HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Drying Kinetics
2.2. Volatile Constituents Profile of Fresh True Lavender Leaves
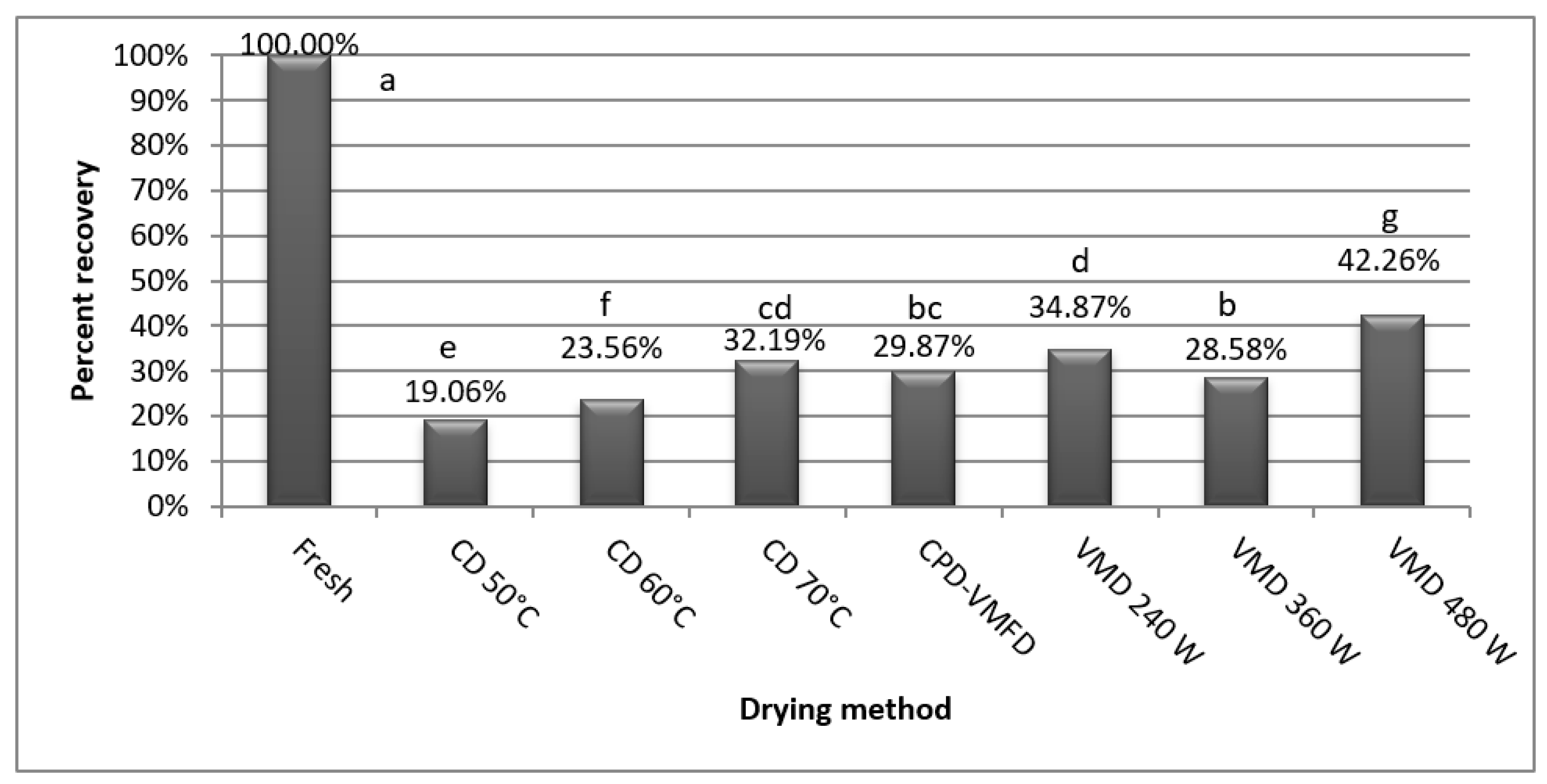
2.3. Effect of the Drying Methods on the Quantity of True Lavender Leaves Volatile Constituents
3. Materials and Methods
3.1. Plant Material
3.2. Drying Methods
3.2.1. Convective Drying (CD)
3.2.2. Vacuum-Microwave Drying (VMD)
3.2.3. Combined Drying—Pre-Drying by Convective Drying with Vacuum-Microwave Finishing-Drying (CPD-VMFD)
3.3. Modeling of Drying Kinetics
3.4. Solid-Phase Micro Extraction (SPME) Analysis
3.5. GC-MS Analysis
3.6. Hydrodistillation of Essential Oil (EO)
3.7. Identification and Quantification of Volatile Compounds
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Preedy, V.R. Essential Oils in Food Preservation, Flavor and Safety; Elsevier Inc.: London, UK, 2016; ISBN 9780124166417. [Google Scholar]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: a review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Seol, G.H. Lavandula angustifolia Mill. Oil and Its Active Constituent Linalyl Acetate Alleviate Pain and Urinary Residual Sense after Colorectal Cancer Surgery: A Randomised Controlled Trial. Evidence-based Complement. Altern. Med. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tomi, K.; Kitao, M.; Murakami, H.; Matsumura, Y.; Hayashi, T. Classification of lavender essential oils: sedative effects of Lavandula oils. J. Essent. Oil Res. 2018, 30, 56–68. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 1–44. [Google Scholar] [CrossRef]
- Śmigielski, K.; Prusinowska, R.; Raj, A.; Sikora, M.; Woliñska, K.; Gruska, R. Effect of drying on the composition of essential oil from Lavandula angustifolia. J. Essent. Oil-Bearing Plants 2011, 14, 532–542. [Google Scholar] [CrossRef]
- Husnu Can Baser, K.; Buchbauer, G. Handbook of Essential Oils. Science, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18–27. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Kirimer, N.; Mokhtarzadeh, S.; Demirci, B.; Goger, F.; Khawar, K.M.; Demirci, F. Phytochemical profiling of volatile components of Lavandula angustifolia Miller propagated under in vitro conditions. Ind. Crops Prod. 2017, 96, 120–125. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Arrillaga, I.; Eisenreich, W. Dynamics of monoterpene formation in spike lavender plants. Metabolites 2017, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.P.; Melo, E.C.; Radünz, L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plants Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K. Losses of essential oils and antioxidants during the drying of herbs and spices. A review. Eng. Sci. Technol. 2015, 2, 51–62. [Google Scholar] [CrossRef][Green Version]
