Cyclo(l-Pro–l-Leu) of Pseudomonas putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne incognita
Abstract
1. Introduction
2. Results
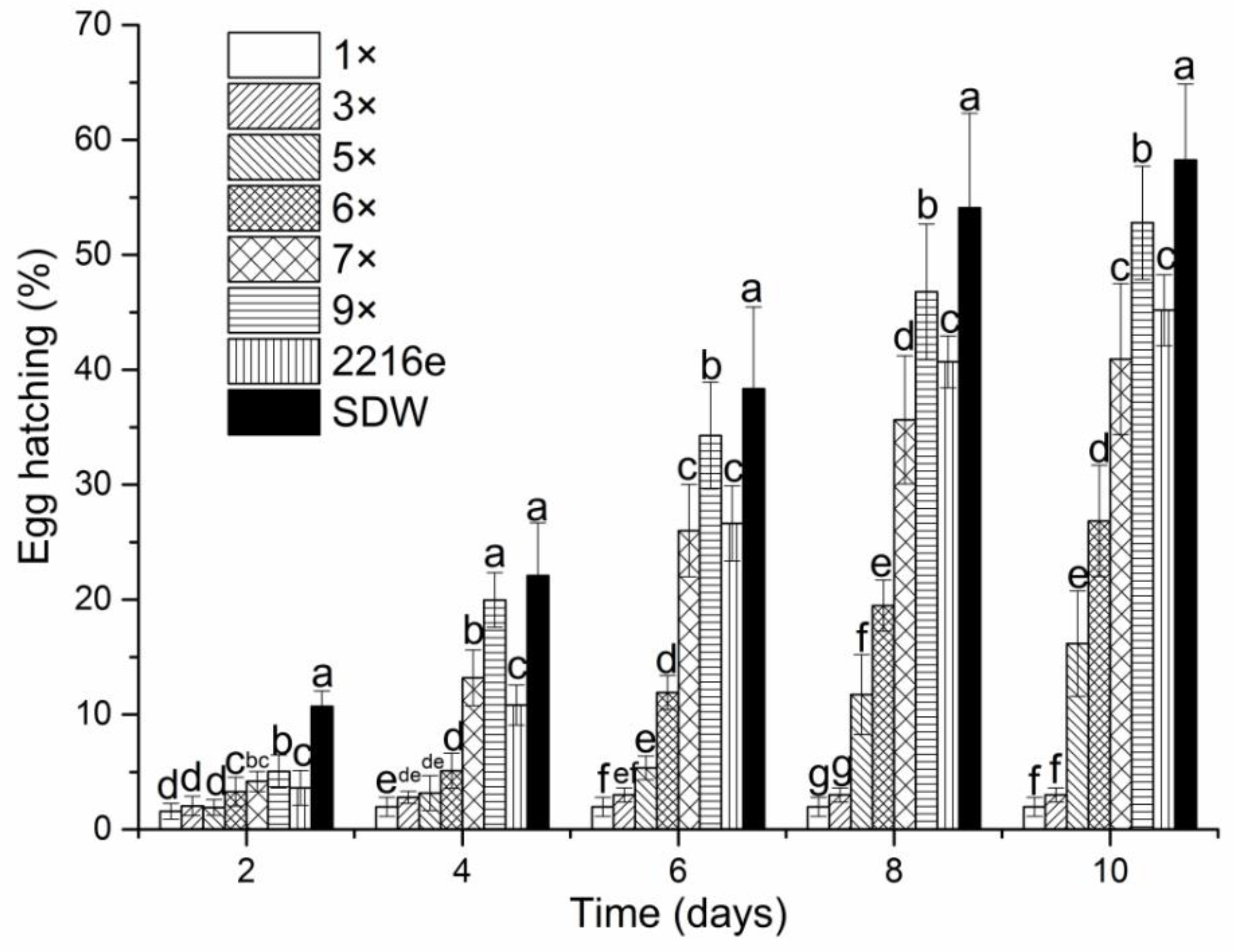
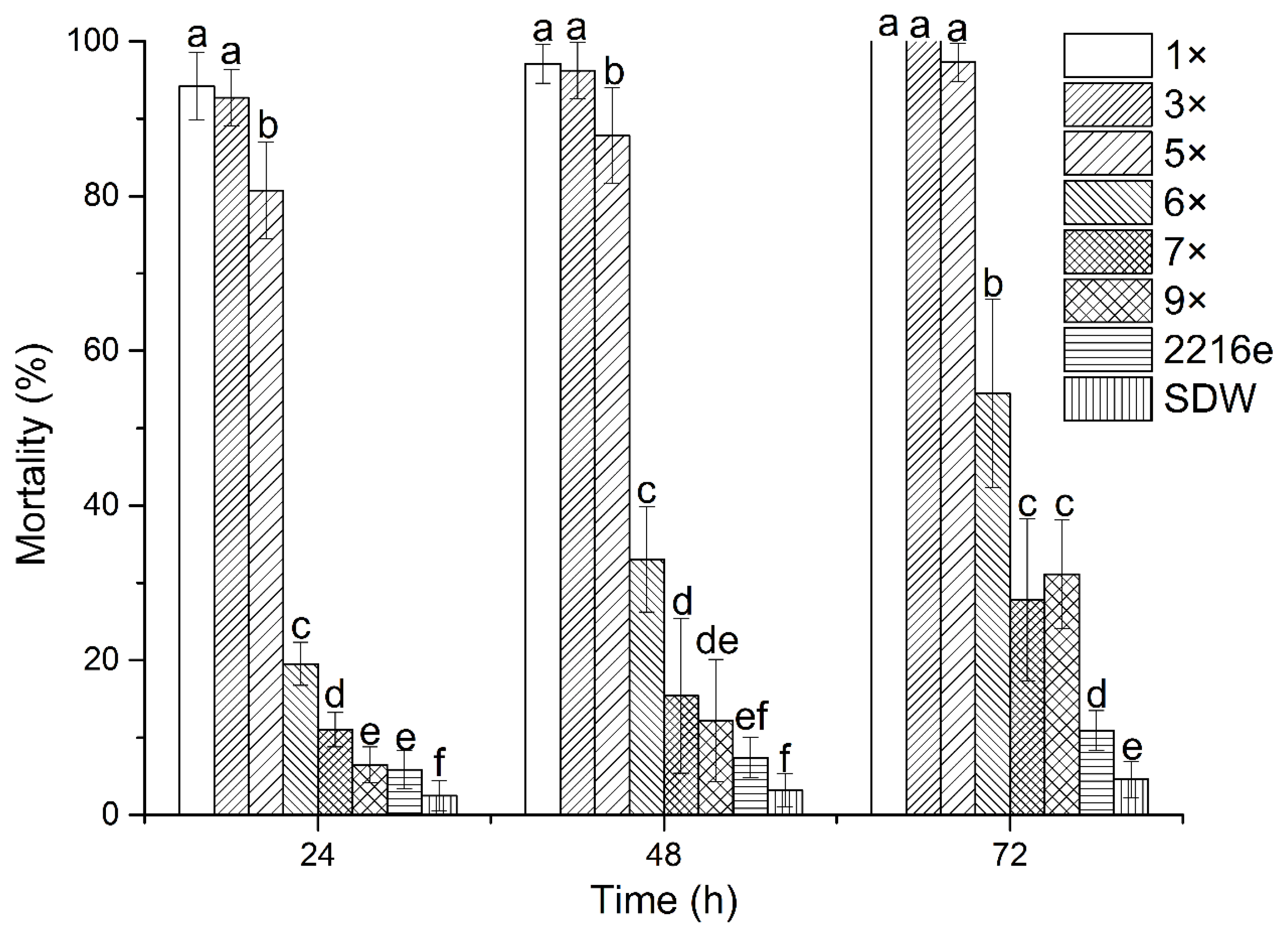
2.1. Nematicidal Activity of Culture Filtrates from P. Putida MCCC 1A00316
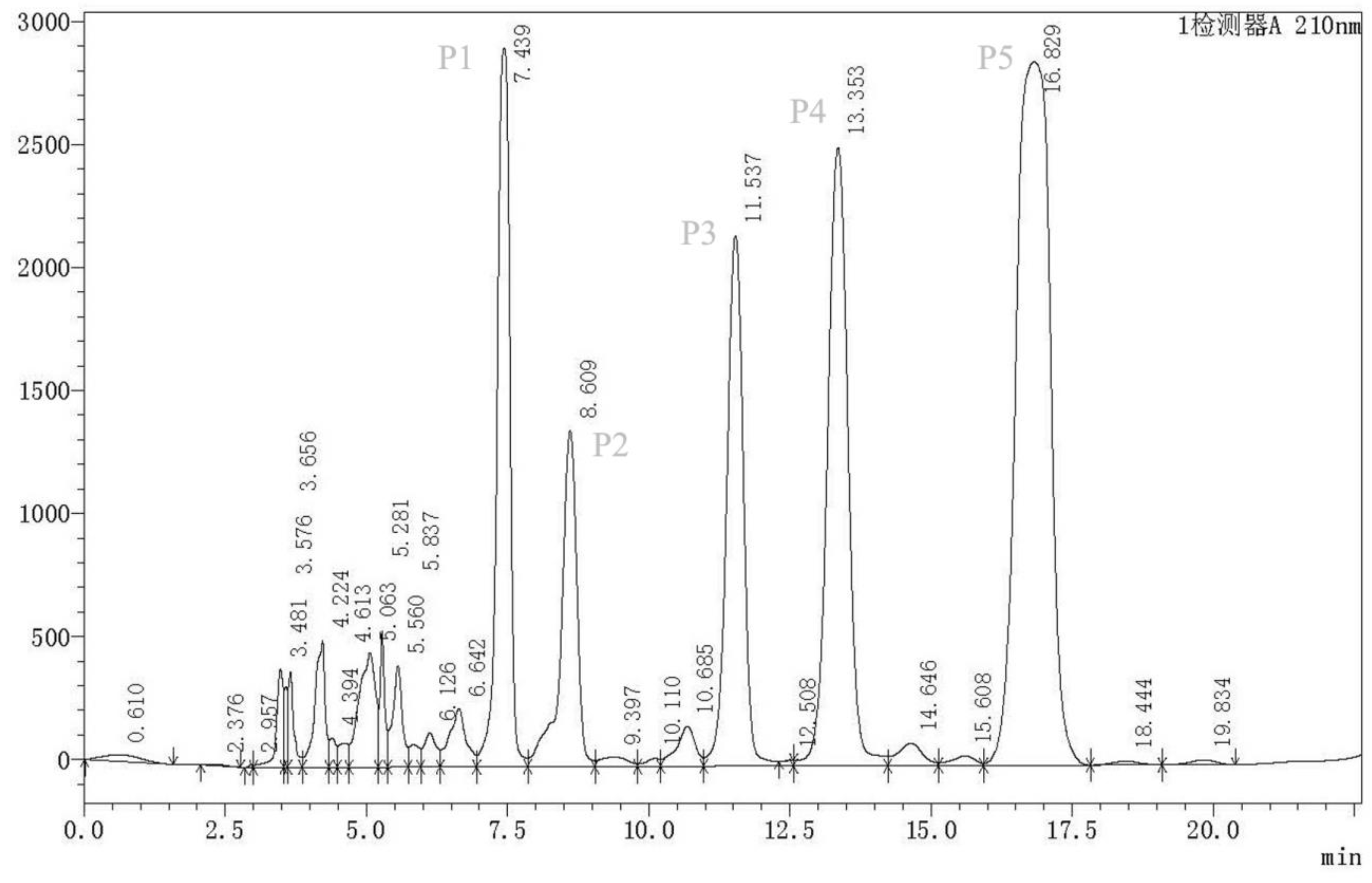
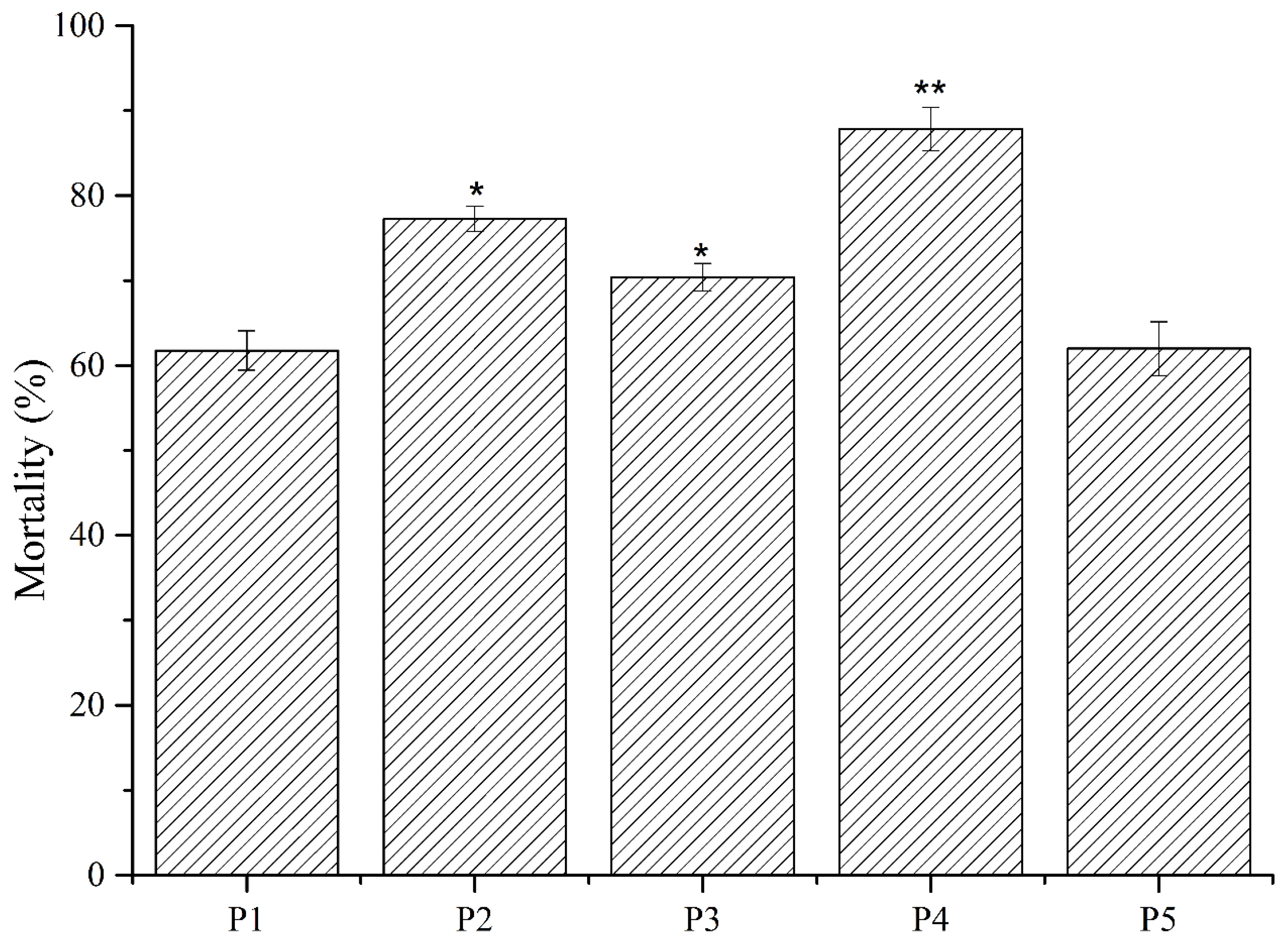
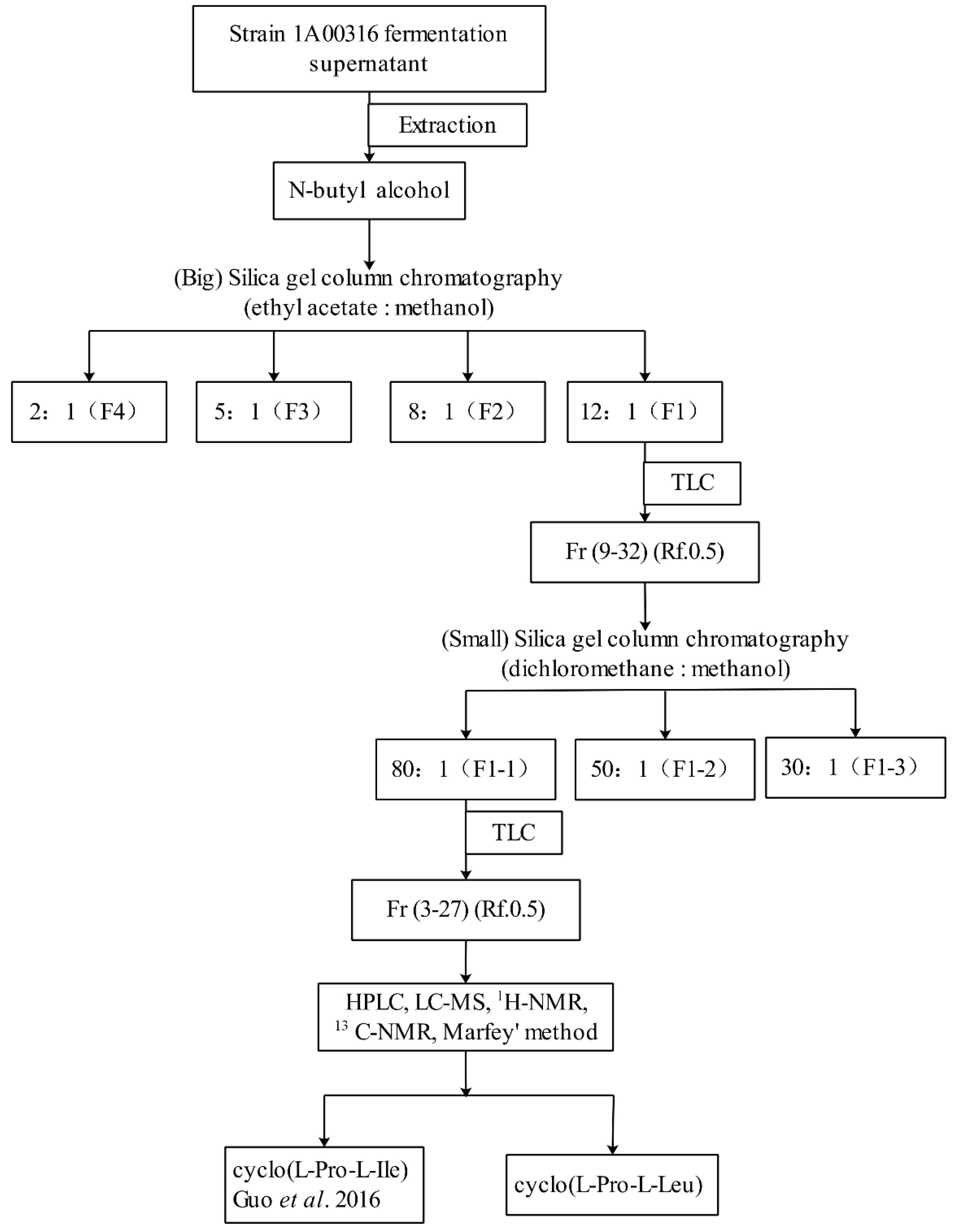
2.2. Purification of Nematicidal Compounds from Strain MCCC 1A00316
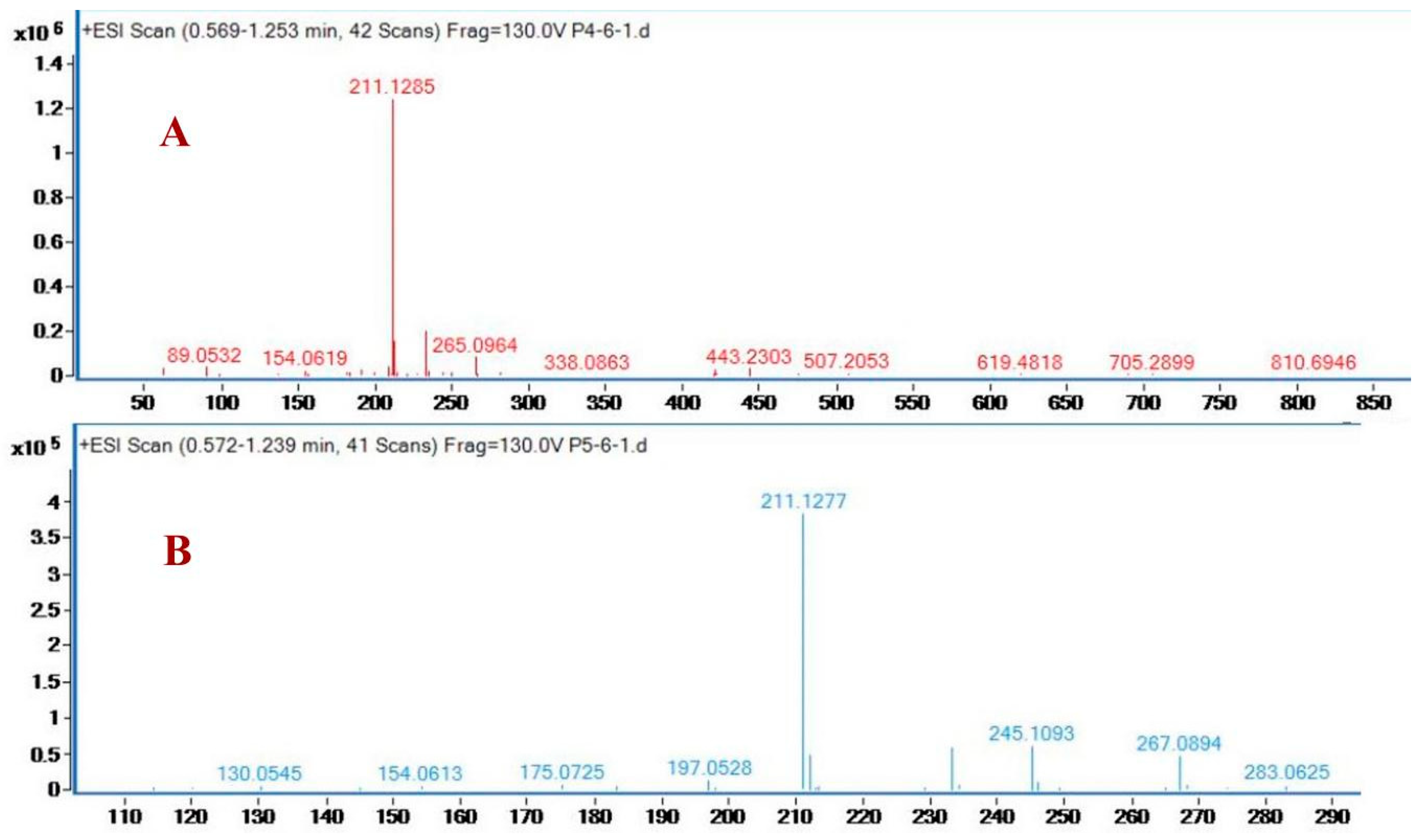
2.3. Identification of Purified Nematicidal Compounds
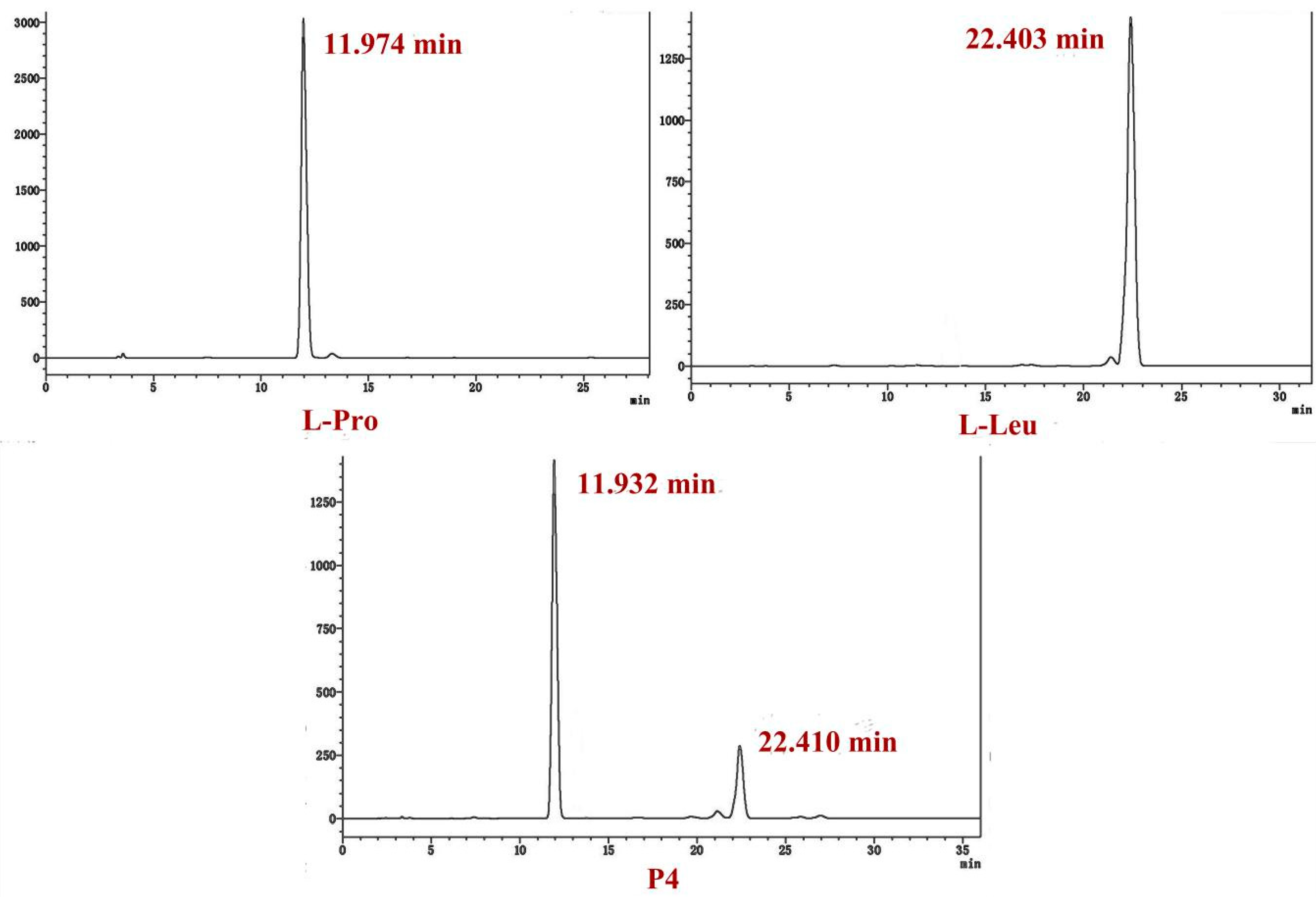
2.4. Determination of the Absolute Configuration of the Two Cyclodipetides
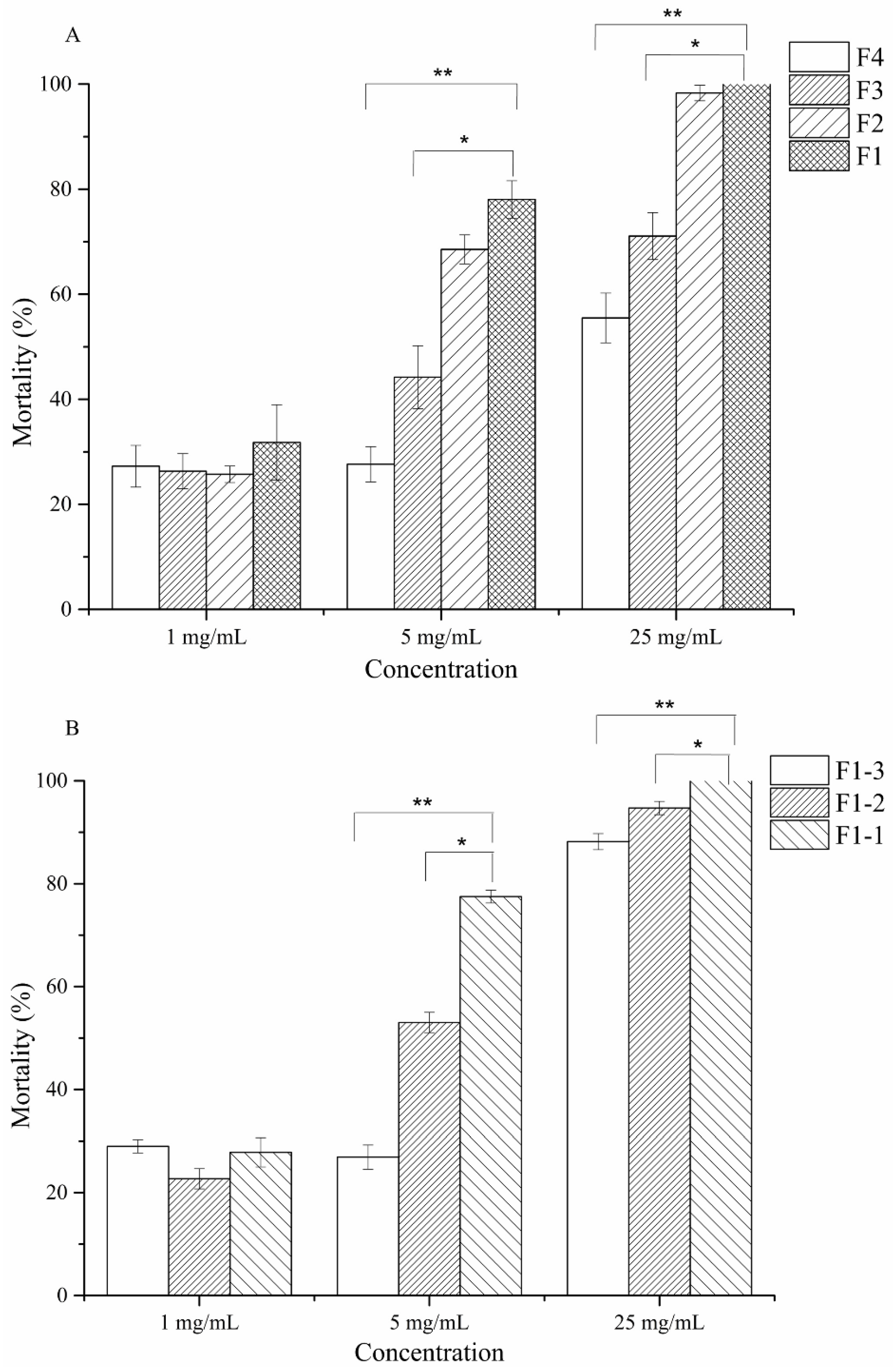
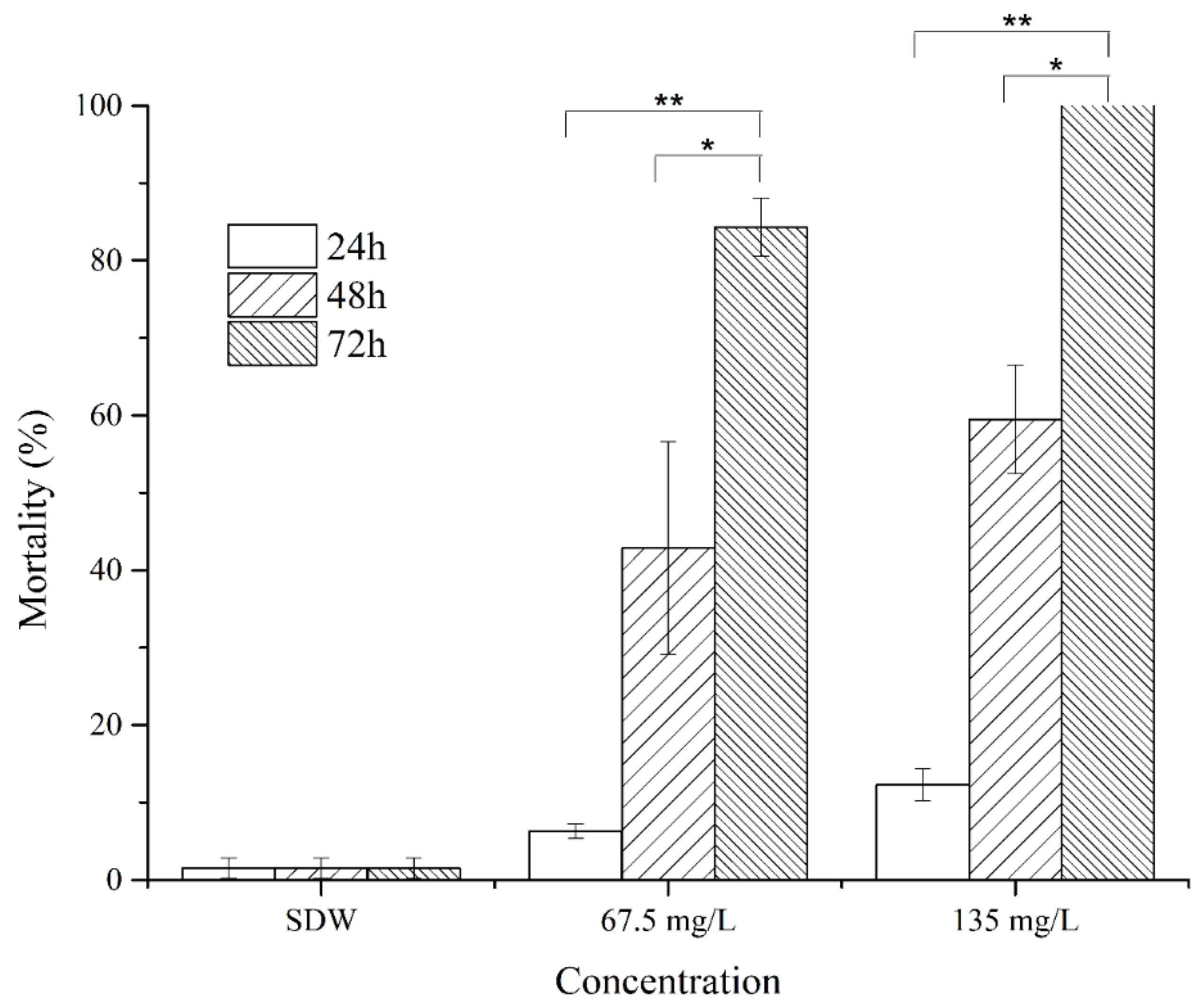
2.5. Anti-M. Incognita Characteristics of Cyclo(l-Pro–l-Leu) from P. putida MCCC 1A00316
3. Discussion
4. Materials and Methods
4.1. Collection of M. Incognita Eggs and J2
4.2. Preparation of the Fermentation Supernatant of P. putida MCCC 1A00316
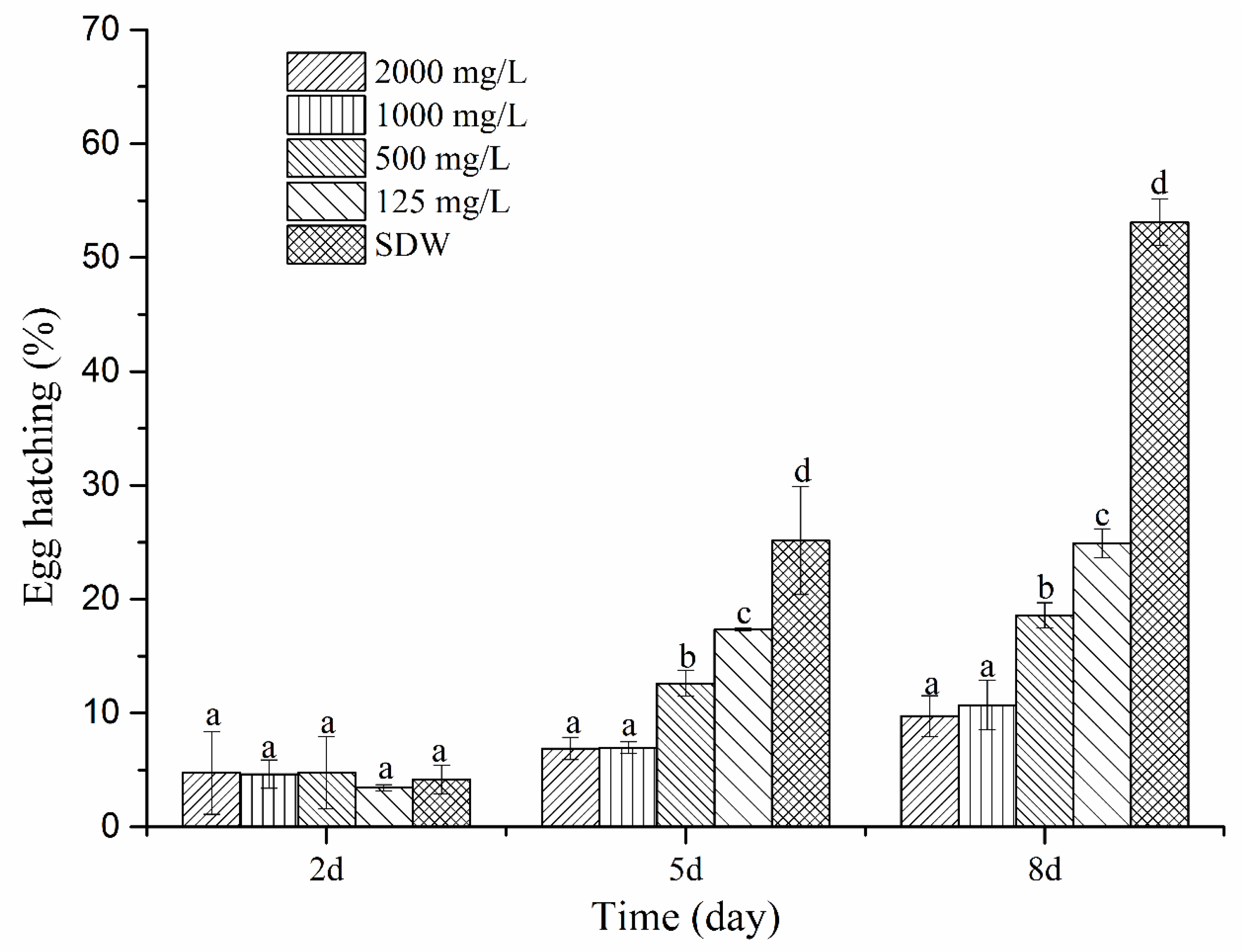
4.3. Effect of the Fermentation Supernatant of P. putida MCCC 1A00316 on Egg-Hatching
