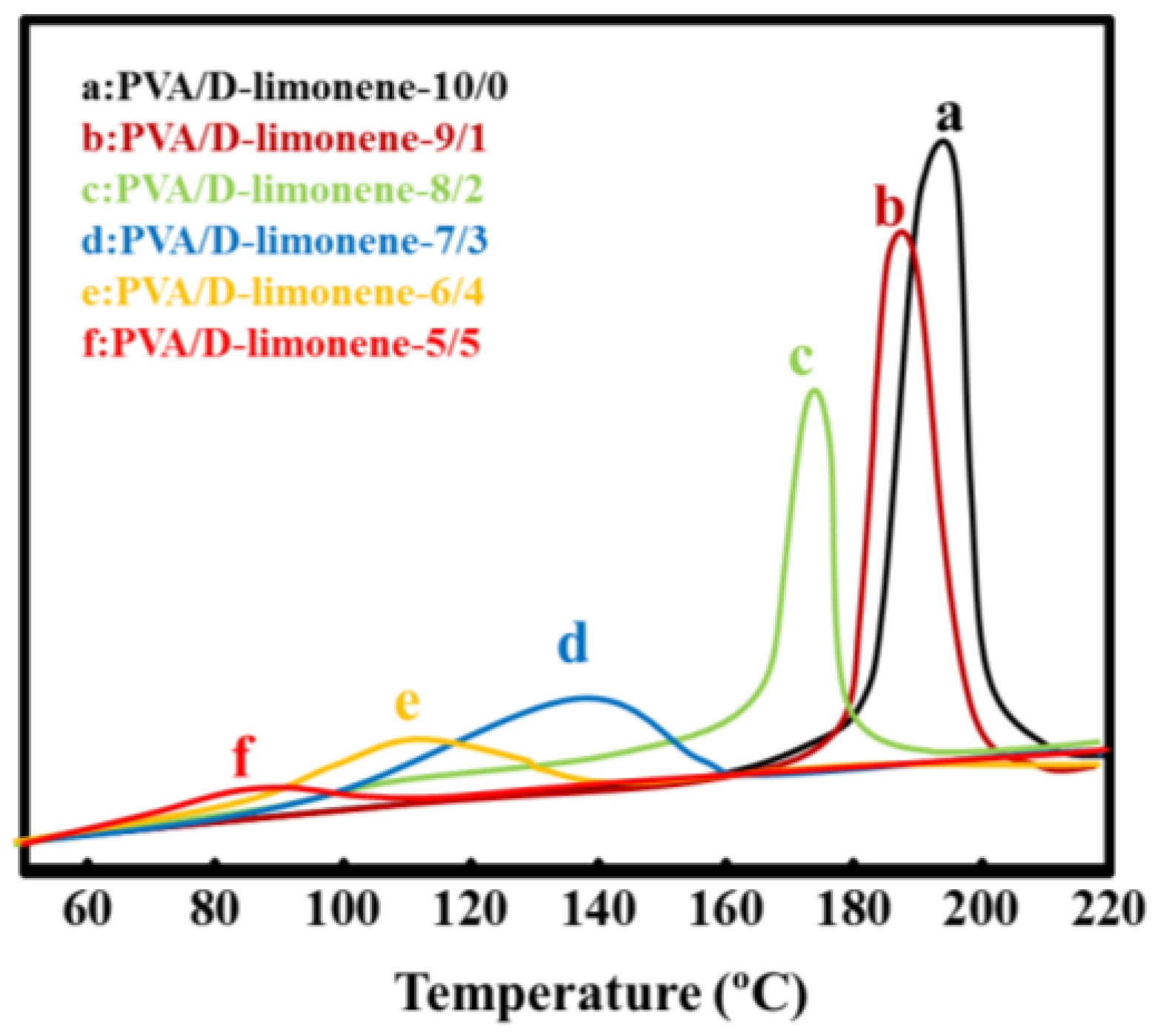
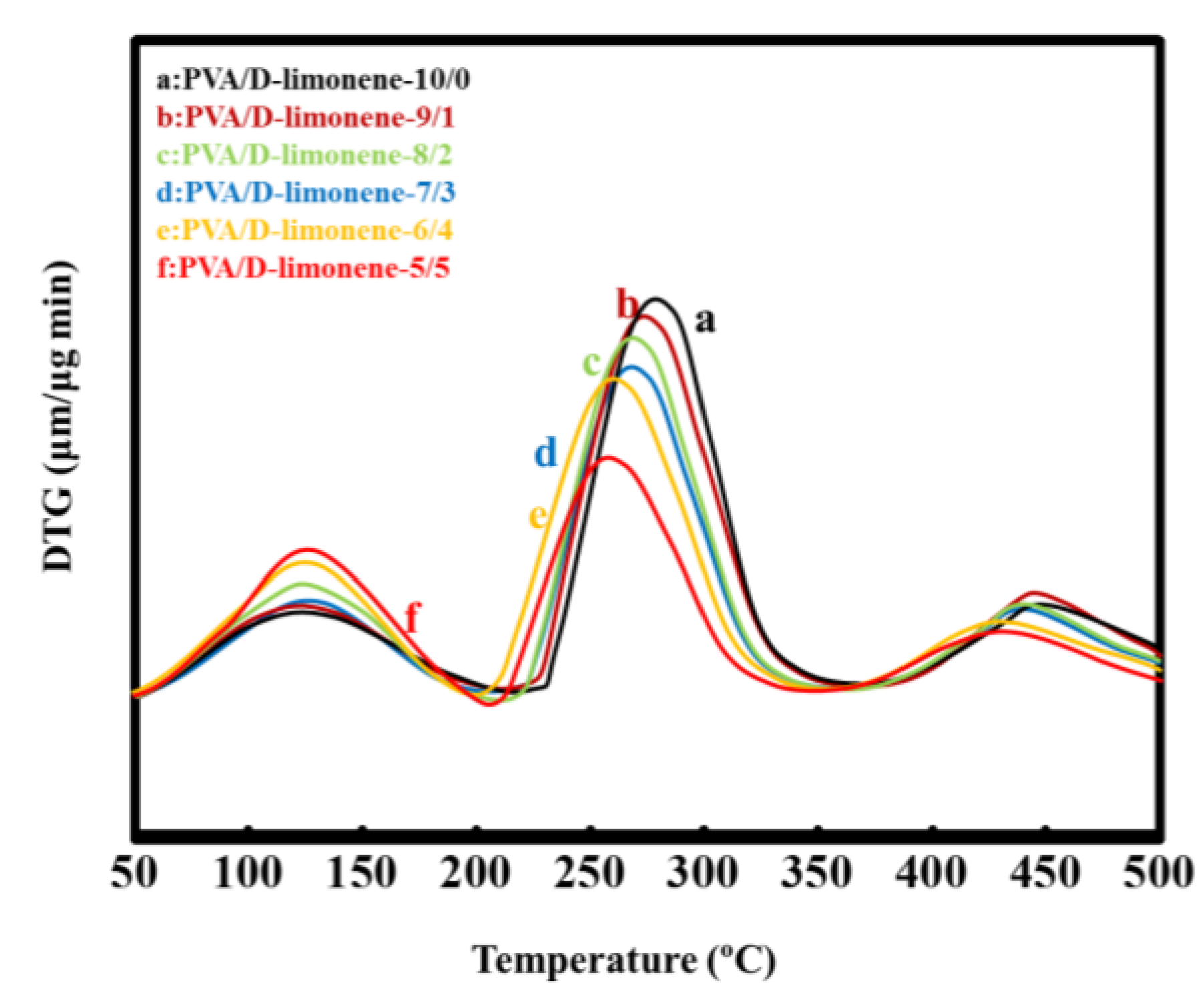
2.2. Thermal Properties
| Samples | Tm/°C | Tc/°C | ΔHm/(J·g−1) |
|---|---|---|---|
| PVA/d-limonene-10/0 | 226.5 | 197.6 | 44.38 |
| PVA/d-limonene-9/1 | 221.3 | 186.4 | 39.26 |
| PVA/d-limonene-8/2 | 218.4 | 173.7 | 35.33 |
| PVA/d-limonene-7/3 | 206.7 | 139.3 | 30.27 |
| PVA/d-limonene-6/4 | 201.3 | 112.5 | 28.25 |
| PVA/d-limonene-5/5 | 192.2 | 87.3 | 25.31 |
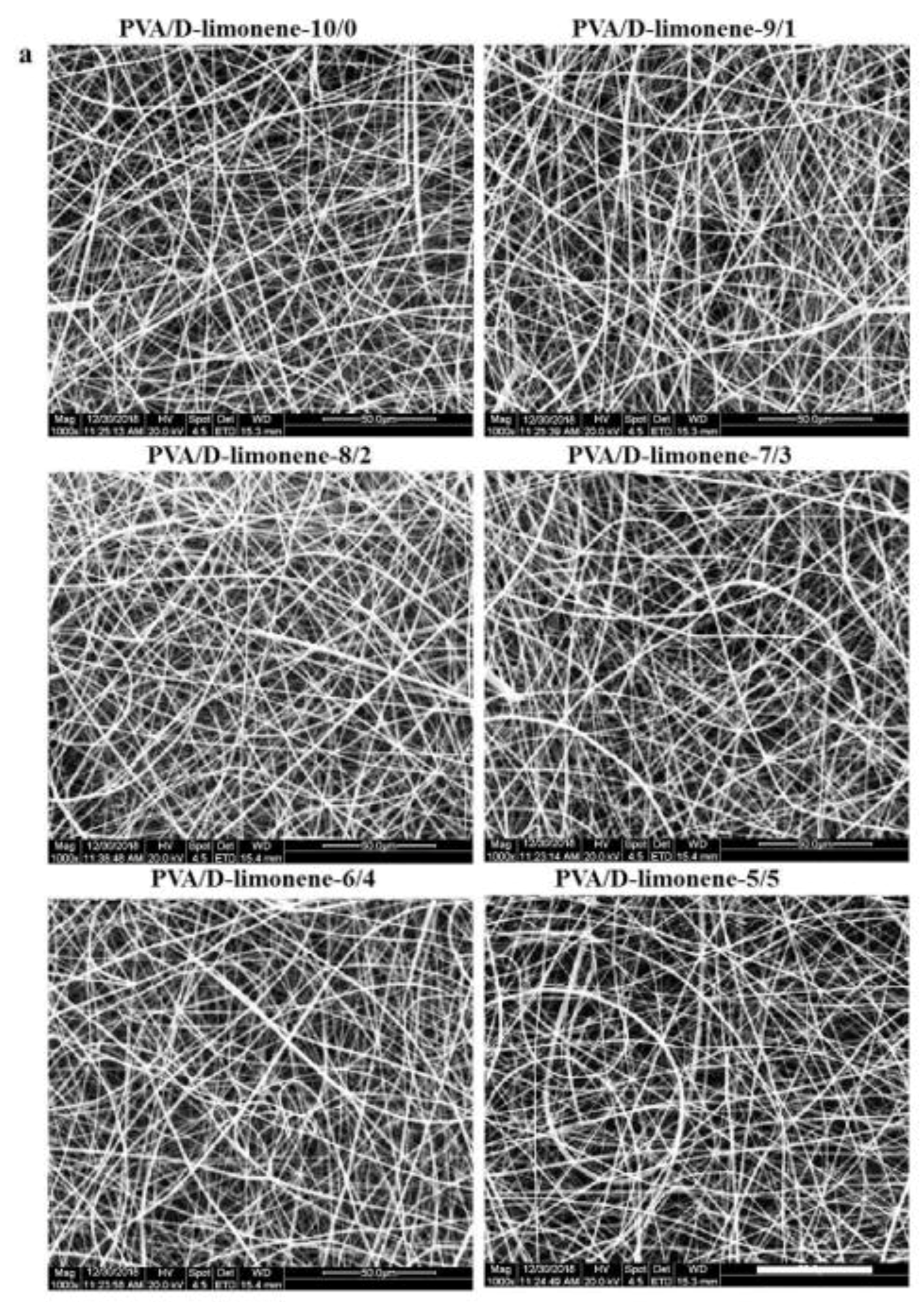
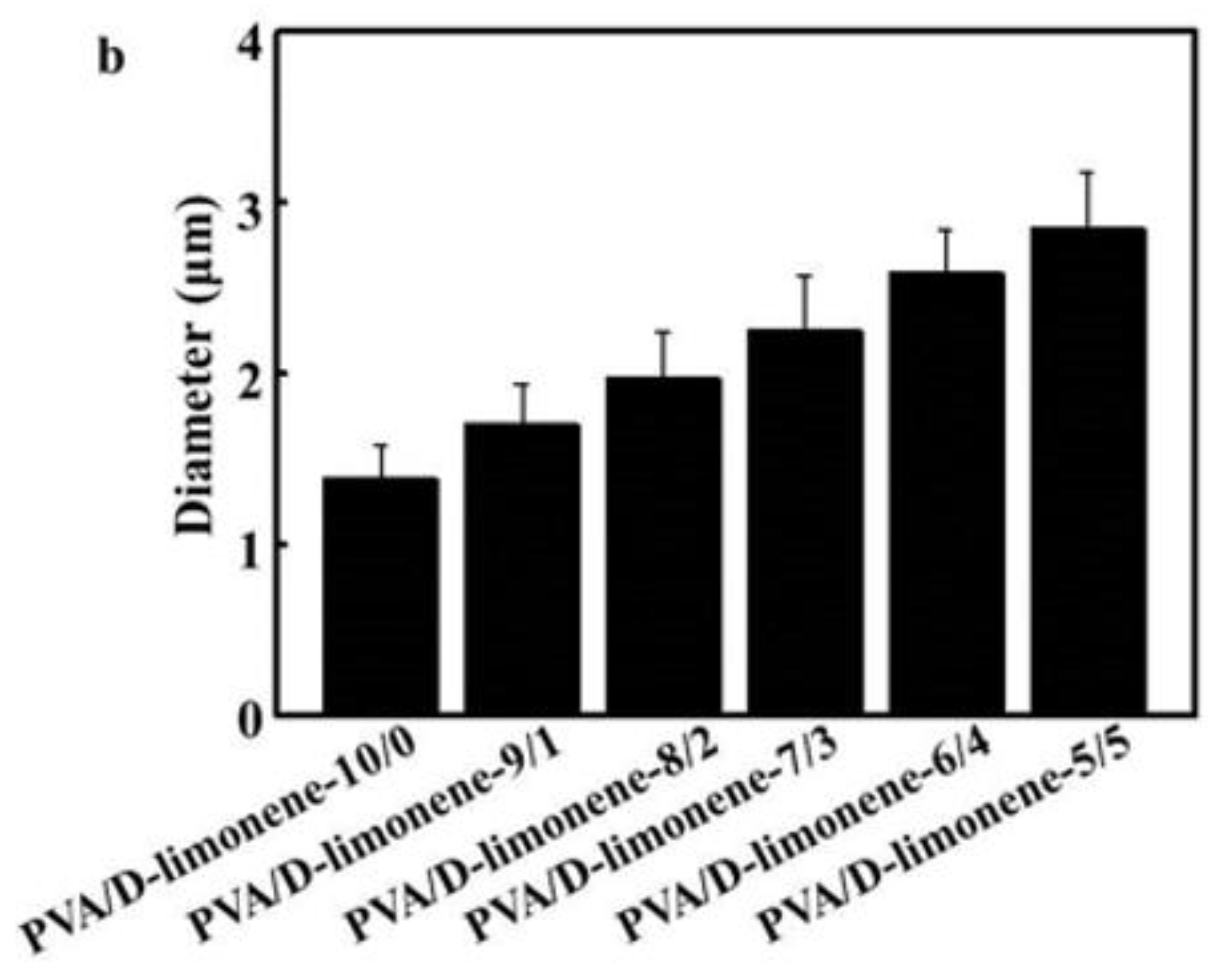
2.3. SEM Analysis
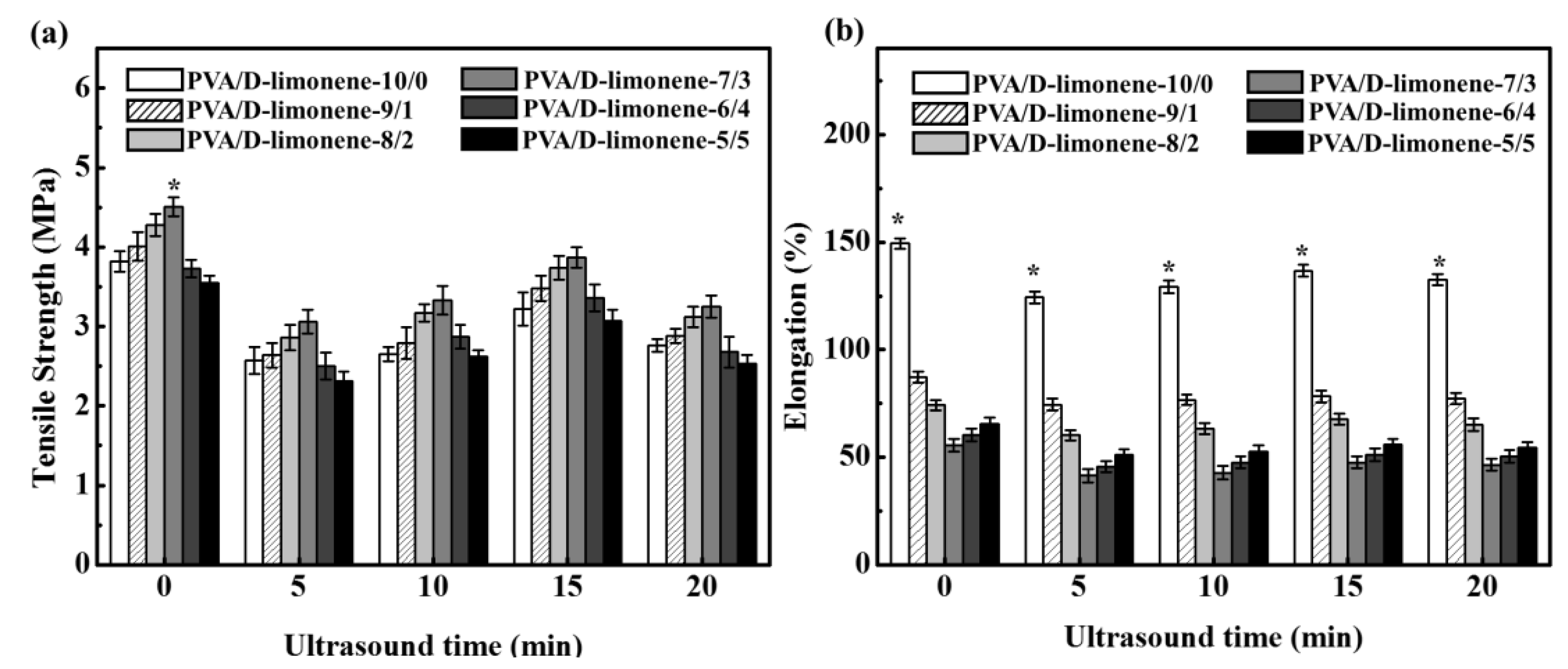
2.4. Mechanical Properties
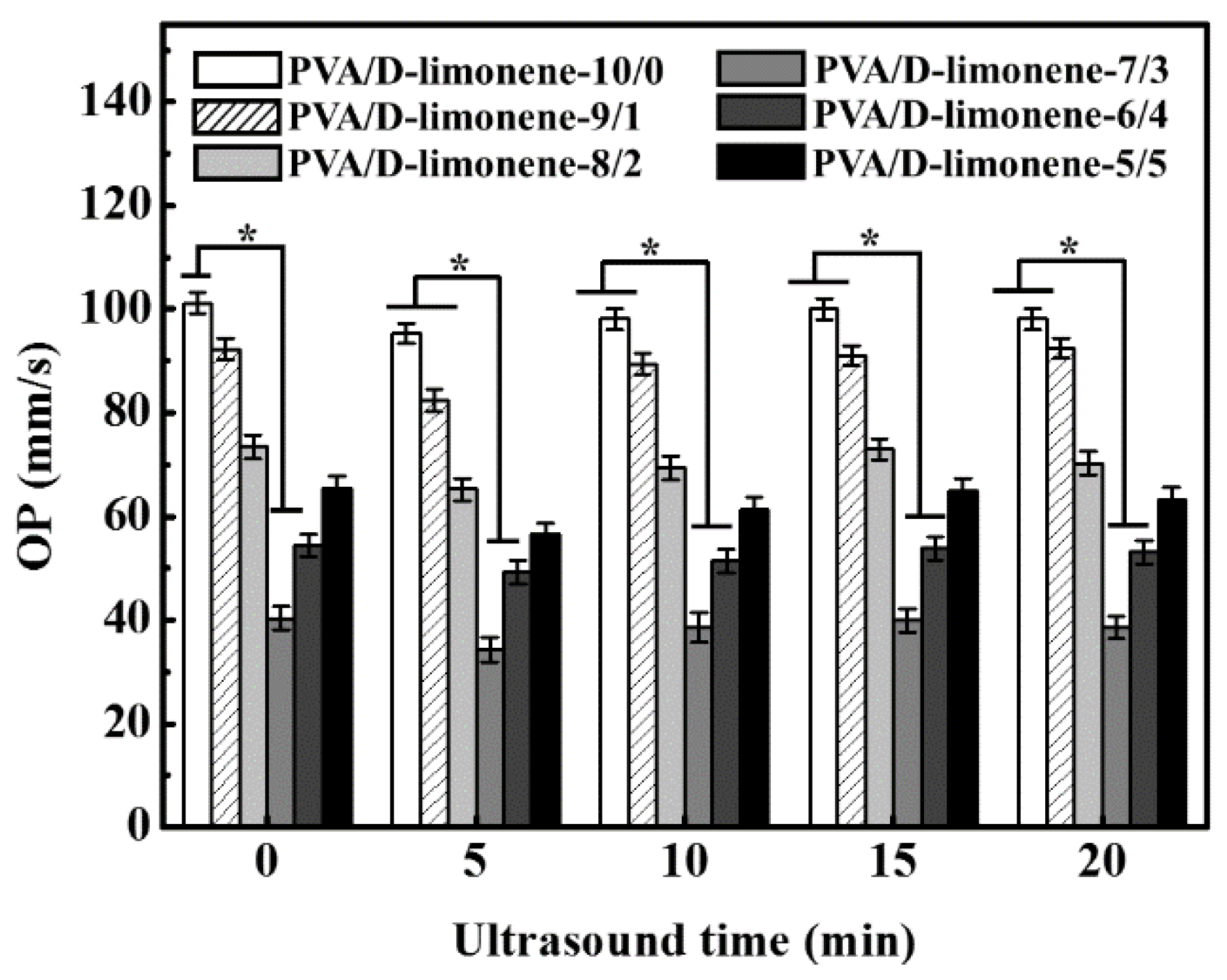
2.5. Oxygen Permeability
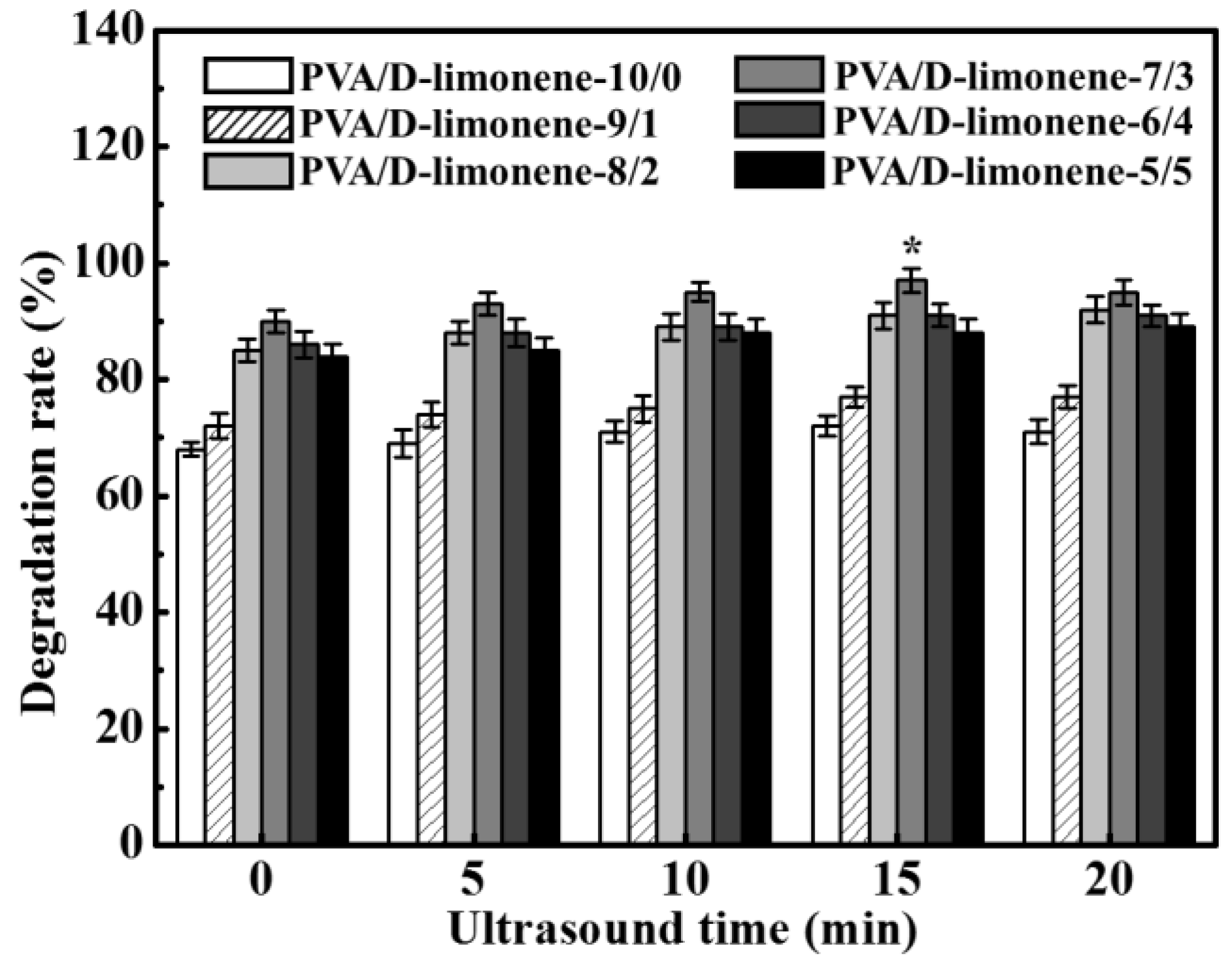
2.6. Biodegradation of the Composite Fibers
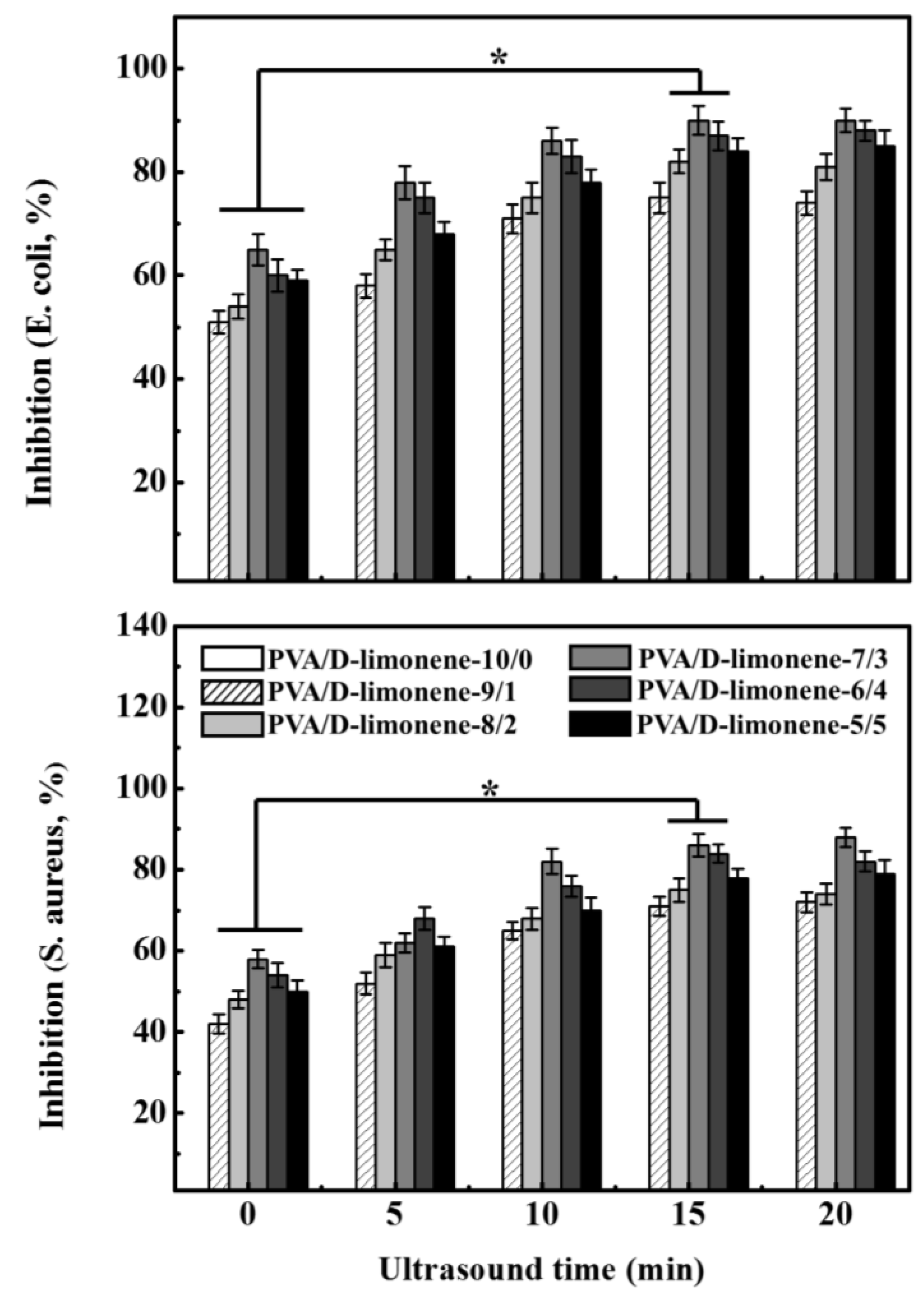
2.7. Antibacterial Properties
3. Materials and Methods
3.1. Materials
3.2. Electrospinning PVA/d-limonene Composite Fibers
| Sample Number | Sample Name | PVA/d-limonene Ratio | Ultrasonication Time |
|---|---|---|---|
| 1 | PVA/d-limonene-10/0-0 | 10/0 | 0 |
| 2 | PVA/d-limonene-10/0-5 | 10/0 | 5 |
| 3 | PVA/d-limonene-10/0-15 | 10/0 | 15 |
| 4 | PVA/d-limonene-10/0-20 | 10/0 | 20 |
| 5 | PVA/d-limonene-9/1-0 | 9/1 | 0 |
| 6 | PVA/d-limonene-9/1-5 | 9/1 | 5 |
| 7 | PVA/d-limonene-9/1-15 | 9/1 | 15 |
| 8 | PVA/d-limonene-9/1-20 | 9/1 | 20 |
| 9 | PVA/d-limonene-8/2-0 | 8/2 | 0 |