- Figiel, A.; Michalska, A. Overall Quality of Fruits and Vegetables Products Affected by the Drying Processes with the Assistance of Vacuum-Microwaves. Int. J. Mol. Sci. 2017, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of oregano essential oil (Origanum vulgare) as affected by drying method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Szumny, A.; Figiel, A.; Carbonell-barrachina, A.A. Composition of rosemary essential oil (Rosmarinus officinalis) as affected by drying method. J. Food Eng. 2010, 97, 253–260. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Szumny, A.; Figiel, A.; Jałoszyński, K.; Adamski, M.; Carbonell-barrachina, Á.A. Effects of vacuum level and microwave power on rosemary volatile composition during vacuum—microwave drying. J. Food Eng. 2011, 103, 219–227. [Google Scholar]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food Chem. 2011, 126, 691–697. [Google Scholar] [CrossRef]
- Motevali, A.; Younji, S.; Chayjan, R.A.; Aghilinategh, N.; Banakar, A. Drying kinetics of dill leaves in a convective dryer. Int. Agrophysics 2013, 27, 39–47. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Sánchez-Rodríguez, L.; Szumny, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of Cantharellus cibarius Fr. as affected by drying method. J. Sci. Food Agric. 2017, 97, 5223–5232. [Google Scholar] [CrossRef] [PubMed]
- Tulek, Y. Drying kinetics of oyster mushroom (Pleurotus ostreatus) in a convective hot air dryer. J. Agric. Sci. Technol. 2011, 13, 655–664. [Google Scholar]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Nöfer, J.; Lech, K.; Figiel, A.; Szumny, A.; Carbonell-Barrachina, Á.A. The Influence of Drying Method on Volatile Composition and Sensory Profile of Boletus edulis. J. Food Qual. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Wojdyło, A.; Szarycz, M.; Carbonell-Barrachina, Á.A. Drying of Garlic Slices Using Convective Pre-drying and Vacuum-Microwave Finishing Drying: Kinetics, Energy Consumption, and Quality Studies. Food Bioprocess Technol. 2014, 7, 398–408. [Google Scholar] [CrossRef]
- Calín-Sanchez, Á.; Figiel, A.; Szarycz, M.; Lech, K.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A. Drying Kinetics and Energy Consumption in the Dehydration of Pomegranate (Punica granatum L.) Arils and Rind. Food Bioprocess Technol. 2014, 7, 2071–2083. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of Convective and Vacuum-Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oils by ion trap mass spectroscopy; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Adaszyńska-Skwirzyńska, M.; Śmist, M.; Swarcewicz, M. Comparison of extraction methods for the determination of essential oil content and composition of lavender leaves. Available online: http://ena.lp.edu.ua:8080/handle/ntb/27086 (accessed on 21 January 2019).