4.4. Effects of Strain MCCC 1A00316 Culture Filtrates on M. incognita J2
4.5. Crude Extraction of Nematicidal Compounds
4.6. Purification of Two Cyclodipetide Compounds
4.7. High-Performance Liquid Chromatography
4.8. Liquid Chromatography Time-of-Flight Mass Spectrometry
4.9. Identification of Nematicidal Compounds through Nuclear Magnetic Resonance Spectroscopy
4.10. Determination of the Absolute Configuration of Compounds
4.11. Nematicidal Activity of Cyclodipetides from Strain MCCC 1A00316
4.12. Effect of Cyclo(l-Pro–l-Leu) on the Egg-Hatching of M. incognita
4.13. Chemotaxis of M. incognita J2 to cyclo(l-Pro–l-Leu)
4.14. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lahm, G.P.; Desaeger, J.; Smith, B.K.; Pahutski, T.F.; Rivera, M.A.; Meloro, T.; Kucharczyk, R.; Lett, R.M.; Daly, A.; Smith, B.T.; et al. The discovery of fluazaindolizine: A new product for the control of plant parasitic nematodes. Bioorg. Med. Chem. Lett. 2017, 27, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.A.; Iqbal, A.; Mahmood, I. Effects of Pseudomonas fluorescens and fertilizers on the reproduction of Meloidogyne incognita and growth of tomato. Appl. Soil. Ecol. 2001, 16, 179–185. [Google Scholar] [CrossRef]