| 10 | PVA/d-limonene-8/2-5 | 8/2 | 5 |
| 11 | PVA/d-limonene-8/2-15 | 8/2 | 15 |
| 12 | PVA/d-limonene-8/2-20 | 8/2 | 20 |
| 13 | PVA/d-limonene-7/3-0 | 7/3 | 0 |
| 14 | PVA/d-limonene-7/3-5 | 7/3 | 5 |
| 15 | PVA/d-limonene-7/3-15 | 7/3 | 15 |
| 16 | PVA/d-limonene-7/3-20 | 7/3 | 20 |
| 17 | PVA/d-limonene-6/4-0 | 6/4 | 0 |
| 18 | PVA/d-limonene-6/4-5 | 6/4 | 5 |
| 19 | PVA/d-limonene-6/4-15 | 6/4 | 15 |
| 20 | PVA/d-limonene-6/4-20 | 6/4 | 20 |
| 21 | PVA/d-limonene-5/5-0 | 5/5 | 0 |
| 22 | PVA/d-limonene-5/5-20 | 5/5 | 5 |
| 23 | PVA/d-limonene-5/5-15 | 5/5 | 15 |
| 24 | PVA/d-limonene-5/5-20 | 5/5 | 20 |
3.3. Characterization of Fibers
3.4. Biodegradation of Fibers
3.5. Antimicrobial Tests
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tavares, L.B.; Ito, N.M.; Salvadori, M.C.; Santos, D.J.D.; Rosa, D.S. PBAT/kraft lignin blend in flexible laminated food packaging: Peeling resistance and thermal degradability. Polym. Test. 2018, 67, 169–176. [Google Scholar] [CrossRef]
- Laidson, P.G.; Hiléia, K.S.S.; José, M.C.; Cristina, T.A.; António, F.S.; Maria, P.G.; Vania, M.F.P. Edible chitosan films and their nanosized counterparts exhibit antimicrobial activity and enhanced mechanical and barrier properties. Molecules 2019, 24, 127–141. [Google Scholar]
- Giuseppe, C.; Anna, D.; Vladimir, E.; Giuseppe, L.; Stefana, M.; Filippo, P.; Elvira, R.; Rawil, F. Halloysite nanotubes: Controlled access and release by smart gates. Nanomaterials 2017, 7, 199–211. [Google Scholar]