- Hassanpouraghdam, M.B.; Hassani, A.; Vojodi, L.; Asl, B.H.; Rostami, A. Essential oil constituents of Lavandula ofcinalis Chaix. from Northwest Iran. Chemija 2011, 22, 167–171. [Google Scholar]
- Nurzyńska-Wierdak, R.; Zawiślak, G. Chemical composition and antioxidant activity of lavender (Lavandula angustifolia Mill.) aboveground parts. Acta Sci. Pol., Hortorum Cultus 2016, 15, 225–241. [Google Scholar]
- An, M.; Haig, T.; Hatfield, P. On-site field sampling and analysis of fragrance from living Lavender (Lavandula angustifolia L.) flowers by solid-phase microextraction coupled to gas chromatography and ion-trap mass spectrometry. J. Chromatogr. A 2001, 917, 245–250. [Google Scholar] [CrossRef]
- Afifi, F.U.; Abu-Dahab, R.; Beltrán, S.; Alcalde, B.B.; Abaza, I.F. GC-MS composition and antiproliferative activity of Lavandula angustifolia Mill. essential oils determined by hydro-distillation, SFE and SPME. Arab. J. Med. Aromat. Plants 2016, 2, 71–85. [Google Scholar]
- Fu, J.; Zhao, J.; Zhu, Y.; Tang, J. Rapid Analysis of the Essential Oil Components in Dried Lavender by Magnetic Microsphere-Assisted Microwave Distillation Coupled with HS-SPME Followed by GC-MS. Food Anal. Methods 2017, 10, 2373–2382. [Google Scholar] [CrossRef]
- Torabbeigi, M.; Aberoomand Azar, P. Analysis of essential oil compositions of Lavandula angustifolia by HS-SPME and MAHS-SPME followed by GC and GC-MS. Acta Chromatogr. 2013, 25, 571–579. [Google Scholar] [CrossRef]
- Mirahmadi, S.F.; Norouzi, R. Influence of Thin Layer Drying on the Essential Oil Content and Composition of Lavandula officinalis. J. Essent. Oil-Bearing Plants 2016, 19, 1537–1546. [Google Scholar] [CrossRef]
- Milojevic, S.; Radosavljevic, D.; Pavicevic, V.; Pejanovic, S.; Veljkovic, V. Modeling the kinetics of essential oil hydrodistillation from plant materials. Hem. Ind. 2013, 67, 843–859. [Google Scholar] [CrossRef]
- Baydar, H.; Erbaş, S. Effects of harvest time and drying on essential oil properties in lavandin (Lavandula × intermedia Emeric ex Loisel.). Acta Hortic. 2009, 826, 377–382. [Google Scholar] [CrossRef]
- Ghasemi, A.; Salehi, S.; Craker, L. Effect of drying methods on qualitative and quantitative properties of essential oil from the aerial parts of coriander. J. Dermatol. Sci. 2017, 4, 35–40. [Google Scholar]
