Properties and Digestibility of Octenyl Succinic Anhydride-Modified Japonica-Type Waxy and Non-Waxy Rice Starches
Abstract
:1. Introduction
2. Results and Discussion
2.1. DS of OSA-Modified Rice Starch
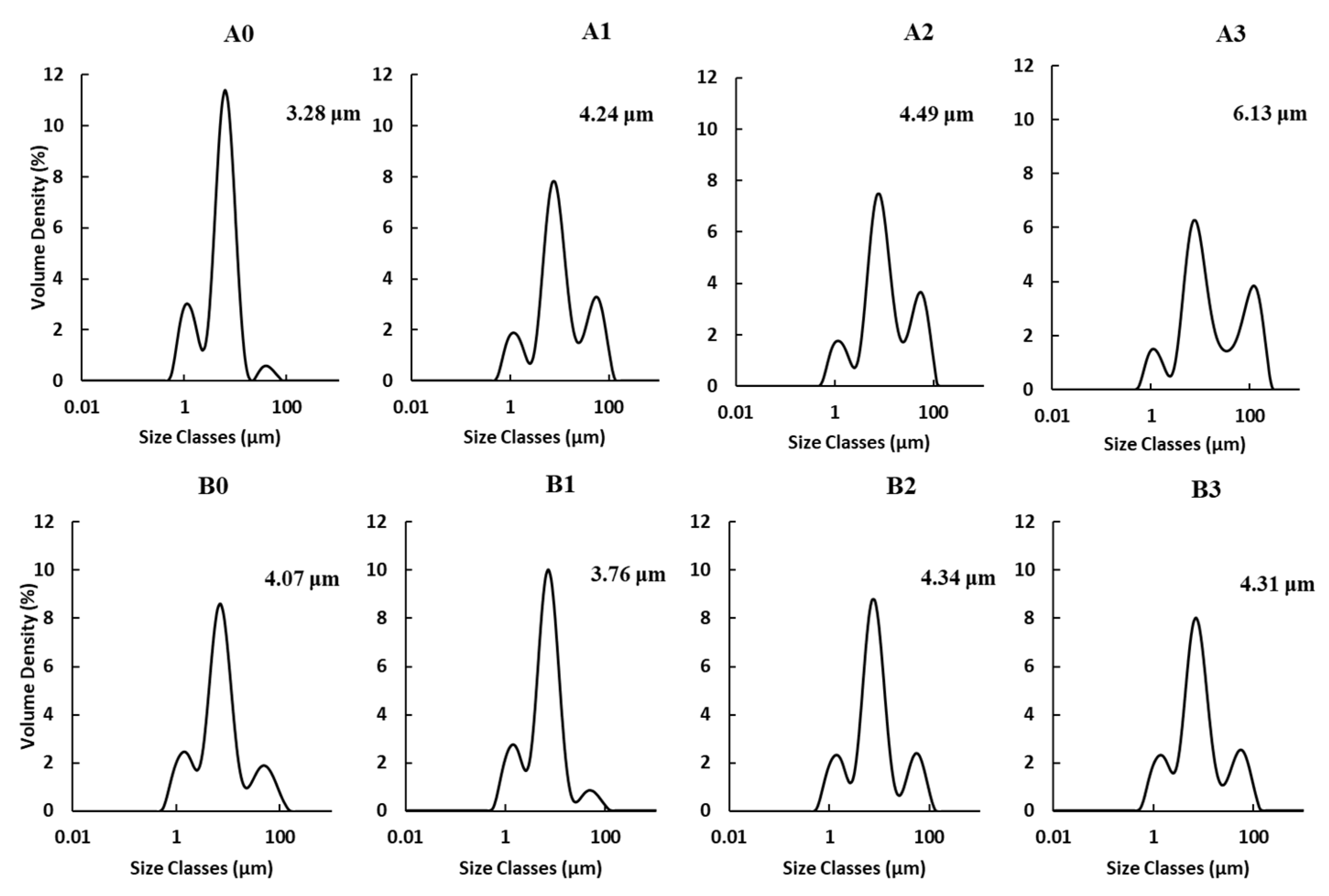
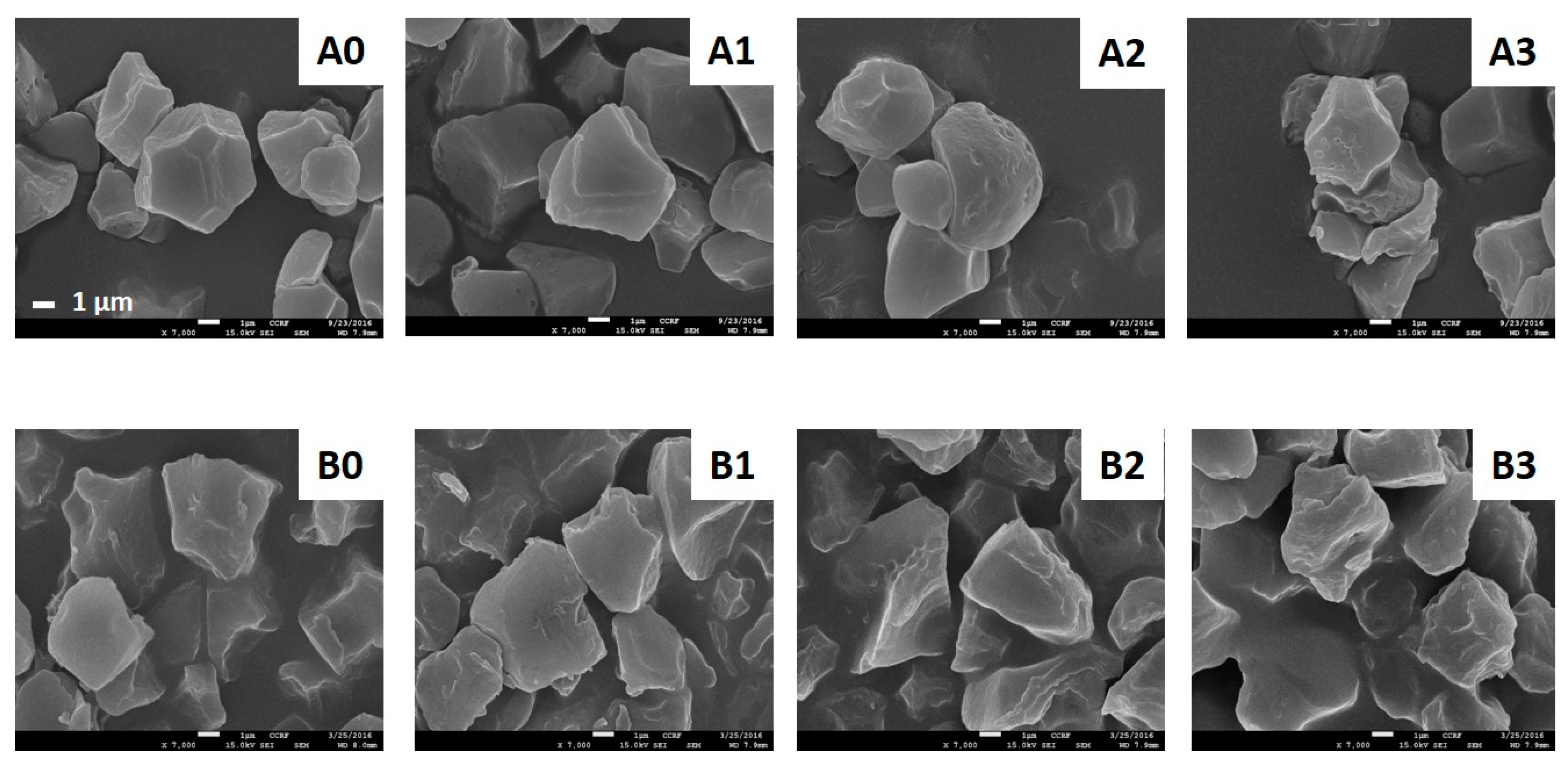
2.2. Particle Size Distribution and Morphology
2.2.1. Particle Size Distribution
2.2.2. Scanning Electron Microscopy (SEM)
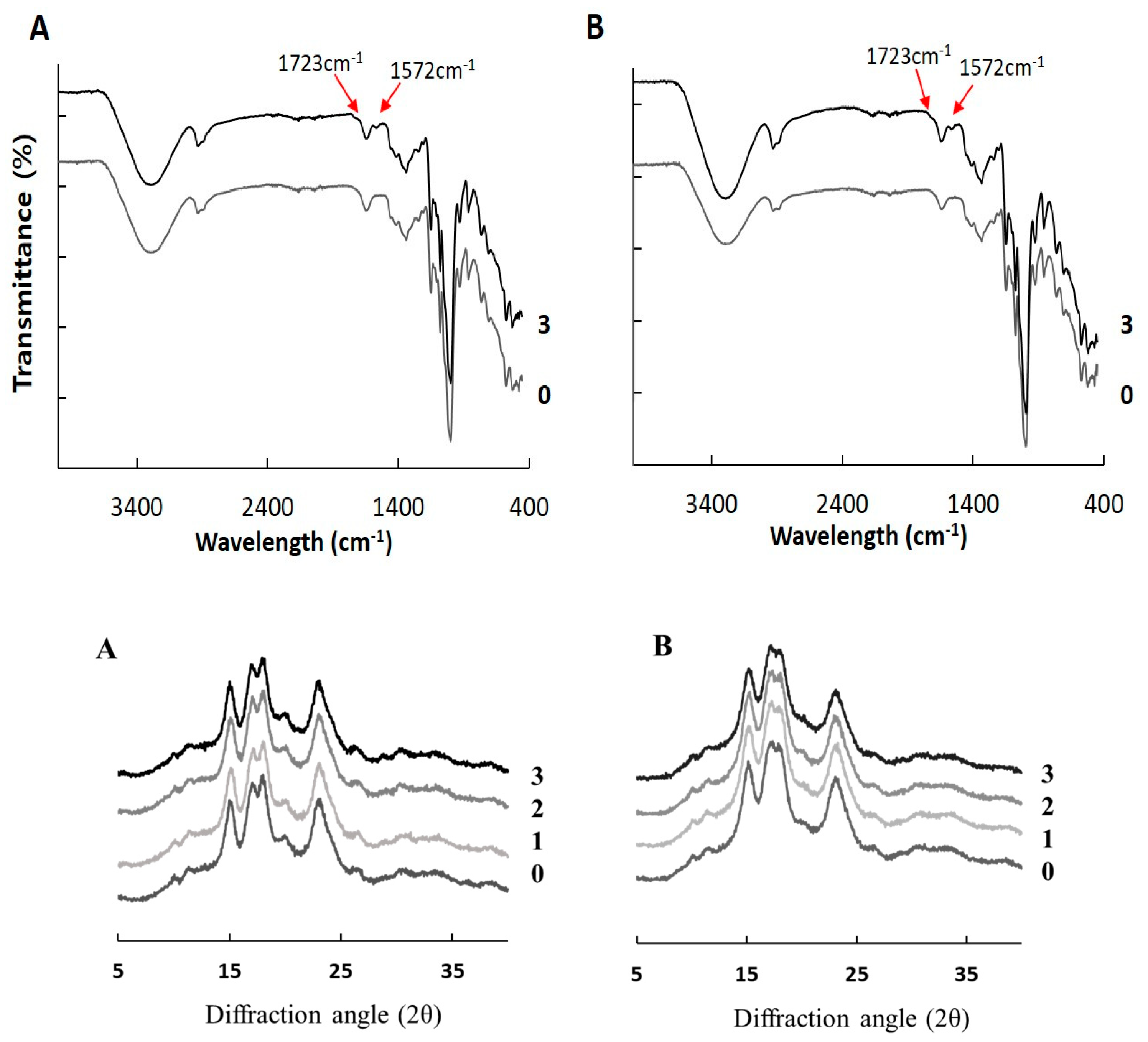
2.3. Crystalline Structure
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. X-ray Diffraction
2.4. Chain Length Distribution of Amylopectin
2.5. In Vitro Digestibility
2.6. Pasting and Thermal Properties
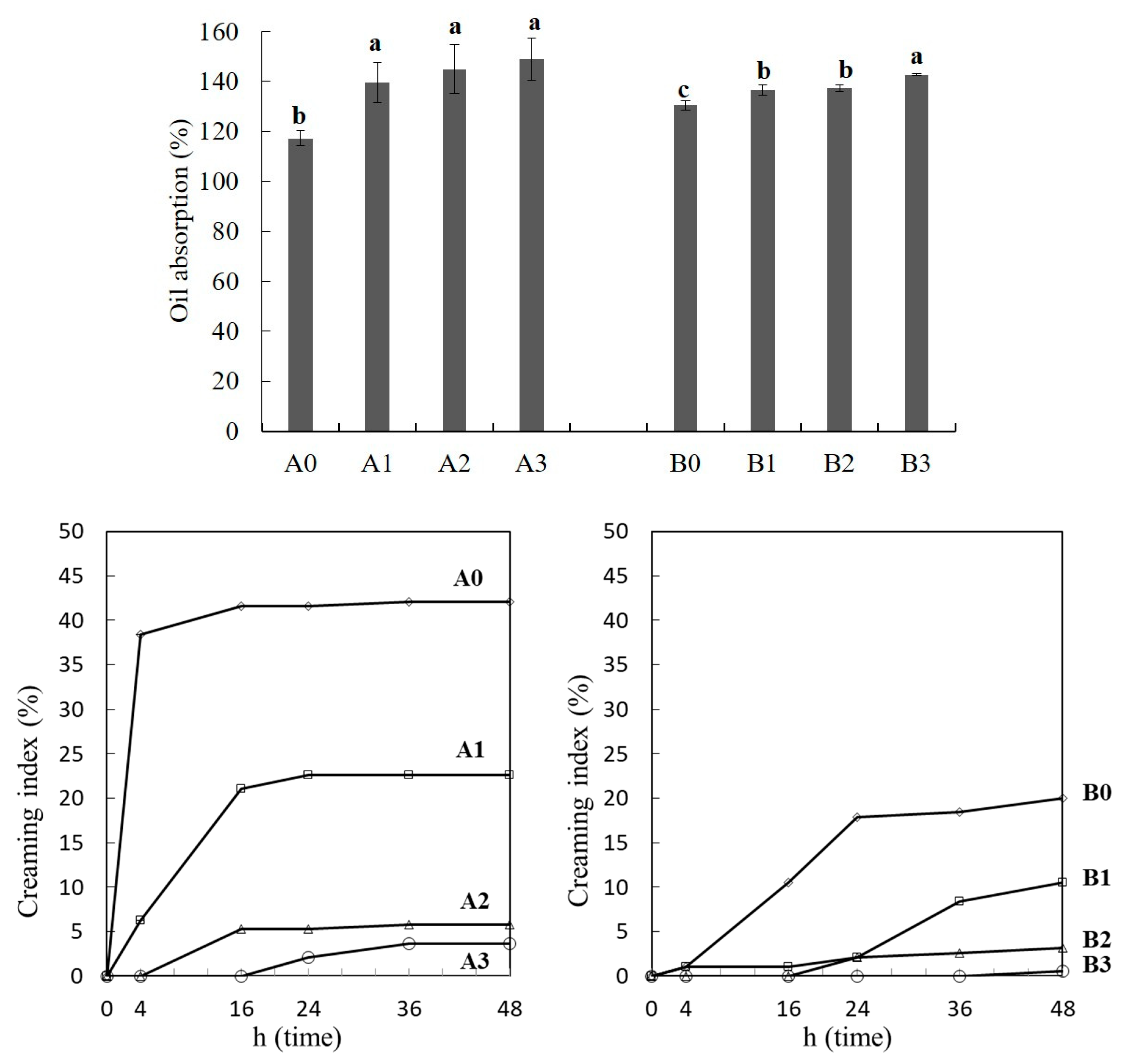
2.7. Emulsion Stability and Oil Absorption Capacity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Purification of Rice Starch
4.3. Preparation of OSA-Modified Starch and Determination of DS
4.4. Particle Size Distribution
4.5. Digestibility of OSA-Modified Rice Starch
4.6. Morphology
4.7. Crystalline Structure Analysis
4.7.1. Fourier-Transform Infrared Spectroscopy
4.7.2. X-ray Diffractometry
4.8. Determination of Chain Length Distribution of Amylopectin
4.9. Analysis of Pasting and Thermal Properties
4.10. Determination of Oil Absorption Capacity and Emulsion Stability
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Zhao, S.; Xiong, S. Molecular properties of octenyl succinic esters of mechanically activated Indica rice starch. Starch/Stärke 2013, 65, 453–460. [Google Scholar] [CrossRef]