- Maziyar, M.; Pooria, P.; Giuseppe, C.; Giuseppe, L.; Yoong, K.A.; Sui, M.L.; Stefana, M. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Inter. 2017, 9, 17476–17488. [Google Scholar]
- Gandhi, N.; Ahluwalia, P.; Bhise, S.; Singh, B. Recent advances in antioxidant active food packaging. 2016. [Google Scholar]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendon, R.; de Quiros, A.R.B.; Ares, A.; Castro-López, M.; José Abad, M.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extend the shelf life of fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Croteau, R. Biosynthesis and catabolism of monoterpenes. Chem. Rev. 1987, 87, 929–954. [Google Scholar] [CrossRef]
- Maltzman, T.H.; Hurt, L.M.; Elson, C.E.; Tanner, M.A.; Gould, M.N. The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis 1989, 10, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, L.W.; Sparnins, V.L.; Barany, G. Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res. 1989, 49, 2689–2692. [Google Scholar] [PubMed]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Kumar, T.R.S.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Frag. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Ochocka, J.R.; Lis-Balchin, M.; Deans, S.; Asztemborska, M. Differences in bioactivity between the enantiomers of α-pinene and limonene. Eur. J. Pharm. Sci. 1996, 4, S105. [Google Scholar] [CrossRef]