- Kim, N.S.; Lee, D.S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography-mass spectrometry. J. Chromatogr. A 2002, 982, 31–47. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D. Analysis of the volatile compounds of flowers and essential oils from Lavandula angustifolia cultivated in northeastern Italy by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry. Planta Med. 2008, 74, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Aghbashlo, M.; kianmehr, M.H.; Samimi-Akhijahani, H. Influence of drying conditions on the effective moisture diffusivity, energy of activation and energy consumption during the thin-layer drying of berberis fruit (Berberidaceae). Energy Convers. Manag. 2008, 49, 2865–2871. [Google Scholar] [CrossRef]
- Alibas, I. Characteristics of chard leaves during microwave, convective, and combined microwave-convective drying. Dry. Technol. 2006, 24, 1425–1435. [Google Scholar] [CrossRef]
- Dadali, G.; Apar, D.K.; Özbek, B. Microwave drying kinetics of okra. Dry. Technol. 2007, 25, 917–924. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
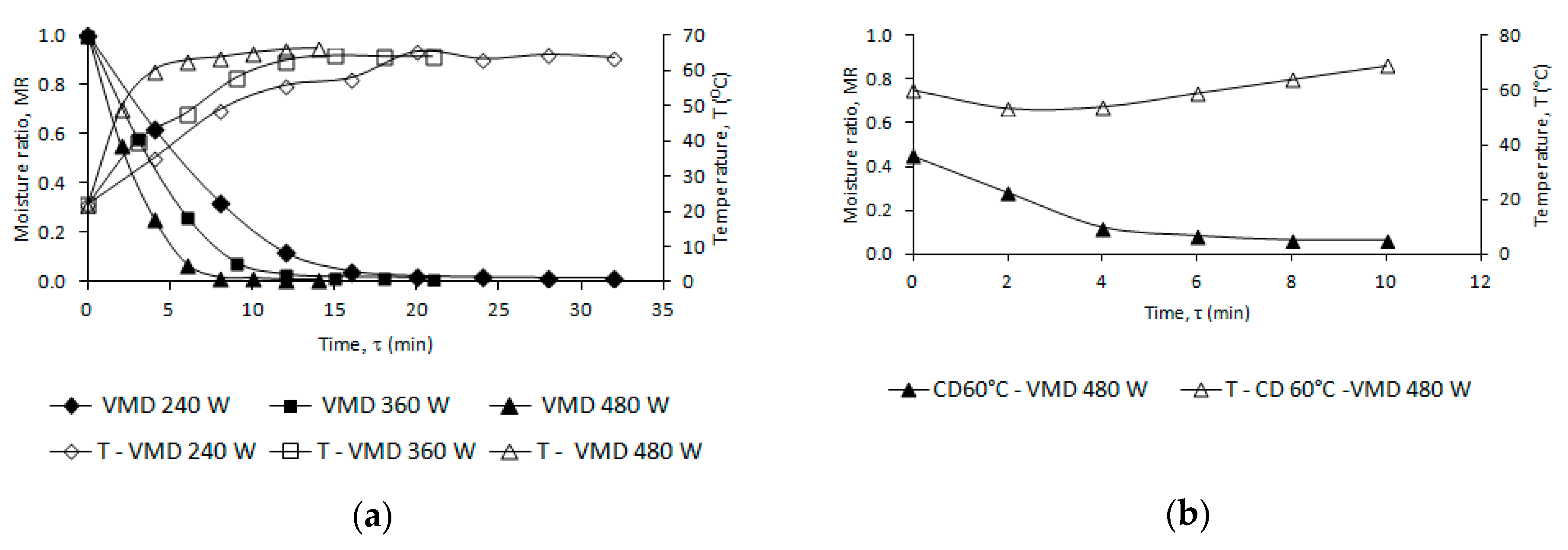
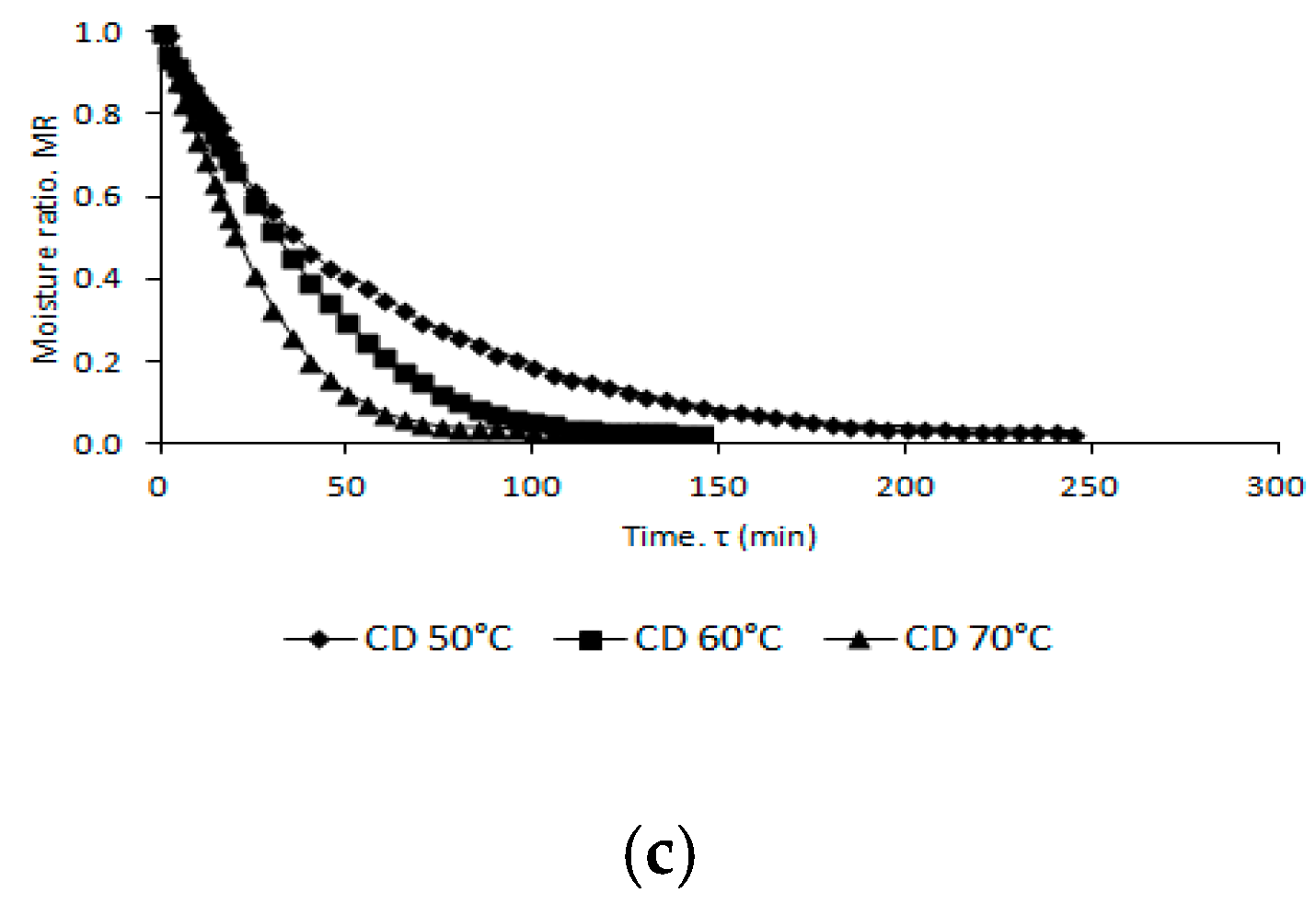
| Drying Conditions | A | Constants K | n | R2 | RMSE | τ | τ1 | T (°C) | Mfwb (%) |
|---|---|---|---|---|---|---|---|---|---|
| CD 50 °C | 1.000 | 0.0201 | 0.953 | 0.9984 | 0.0125 | 245 | - | 50 | 7.18 |
| CD 60 °C | 1.000 | 0.0125 | 1.173 | 0.9991 | 0.0104 | 145 | - | 60 | 7.09 |
| CD 70 °C | 1.000 | 0.0202 | 1.150 | 0.9983 | 0.0156 | 135 | - | 70 | 7.42 |
| VMD 240 W | 1.000 | 0.0736 | 1.328 | 0.9989 | 0.0127 | - | 32 | 64 | 6.78 |
| VMD 360 W | 1.000 | 0.1205 | 1.358 | 0.9991 | 0.0104 | - | 21 | 65 | 6.90 |
| VMD 480 W | 1.000 | 0.2339 | 1.300 | 0.9991 | 0.0111 | - | 14 | 66 | 6.87 |
| CPD 60°C + VMFD 480 W | 0.449 | 0.2895 | 0.893 | 0.9982 | 0.0155 | 60 | 10 | 64 | 7.02 |
| Compound | RT (min) | Retention Indeces (RI) | Content [%] 4 | ||
|---|---|---|---|---|---|
| RI_lit 1 | RI_lit 2 | RI_exp 3 | |||
| 1-Penten-3-ol | 2.407 | - | 684 | 686 | Tr 5 |
| (Z)-3-Hexenal | 3.755 | 797 | 810 | 808 | 0.23 ± 0.14 |
| (E)-2-Hexenal | 4.765 | 846 | 854 | 857 | 0.33 ± 0.17 |