- Hui, R.; Qi-he, C.; Ming-liang, F.; Qiong, X.; Guo-qing, H. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Hasjim, J.; Schäfer, C.; Gilbert, R.G. Structures of octenyl succinylated starches: Effects on emulsions containing β-carotene. Carbohyd. Polym. 2014, 112, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohyd. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Li, G.Y.; Xie, Q.T.; Zhang, B.; Li, X.M.; Pan, Y.; Chen, H.Q. Evaluation studies on the combined effect of hydrothermal treatment and octenyl succinylation on the physic-chemical, structure and digestibility characteristics of sweet potato starch. Food Chem. 2018, 256, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohyd. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Song, X.; He, G.; Ruan, H.; Chen, Q. Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch/Stärke 2006, 58, 109–117. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Shi, J.; Huang, Z.; Zhang, Y.; Huang, A.; Yang, M.; Qin, X.; Shen, F. Structure and functional properties of octenyl succinic anhydride modified starch prepared by a non-conventional technology. Starch/Stärke 2016, 68, 151–159. [Google Scholar] [CrossRef]
- Jiang, S.; Dai, L.; Qin, Y.; Xiong, L.; Sun, Q. Preparation and characterization of octenyl succinic anhydride modified taro starch nanoparticles. PLoS ONE 2016, 11, e0150043. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cui, F.; Ping, L.; Song, J.; Ravee, Y.; Jin, L.; Xue, Y.; Xu, J.; Li, G.; et al. Production of octenyl succinic anhydride-modified waxy corn starch and its characterization. J. Agric. Food Chem. 2008, 56, 11499–11506. [Google Scholar] [CrossRef]