- Adekunle, O.K.; Akinlua, A. Nematicidal effects of Leucaena leucocephala and Gliricidia sepium extracts on Meloidogyne incognita infecting okra. J. Agric. Sci. 2007, 52, 53–63. [Google Scholar] [CrossRef]
- Ali, S.S. Estimation of unavoidable yield losses in certain rabi pulse crops due to the root-knot nematode, Meloidogyne javanica. Trends Biosci. 2009, 2, 48–49. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Den nijs, L.; Tahna Maafi, Z. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Noling, J.W.; Becker, J.O. The challenge of research and extension to define and implement alternatives to methyl bromide. J. Nematol. 1994, 26, 573. [Google Scholar] [PubMed]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Aoudia, H.; Ntalli, N.; Aissani, N.; Yahiaoui-Zaidi, R.; Caboni, P. Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. J. Agric. Food Chem. 2012, 60, 11675–11680. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.M.; Roberts, P.A.; Perry, R.N. 13. Mechanisms and genetics of resistance. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI: Oxfordshire, UK, 2009; Volume 301, ISBN 9781845934927. [Google Scholar]
- Zhou, L.; Yuen, G.; Wang, Y.; Wei, L.; Ji, G. Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop Prot. 2016, 84, 8–13. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Kirienko, N.V.; Ausubel, F.M.; Ruvkun, G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 1821–1826. [Google Scholar] [CrossRef]
- Chen, Z.X.; Dickson, D.W. Review of Pasteuria penetrans: Biology, ecology, and biological control potential. J. Nematol. 1998, 30, 313. [Google Scholar] [PubMed]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; li, C.; Zhao, X. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci. Rep. UK 2016, 6, 28756. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, W.; Zhang, P.; Ruan, W.; Zhu, X. nematicidal activity of chaetoglobosin A poduced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2012, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jing, X.; Peng, W.L.; Nie, Q.; Zhai, Y.; Shao, Z.; Zheng, L.; Cai, M.; Li, G.; Zou, H. Comparative