- Zhao, J.L.; Cao, Z.S.; Zhang, H.X. Effect of limonene compound liquid on fresh-keeping nectarine after harvest. Food Nutr. China 2015, 21, 35–37. (In Chinese) [Google Scholar]
- Zhao, J.L.; Shui, J.J. Effect of limonene compound liquid on fresh-keeping strawberry after harvest. Food Nutr. China 2014, 20, 51–54. (In Chinese) [Google Scholar]
- Lu, H.Y.; Liang, Y.; Liu, X.J. Effect of limonene emulsion on post-harvest physiology and storage quality of spinaches. Sci. Tech. Food Ind. 2013, 34, 338–341. [Google Scholar]
- Liu, Y.W.; Wang, S.Y.; Lan, W.T. Fabrication of antibacterial chitosan-PVA blended film using electrospray technique for food packaging applications. Int. J. Biol. Macromol. 2018, 107, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kavyanifar, S.; Shamspur, T.; Fathirad, F.; Mostafavi, A. Design and performance investigation of electrospun PVA nanofibers containing core-shell nanostructures for anticancer drug delivery. Nanomed. Res. J. 2014, 3, 31–36. [Google Scholar]
- Rebia, R.A.; Rozet, S.; Tamada, Y.; Tanaka, T. Biodegradable PHBH/PVA blend nanofibers: Fabrication, characterization, in vitro degradation, and in vitro biocompatibility. Polym. Degrad. Stabil. 2018, 154, 124–136. [Google Scholar] [CrossRef]