- Webb, B.D.; Bollich, C.N.; Adair, C.R.; Jonston, T.H. Characteristics of rice varieties in the U.S. department of agriculture collection. Crop Sci. 1968, 8, 361–365. [Google Scholar] [CrossRef]
- Takeda, K.; Nakajima, K.; Saito, K. Difference between the size of waxy and non-waxy kernel in the F1 rice plant. Jpn. J. Breed. 1978, 28, 225–233. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, L.; Xie, F.; Yu, L.; Li, B. Preparation and characterisation of octenyl succinate starch as a delivery carrier for bioactive food components. Food Chem. 2011, 126, 1218–1225. [Google Scholar] [CrossRef]
- Jung, M.H.; Youn, K.S. Preparation and physicochemical characteristics of octenyl succinated rice starches based on amylose content. Korean J. Food Sci. Technol. 2012, 44, 577–582. [Google Scholar] [CrossRef]
- Cheuk, S.Y.; Shih, F.F.; Champagne, E.T.; Daigle, K.W.; Patindol, J.A.; Mattison, C.P.; Boue, S.M. Nano-encapsulation of coenzyme Q10 using octenyl succinic anhydride modified starch. Food Chem. 2015, 174, 585–590. [Google Scholar] [CrossRef]
- Peng, S.; Xue, L.; Leng, X.; Yang, R.; Zhang, G.; Hamaker, B.R. Slow digestion property of octenyl succinic anhydride modified waxy maize starch in the presence of tea polyphenols. J. Agric. Food Chem. 2015, 63, 2820–2829. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martinez, M.; Marefati, A.; Sjӧӧ, M.; Rayner, M. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- No, J.; Shin, M. Physicochemical, pasting, and emulsification properties of octenyl succinic anhydride modified waxy rice starch. Korean J. Food Sci. Technol. 2017, 49, 463–468. [Google Scholar]