genomic and functional analyses: Unearthing the diversity and specificity of nematicidal factors in Pseudomonas putida strain MCCC 1A00316. Sci. Rep. UK 2016, 6, 29211. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Huang, D.; Cheng, W.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Multiple modes of nematode control by volatiles of Pseudomonas putida MCCC 1A00316 from Antarctic soil against Meloidogyne incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.D.; Hsu, P.C.L.; Richardson, A.E.; Hurst, M.R.H.; Hill, J.E. Plant assimilation of phosphorus from an insoluble organic form is improved by addition of an organic anion producing Pseudomonas sp. Soil Biol. Biochem. 2014, 68, 263–269. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Pu, S.; Qian, S. Chemical constituents from scolopendra multidens (I). Chin. Tradit. Herb. Drugs 2013, 44, 1726–1729. [Google Scholar]
- Lee, Y.S.; Naning, K.W.; Nguyen, X.H.; Kim, S.B.; Moon, J.H.; Kim, K.Y. Ovicidal activity of lactic acid produced by Lysobacter capsici YS1215 on eggs of root-knot nematode, Meloidogyne incognita. J. Microbiol. Biotechnol. 2014, 24, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Regaieg, H.; Ciancio, A.; Raouani, N.H.; Grasso, G.; Rosso, L. Effects of culture filtrates from the nematophagous fungus Verticillium leptobactrum on viability of the root-knot nematode Meloidogyne incognita. World J. Microb. Biot. 2010, 26, 2285–2289. [Google Scholar] [CrossRef]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Rhee, K.H. Purification and identification of an antifungal agent from Streptomyces sp. KH-614 antagonistic to rice blast fungus, Pyricularia oryzae. J. Microbiol. Biotechnol. 2003, 13, 984–988. [Google Scholar]
- Yan, P.S.; Song, Y.; Sakuno, E.; Nakajima, H.; Nakagawa, H.; Yabe, K. Cyclo (l-leucyl-l-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microb. 2004, 70, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.H. Isolation and characterization of Streptomyces sp. KH-614 producing anti-VRE (vancomycin-resistant enterococci) antibiotics. J. Gen. Appl. Microbiol. 