- Hu, D.; Xiao, Y.; Liu, H.; Wang, H.; Li, J.; Zhou, B.; Liu, P.; Shen, M.; Shi, X. Loading of Au/Ag bimetallic nanoparticles within electrospun PVA/PEI nanofibers for catalytic applications. Colloid. Surfaces A 2018, 552, 9–15. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Ge, L.; Zhao, Y.S.; Mo, T.; Li, J.R.; Li, P. Immobilization of glucose oxidase in electrospun nanofibrous membranes for food preservation. Food Control 2012, 26, 188–193. [Google Scholar] [CrossRef]
- Wang, X.; Yue, T.; Lee, T.C. Development of Pleurocidin-poly(vinyl alcohol) electrospun antimicrobial nanofibers to retain antimicrobial activity in food system application. Food Control 2015, 54, 150–157. [Google Scholar] [CrossRef]
- Chae, J.M.; Lee, K.O.; Amanov, A. Gradient nanostructured tantalum by thermal-mechanical ultrasonic impact energy. Materials 2018, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Ali, S.; Grøndahl, L. Evaluation of an ultrasonic-assisted mechanical stirring technique for the synthesis of an efficient nano-photocatalyst. Res. Chem. Intermediat. 2018, 44, 4015–4028. [Google Scholar] [CrossRef]
- Ferrell, R.A.; Bhattacharjee, J.K. Critical ultrasonic attenuation in superfluid helium: Mixing of order-parameter and fluctuation contributions. Phys. Rev. B 1980, 23, 2434–2437. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power ultrasoud in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 37, 180–196. [Google Scholar] [CrossRef]