- Shih, F.F.; Daigle, K.W. Gelatinization and pasting properties of rice starch modified with 2-octen–1-ylsuccinic anhydride. Food Nahrung 2003, 47, 64–67. [Google Scholar] [CrossRef]
- He, G.Q.; Song, X.Y.; Ruan, H.; Chen, F. Octenyl succinic anhydride modified early indica rice starches differing in amylose content. J. Agric. Food Chem. 2006, 54, 2775–2779. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Q.; Luo, F.X.; Fu, X.; Jiang, H.; Jane, J.L. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohyd. Polym. 2011, 84, 1276–1281. [Google Scholar] [CrossRef]
- Chen, M.; Yin, T.; Chen, Y.; Xiong, S.; Zhao, S. Preparation and characterization of octenyl succinic anhydride modified waxy rice starch by dry media milling. Starch/Stärke 2014, 66, 985–991. [Google Scholar] [CrossRef]
- Timgren, A.; Rayner, M.; Dejmek, P.; Marku, D.; Sjöö, M. Emulsion stabilizing capacity of intact starch granules modified by heat treatment or octenyl succinic anhydride. Food Sci. Nutr. 2013, 1, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Shogren, R.L.; Viswanathan, A.; Felker, F.; Gross, R.A. Distribution of octenyl succinate groups in octenyl succinic anhydride modified waxy maize starch. Starch/Stärke 2000, 52, 196–204. [Google Scholar] [CrossRef]
- Yusoff, A.; Murray, B.S. Modified starch granules as particle-stabilizers of oil-in-water emulsions. Food Hydrocoll. 2011, 25, 42–55. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Ortega-Ojeda, F.E.; Larsson, H.; Eliasson, A. Gel formation in mixtures of hydrophobically modified potato and high amylopectin potato starch. Carbohyd. Polym. 2005, 59, 313–327. [Google Scholar] [CrossRef]