| (Z)-3-Hexen-1-ol | 4.821 | 850 | 857 | 859 | 1.75 ± 0.35 |
| 1-Hexanol | 5.087 | 863 | 868 | 871 | 0.32 ± 0.09 |
| (E,E)-2,4-Hexadienal | 6.113 | 909 | 911 | 913 | 0.15 ± 0.09 |
| 5.5-Dimethyl-1-vinylbicyclo[2.1.1]hexane | 6.380 | - | 921 | 924 | tr |
| Tricyclene | 6.479 | 921 | 926 | 928 | 0.17 ± 0.03 |
| α-Thujene | 6.591 | 924 | 930 | 932 | 0.12 ± 0.05 |
| α-Pinene | 6.788 | 932 | 939 | 940 | 0.30 ± 0.07 |
| Camphene | 7.209 | 946 | 954 | 955 | 0.92 ± 0.19 |
| 3,7,7-Trimethyl-1.3.5-cycloheptatriene | 7.840 | - | 972 | 976 | tr |
| Sabinene | 7.911 | 696 | 976 | 978 | 0.11 ± 0.03 |
| 1-Octen-3-ol | 8.038 | 974 | 979 | 982 | 0.72 ± 0.06 |
| 3-Octanone | 8.260 | 979 | 986 | 988 | 0.22 ± 0.03 |
| β-Myrcene | 8.415 | 988 | 991 | 993 | 0.52 ± 0.22 |
| Mesitylene | 8.512 | 994 | 995 | 996 | tr |
| n-Decane | 8.681 | 1000 | 1000 | 1000 | 0.19 ± 0.04 |
| α-Phellandrene | 8.850 | 1002 | 1005 | 1007 | 0.49 ± 0.28 |
| 3-Carene | 9.031 | 1008 | 1011 | 1013 | 1.60 ± 0.66 |
| m-Cymene | 9.397 | 1020 | 1024 | 1026 | 2.58 ± 0.33 |
| p-Cymene | 9.482 | 1022 | 1030 | 1028 | 4.81 ± 0.52 |
| Limonene | 9.634 | 1024 | 1030 | 1033 | 3.42 ± 1.16 |
| Eucalyptol | 9.692 | 1026 | 1031 | 1035 | 7.28 ± 1.06 |
| β-cis-Ocimene | 9.902 | 1032 | 1038 | 1042 | 0.16 ± 0.03 |
| β-trans-Ocimene | 10.240 | 1044 | 1050 | 1053 | 0.14 ± 0.04 |
| γ-Terpinene | 10.605 | 1054 | 1059 | 1063 | 0.11 ± 0.03 |
| trans-Sabinene hydrate | 10.886 | 1065 | 1070 | 1071 | 0.23 ± 0.05 |
| cis-Linalool oxide | 11.041 | 1067 | 1074 | 1076 | 0.13 ± 0.03 |
| unknown | 11.167 | - | - | 1079 | tr |
| m-Cymenene | 11.419 | 1082 | 1085 | 1086 | 0.50 ± 0.04 |
| p-Mentha-2.4(8)-diene | 11.519 | 1085 | 1088 | 1089 | 0.34 ± 0.10 |
| p-Cymenene | 11.602 | 1089 | 1091 | 1091 | 0.30 ± 0.03 |
| Camphenone | 11.840 | 1095 | 1096 | 1097 | 0.26 ± 0.02 |
| Linalool | 11.953 | 1095 | 1096 | 1100 | 0.42 ± 0.03 |
| 1.3.8-p-Menthatriene | 12.206 | 1108 | 1110 | 1108 | 0.10 ± 0.02 |
| 1-Octen-3-ol acetate | 12.360 | 1110 | 1112 | 1114 | 3.80 ± 0.52 |
| cis-p-Menth-2-en-1-ol | 12.556 | 1118 | 1121 | 1120 | 0.18 ± 0.04 |
| trans-p-Mentha-2.8-dien-1-ol | 12.724 | 1119 | 1122 | 1125 | 0.64 ± 0.19 |
| cis-p-Mentha-2.8-dien-1-ol | 13.173 | 1133 | 1137 | 1139 | 0.26 ± 0.03 |