- Pereira, S.V.; Colombo, F.B.; de Freitas, L.A. Ultrasound influence on the solubility of solid dispersions prepared for a poorly soluble drug. Ultrason. Sonochem. 2016, 29, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.S.; Chotoowen, C.; Waniska, R.D.; Rooney, L.W. Characterization of starch cooked in alkali by aqueous high-performance size-exclusion chromatography. Cereal Chem. 1988, 65, 493–496. [Google Scholar]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Mrs Bull. 1995, 20, 29–34. [Google Scholar] [CrossRef]
- Hadipour-Goudarzi, E.; Montazer, M.; Latifi, M.; Aghaji, A.A. Electrospinning of chitosan/sericin/PVA nanofibers incorporated with in situ synthesis of nano silver. Carbohyd. Polym. 2014, 113, 231–239. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Razi, F.; Sawada, I.; Takagi, R.; Ohmukai, Y.; Matsuyama, H. Improvement of antibiofouling performance of a reverse osmosis membrane through biocide release and adhesion resistance. Sep. Purif. Technol. 2013, 105, 106–113. [Google Scholar] [CrossRef]
- Zahi, M.R. Fabrication of novel d-Limonene nano-scale systems and evaluation of their antimicrobial activity. Beijing University of Chemical Technology: Beijing, China, Unpublished work. 2015. [Google Scholar]
- Satoshi, K.; John, F.K. The formation of strong intermolecular interactions in immiscible blends of Poly(vinyl alcohol) (PVA) and Lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar]