- Thirathumthavorn, D.; Charoenrein, S. Thermal and pasting properties of native and acid-treated starches derivatized by 1-octenyl succinic anhydride. Carbohyd. Polym. 2006, 66, 258–265. [Google Scholar] [CrossRef]
- Bao, J.; Xing, J.; Phillips, D.L.; Corke, H. Physical properties of octenyl succinic anhydride modified rice, wheat, and potato starches. J. Agric. Food Chem. 2003, 51, 2283–2287. [Google Scholar] [CrossRef]
- Han, J.A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modified food starches. Carbohyd. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Carlos-Amaya, F.; Osorio-Diaz, P.; Agama-Acevedo, E.; Yee-Madeira, H.; Bello-Pérez, L.A. Physicochemical and digestibility properties of double-modified banana (Musa paradisiaca L.) starches. J. Agric. Food Chem. 2011, 59, 1376–1382. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Nikolić, I.; Milanović, M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015, 45, 9–17. [Google Scholar] [CrossRef]
- McClements, D.J. Comments on viscosity enhancement and depletion flocculation by polysaccharides. Food Hydrocoll. 2000, 14, 173–177. [Google Scholar] [CrossRef]
- Jeong, O.; Shin, M. Preparation and stability of resistant starch nanoparticles, using acid hydrolysis and cross-linking of waxy rice starch. Food Chem. 2018, 256, 77–84. [Google Scholar] [CrossRef]
- Williams, P.C.; Kuzina, F.D.; Hlynka, I. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970, 47, 411–420. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 2, S33–S50. [Google Scholar]
- Van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–204. [Google Scholar] [CrossRef]