| trans-p-Menth-2-en-1-ol | 13.327 | 1136 | 1140 | 1144 | 0.49 ± 0.08 |
| Camphor | 13.496 | 1141 | 1146 | 1149 | 2.09 ± 0.29 |
| Tetrahydrolavandulol | 13.960 | 1157 | 1161 | 1162 | 0.48 ± 0.09 |
| Borneol + Lavandulol | 14.240 | 1165 | 1169 | 1170 | 4.66 ± 0.69 |
| Melilotal | 14.450 | 1179 | 1182 | 1176 | tr |
| Terpinen-4-ol | 14.631 | 1174 | 1177 | 1181 | 0.59 ± 0.07 |
| m-Cymen-8-ol | 14.774 | 1176 | 1179 | 1184 | 2.09 ± 0.25 |
| p-Cymen-8-ol | 14.914 | 1179 | 1182 | 1188 | 4.09 ± 0.67 |
| α-Terpineol | 15.082 | 1186 | 1189 | 1193 | 0.31 ± 0.06 |
| Myrtenol | 15.278 | 1194 | 1195 | 1198 | 0.20 ± 0.14 |
| trans-Piperitol | 15.671 | 1207 | 1208 | 1210 | 0.65 ± 0.07 |
| cis-Carveol | 16.035 | 1215 | 1217 | 1222 | 0.37 ± 0.07 |
| (Z)-Ocimenone | 16.159 | 1226 | 1229 | 1226 | 0.26 ± 0.07 |
| exo-Fenchyl acetate | 16.356 | 1229 | 1232 | 1232 | 0.49 ± 0.04 |
| cis-Verbenol | 16.623 | 1237 | 1244 | 1240 | tr |
| Cumin aldehyde | 16.748 | 1238 | 1241 | 1244 | 1.92 ± 0.59 |
| Carvone | 16.874 | 1246 | 1243 | 1247 | 1.08 ± 0.28 |
| Geraniol | 17.055 | 1249 | 1252 | 1253 | 0.33 ± 0.29 |
| Linalyl acetate | 17.263 | 1254 | 1257 | 1259 | 2.21 ± 0.73 |
| Geranial | 17.529 | 1264 | 1267 | 1267 | 0.10 ± 0.08 |
| trans-Carvone oxide | 18.021 | 1273 | 1276 | 1281 | 0.33 ± 0.07 |
| Bornyl acetate | 18.301 | 1284 | 1285 | 1288 | 5.57 ± 0.82 |
| Lavandulyl acetate | 18.428 | 1288 | 1290 | 1292 | 1.72 ± 0.25 |
| Terpinen-4-ol acetate | 18.761 | 1299 | 1299 | 1301 | 0.18 ± 0.02 |
| unknown | 19.124 | - | - | 1314 | 0.61 ± 0.09 |
| Myrtenyl acetate | 19.435 | 1324 | 1326 | 1326 | 0.16 ± 0.06 |
| δ-Elemene | 19.749 | 1335 | 1337 | 1337 | tr |
| α-Terpinyl acetate | 20.036 | 1346 | 1349 | 1347 | 0.26 ± 0.08 |
| α-Cubebene | 20.179 | 1348 | 1351 | 1351 | tr |
| α-Longipinene | 20.351 | 1350 | 1352 | 1357 | 0.18 ± 0.01 |
| unknown | 20.465 | - | - | 1361 | 0.31 ± 0.05 |
| Silphiperfola-4.7(14)-diene | 20.578 | 1358 | 1362 | 1365 | tr |
| Neryl acetate | 20.748 | 1359 | 1364 | 1371 | 0.26 ± 0.06 |
| α-Copaene | 21.134 | 1374 | 1376 | 1383 | 0.14 ± 0.02 |
| Geranyl acetate | 21.248 | 1379 | 1381 | 1387 | 0.49 ± 0.11 |
| α-Bourbonene | 21.375 | 1387 | 1388 | 1391 | tr |
| unknown | 21.461 | 1394 | 1396 | 1394 | tr |