2002, 48, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Degrassi, G.; Aguilar, C.; Bosco, M.; Zahariev, S.; Pongor, S.; Venturi, V. Plant growth-promoting Pseudomonas putida WCS358 produces and secretes four cyclic dipeptides: Cross-talk with quorum sensing bacterial sensors. Curr. Microbiol. 2002, 45, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Jamal, Q.; Cho, J.Y.; Moon, J.H.; Munir, S.; Anees, M.; Kim, K.Y. Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita. Molecules 2017, 22, 1839. [Google Scholar] [CrossRef]
- Barker, K.R.; Carter, C.C.; Sasser, J.N. An Advanced Treatise on Meloidogyne. Volume II: Methodology; United States Agency for International Development: Washington, DC, USA, 1985; Volume 223, ISBN 0931901022.
- Southey, J.F. Laboratory Methods for Work with Plant and Soil Nematodes, 6th ed.; HMSO: London, UK, 1986; Agriculture, Fisheries and Food, 202. Reference Book 402.
- Morisaki, H.; Nagai, S.; Ohshima, H.; Ikemoto, E.; Kogure, K. The effect of motility and cell-surface polymers on bacterial attachment. Microbiology 1999, 145, 2797–2802. [Google Scholar] [CrossRef]
- Marfey, P. Determination of D-amino acids. II. Use of a bifunctional reagent,1,5-difluoro-2, 4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Saeki, S.; Yamamoto, M.; Iino, Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 2001, 204, 1757–1764. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Yu, Z.; Zhang, J. Cyclo(l-Pro–l-Leu) of Pseudomonas putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne incognita. Molecules 2019, 24, 768. https://doi.org/10.3390/molecules24040768
Zhai Y, Shao Z, Cai M, Zheng L, Li G, Yu Z, Zhang J. Cyclo(l-Pro–l-Leu) of Pseudomonas putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne incognita. Molecules. 2019; 24(4):768. https://doi.org/10.3390/molecules24040768
Chicago/Turabian StyleZhai, Yile, Zongze Shao, Minmin Cai, Longyu Zheng, Guangyu Li, Ziniu Yu, and Jibin Zhang. 2019. "Cyclo(l-Pro–l-Leu) of Pseudomonas putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne incognita" Molecules 24, no. 4: 768. https://doi.org/10.3390/molecules24040768
APA StyleZhai, Y., Shao, Z., Cai, M., Zheng, L., Li, G., Yu, Z., & Zhang, J. (2019). Cyclo(l-Pro–l-Leu) of Pseudomonas putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne incognita. Molecules, 24(4), 768. https://doi.org/10.3390/molecules24040768