- You, S.Y.; Lim, S.T.; Lee, J.H.; Chung, H.J. Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohyd. Polym. 2014, 112, 729–735. [Google Scholar] [CrossRef]
- Mun, S.; Shin, M. Physicochemical properties and molecular structure of starches purified from rice flours with different processing qualities. Food Sci. Biotechnol. 2018, 27, 1007–1014. [Google Scholar] [CrossRef]
- No, J.; Shin, M. Textural properties of mung bean starch gels prepared from whole seeds. Food Sci. Biotechnol. 2016, 25, 729–734. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| OSA Substitution of Rice Starches | DS Value | Average Chain Length | Distribution (%) | In Vitro Digestibility | Relative Crystallinity (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DP 6–12 | DP 13–24 | DP 25–36 | DP ≥ 37 | RDS (%) | SDS (%) | RS (%) | ||||
| Hopyeong 0% | - | 21.78 ± 0.14 a | 27.20 ± 0.62 a | 45.08 ± 0.55 a | 12.33 ± 0.06 a | 15.40 ± 0.01 a | 61.85 ± 0.00 a | 34.96 ± 0.23 a | 3.20 ± 0.23 d | 36.89 ± 0.46 a |
| Hopyeong 1% | 0.0147 ± 0.0000 b | 21.20 ± 0.11 b | 27.77 ± 0.77 a | 45.78 ± 0.54 a | 12.26 ± 0.16 a | 14.18 ± 0.07 b | 60.22 ± 0.69 b | 35.00 ± 0.29 a | 4.78 ± 0.40 c | 36.26 ± 0.03 a |
| Hopyeong 2% | 0.0213 ± 0.0000 a | 20.82 ± 0.08 c | 28.41 ± 0.75 a | 46.20 ± 0.65 a | 12.05 ± 0.08 a | 13.34 ± 0.01 c | 58.64 ± 0.17 c | 32.15 ± 0.29 b | 9.21 ± 0.11 b | 35.41 ± 0.03 b |
| Hopyeong 3% | 0.0258 ± 0.0000 a | 20.79 ± 0.02 c | 28.99 ± 0.72 a | 45.75 ± 0.66 a | 11.94 ± 0.17 a | 13.31 ± 0.11 c | 49.86 ± 0.29 d | 35.08 ± 0.06 a | 15.06 ± 0.34 a | 34.42 ± 0.26 c |
| Sinseonchal 0% | - | 21.91 ± 0.32 a | 27.07 ± 0.76 b | 44.34 ± 0.22 a | 12.94 ± 0.09 a* | 15.65 ± 0.45 a | 67.46 ± 0.11 a* | 32.52 ± 0.34 a* | 0.03 ± 0.23 d* | 37.48 ± 0.02 a |