- Guo, D.; Wang, Q.; Bai, S. Poly(vinyl alcohol)/melamine phosphate composites prepared through thermal processing: Thermal stability and flame retardancy. Polym. Advan. Technol. 2013, 24, 339–347. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of poly(vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Elhamalsadat, S.; Ana, M.; Vanessa, B.; Beeren, S.R.; Chronakis, I.S. Electrospun Phospholipid Fibers as Micro-Encapsulation and Antioxidant Matrices. Molecules 2017, 22, 1708–1724. [Google Scholar]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan-tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Layek, R.K.; Samanta, S.; Nandi, A.K. The physical properties of sulfonated graphene/poly(vinyl alcohol) composites. Carbon 2012, 50, 815–827. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Holley, R.A. Active packaging and nonthermal processing. In Packaging for Nonthermal Processing of Food, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2018. [Google Scholar]
- Azahari, N.A.; Othman, N.; Ismail, H. Effect of attapulgite clay on biodegradability and tensile properties of polyvinyl alcohol/corn starch blend film. Int. J. Polym. 2012, 61, 1065–1078. [Google Scholar] [CrossRef]
- Rhayour, K.; Bouchikhi, T.; Elaraki, A.T.; Sendide, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on escherichia coli and bacillus subtilis. J. Essent. Oil Res. 2003, 15, 286–292. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Ma, Z.; Li, N.; Niu, S.; Li, Y. Preparation of polyvinyl alcohol graphene oxide phosphonate film and research of thermal stability and mechanical properties. Ultrason. Sonochem. 2018, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, R.; Kang, X.; Cui, B.; Yu, B. Effects of ultrasonic treatment on amylose-lipid complex formation and properties of sweet potato starch-based films. Ultrason. Sonochem. 2018, 44, 215–222. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the PVA and d-limonene are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.; Liang, X.; Lan, W.; Ahmed, S.; Liu, Y.; Qin, W. Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules 2019, 24, 767. https://doi.org/10.3390/molecules24040767
Lan W, Liang X, Lan W, Ahmed S, Liu Y, Qin W. Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules. 2019; 24(4):767. https://doi.org/10.3390/molecules24040767
Chicago/Turabian StyleLan, Weijie, Xue Liang, Wenting Lan, Saeed Ahmed, Yaowen Liu, and Wen Qin. 2019. "Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material" Molecules 24, no. 4: 767. https://doi.org/10.3390/molecules24040767
APA StyleLan, W., Liang, X., Lan, W., Ahmed, S., Liu, Y., & Qin, W. (2019). Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules, 24(4), 767. https://doi.org/10.3390/molecules24040767