| β-Longipinene | 21.634 | 1400 | 1400 | 1399 | 0.26 ± 0.06 |
| Sesquithujene | 21.833 | 1405 | 1405 | 1409 | tr |
| α-Cedrene | 22.049 | 1410 | 1411 | 1420 | 1.01 ± 0.25 |
| (E)-Caryophyllene | 22.176 | 1417 | 1419 | 1427 | 6.11 ± 1.48 |
| α-Bergamotene | 22.506 | 1432 | 1435 | 1443 | 0.87 ± 0.33 |
| Cadina-3.5-diene | 22.745 | - | 1458 | 1455 | 1.12 ± 0.40 |
| (E)-β-Farnesene | 22.889 | 1454 | 1457 | 1462 | 1.35 ± 0.38 |
| cis-Muurola-4(15).5-diene | 23.084 | 1465 | 1466 | 1472 | 1.44 ± 0.45 |
| 4-epi-α-Acoradiene | 23.155 | 1474 | 1475 | 1475 | 0.19 ± 0.00 |
| Germacrene D | 23.441 | 1484 | 1481 | 1489 | 0.58 ± 0.17 |
| β-Himachalene | 23.629 | 1500 | 1500 | 1498 | tr |
| unknown | 23.741 | 1502 | - | 1505 | tr |
| α-Bulnesene | 23.840 | 1509 | 1509 | 1511 | 0.92 ± 0.16 |
| γ-Cadinene | 24.023 | 1513 | 1513 | 1523 | 10.53 ± 1.51 |
| cis-Calamenene | 24.149 | 1528 | 1529 | 1531 | 0.65 ± 0.07 |
| 10-epi-Cubebol | 24.290 | 1533 | 1535 | 1540 | 0.11 ± 0.05 |
| α-Cadinene | 24.402 | 1537 | 1538 | 1547 | 0.12 ± 0.02 |
| Cadala-1(10).3.8-triene | 24.473 | - | 1555 | 1552 | tr |
| trans-Cadinene ether | 24.669 | 1557 | - | 1564 | 0.35 ± 0.09 |
| unknown | 24.851 | - | - | 1576 | 0.13 ± 0.05 |
| Spathulenol | 24.950 | 1577 | 1578 | 1582 | 0.25 ± 0.05 |
| Caryophyllene oxide | 25.158 | 1582 | 1583 | 1595 | 3.31 ± 0.18 |
| 1-epi-Cubenol | 25.552 | 1627 | 1628 | 1628 | 0.56 ± 0.03 |
| τ-Cadinol | 25.860 | 1635 | 1340 | 1656 | 2.04 ± 0.55 |
| unknown | 26.056 | - | - | 1673 | 0.11 ± 0.03 |
| 14-Hydroxy-4.5-dihydrocaryophyllene | 26.407 | 1706 | 1706 | 1706 | 0.21 ± 0.11 |
| unknown | 26.911 | 1760 | 1761 | 1764 | 0.23 ± 0.02 |
| Compound | Drying Method | |||||||
|---|---|---|---|---|---|---|---|---|
| Fresh | CD 50 °C | CD 60 °C | CD 70 °C | CPD-VMFD | VMD 240 W | VMD 360 W | VMD 480 W | |
| Content [%] 1 | ||||||||
| p-Cymene | 2.58 a | 2.73 c | 2.72 c | 1.76 d | 2.01 d,e | 2.15 e | 2.27 e | 3.50 b |
| o-Cymene | 4.81 a | 6.26 c | 5.65 d | 3.05 f | 3.69 e | 4.62 g | 4.73 g | 8.08 b |
| Limonene | 3.42 a | 3.27 f | 3.47 f | 1.31 e | 3.08 c,f | 1.97 d,e | 2.41 c,d | 6.99 b |
| Eucalyptol | 7.28 a | 5.01 b,c | 3.71 d | 5.12 b | 3.98 c,d | 3.25 d | 3.74 d | 3.44 d |