| Sinseonchal 1% | 0.0140 ± 0.0000 b | 20.76 ± 0.19 b | 29.94 ± 0.76 a | 44.42 ± 0.36 a | 12.07 ± 0.13 b | 13.57 ± 0.27 bc | 66.64 ± 0.11 a* | 32.23 ± 0.06 a* | 1.12 ± 0.06 c* | 33.08 ± 0.80 b |
| Sinseonchal 2% | 0.0246 ± 0.0000 a | 21.03 ± 0.05 b | 29.40 ± 0.17 a | 44.42 ± 0.29 a | 12.12 ± 0.05 b | 14.07 ± 0.06 b* | 62.86 ± 2.13 b | 35.08 ± 1.78 a | 2.06 ± 0.34 b* | 32.27 ± 0.15 b* |
| Sinseonchal 3% | 0.0262 ± 0.0000 a | 20.64 ± 0.10 b | 30.18 ± 0.51 a | 44.47 ± 0.28 a | 12.15 ± 0.13 b | 13.20 ± 0.10 c | 61.20 ± 1.61 c | 33.61 ± 1.32 a | 5.19 ± 0.29 a* | 30.78 ± 0.39 c* |
| OSA Substitution of Rice Starches | Pasting Temp. (°C) | Viscosity (RVU) | DSC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak (P) | Final (F) | Breakdown (P-T) | Setback (F-T) | To (°C) | Tp (°) | Tc (°C) | ∆H (J/g) | ||
| Hopyeong 0% | 83.74 ± 0.62 a | 144.42 ± 0.47 d | 217.79 ± 7.48 d | 42.42 ± 1.30 d | 115.79 ± 8.31 b | 60.70 ± 0.19 a | 67.65 ± 0.12 a | 73.50 ± 0.18 a | 7.50 ± 076 a |
| Hopyeong 1% | 72.32 ± 0.05 b | 245.63 ± 1.83 c | 254.08 ± 1.41 c | 109.88 ± 2.06 c | 118.33 ± 1.18 b | 60.01 ± 0.40 a | 67.12 ± 0.14 b | 73.74 ± 0.18 a | 6.89 ± 0.36 a |
| Hopyeong 2% | 70.10 ± 0.59 c | 325.29 ± 1.00 b | 269.00 ± 0.12 b | 183.21 ± 0.77 b | 126.92 ± 0.12 ab | 59.09 ± 0.01 b | 66.73 ± 0.00 c | 73.86 ± 0.05 a | 6.65 ± 0.23 a |
| Hopyeong 3% | 68.75 ± 0.08 d | 393.29 ± 1.24 a | 280.42 ± 2.47 a | 247.33 ± 1.53 a | 134.46 ± 2.18 a | 58.06 ± 0.22 c | 66.12 ± 0.10 d | 73.69 ± 0.33 a | 7.35 ± 0.64 a |
| Sinseonchal 0% | 71.81 ± 0.21 a* | 279.92 ± 2.83 d* | 193.00 ± 0.59 d | 97.92 ± 3.06 d* | 11.00 ± 0.83 c* | 65.20 ± 0.06 a* | 70.96 ± 0.09 a* | 81.03 ± 3.17 a | 11.99 ± 3.16 a |
| Sinseonchal 1% | 71.00 ± 0.08 b* | 334.67 ± 2.59 c* | 208.92 ± 0.94 c* | 153.50 ± 3.77 b* | 27.75 ± 5.42 ab* | 61.74 ± 0.01 a | 67.78 ± 0.13 b* | 77.02 ± 0.67 a | 8.60 ± 0.40 a * |
| Sinseonchal 2% | 70.98 ± 0.32 b | 352.46 ± 0.53 b* | 255.83 ± 0.71 b* | 125.75 ± 1.41 c* | 29.12 ± 1.59 a* | 59.42 ± 2.57 a | 66.27 ± 1.58 b | 76.30 ± 0.79 a | 7.66 ± 1.32 a |
| Sinseonchal 3% | 66.13 ± 0.21 c* | 492.96 ± 8.54 a* | 282.25 ± 4.01 a | 226.75 ± 5.66 a | 16.04 ± 6.89 bc* | 60.05 ± 1.52 a | 66.30 ± 1.28 b | 74.98 ± 1.16 a | 8.36 ± 0.02 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
No, J.; Mun, S.; Shin, M. Properties and Digestibility of Octenyl Succinic Anhydride-Modified Japonica-Type Waxy and Non-Waxy Rice Starches. Molecules 2019, 24, 765. https://doi.org/10.3390/molecules24040765
No J, Mun S, Shin M. Properties and Digestibility of Octenyl Succinic Anhydride-Modified Japonica-Type Waxy and Non-Waxy Rice Starches. Molecules. 2019; 24(4):765. https://doi.org/10.3390/molecules24040765
Chicago/Turabian StyleNo, Junhee, Saehun Mun, and Malshick Shin. 2019. "Properties and Digestibility of Octenyl Succinic Anhydride-Modified Japonica-Type Waxy and Non-Waxy Rice Starches" Molecules 24, no. 4: 765. https://doi.org/10.3390/molecules24040765
APA StyleNo, J., Mun, S., & Shin, M. (2019). Properties and Digestibility of Octenyl Succinic Anhydride-Modified Japonica-Type Waxy and Non-Waxy Rice Starches. Molecules, 24(4), 765. https://doi.org/10.3390/molecules24040765