| 1-Octen-3-ol. acetate | 3.80 a | 2.70 d,e | 2.82 d,e | 4.23 c | 6.22 b | 2.10 e | 4.42 c | 3.68 c,d |
| Camphor | 2.09 a | 2.32 b | 1.40 d | 1.89 c | 0.40 f | 1.12 e | 0.34 f | 0.39 f |
| Borneol + Lavandulol | 4.66 a | 7.63 b | 5.75 d | 6.07 d | 1.37 e | 4.78 c | 1.46 e | 1.35 e |
| m-Cymen-8-ol | 2.09 a | 3.18 c | 2.48 d | 3.67 b | 0.02 e | 2.74 d | 0.08 e | 0.07 e |
| p-Cymen-8-ol | 4.09 a | 6.31 c | 6.05 c | 7.17 b | 1.10 d | 6.07 c | 1.04 d | 0.89 d |
| Cumin aldehyde | 1.92 a | 3.59 c | 3.74 c | 4.48 b | 0.59 d | 4.30 b | 0.66 d | 0.59 d |
| Linalyl acetate | 2.21 a | 3.46 d | 11.06 b | 4.23 d | 1.60 e | 5.29 c | 1.66 e | 1.75 e |
| Bornyl acetate | 5.57 a | 3.54 c | 2.36 e | 4.04 b | 0.07 f | 3.07 d | 0.07 f | 0.14 f |
| Caryophyllene <(E)-> | 6.11 a | 2.11 d | 2.78 c | 3.51 f | 6.38 b | 4.85 e | 5.28 e | 3.98 f |
| γ-Cadinene | 10.53 a | 3.67 f | 4.43 ef | 4.74 e | 8.48 c,d | 7.80 d | 9.20 c | 5.88 b |
| Caryophyllene oxide | 3.31 a | 2.43 c | 1.63 b | 2.12 b,c | 2.24 b,c | 2.11 b,c | 2.47 c | 1.89 b,c |
| τ-Cadinol | 2.04 a | 1.62 d | 1.44 d | 2.78 c | 2.74 c | 3.37 b,c | 3.85 b | 1.74 d |
| Ʃ | 66.51 a | 59.83 b | 61.42 b | 60.17 b | 43.97 c | 59.59 b | 43.68 c | 42.47 c |
| Linalool | 0.38 a | 4.32 c | 6.33 b | 4.71 c | 0.76 a,d | 6.62 b | 0.73 a,d | 1.16 d |
| TOTAL essential oil [mL 100g−1 dw] 2 | 3.082 a | 0.588 e | 0.726 f | 0.992 c,d | 0.921 b,c | 1.075 d | 0.881 b | 1.302 g |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyczko, J.; Jałoszyński, K.; Surma, M.; Masztalerz, K.; Szumny, A. HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules 2019, 24, 764. https://doi.org/10.3390/molecules24040764
Łyczko J, Jałoszyński K, Surma M, Masztalerz K, Szumny A. HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules. 2019; 24(4):764. https://doi.org/10.3390/molecules24040764
Chicago/Turabian StyleŁyczko, Jacek, Klaudiusz Jałoszyński, Mariusz Surma, Klaudia Masztalerz, and Antoni Szumny. 2019. "HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods" Molecules 24, no. 4: 764. https://doi.org/10.3390/molecules24040764
APA StyleŁyczko, J., Jałoszyński, K., Surma, M., Masztalerz, K., & Szumny, A. (2019). HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules, 24(4), 764. https://doi.org/10.3390/molecules24040764









