Antifungal Activity against Botrytis cinerea of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives
Abstract
1. Introduction
2. Results and Discussion
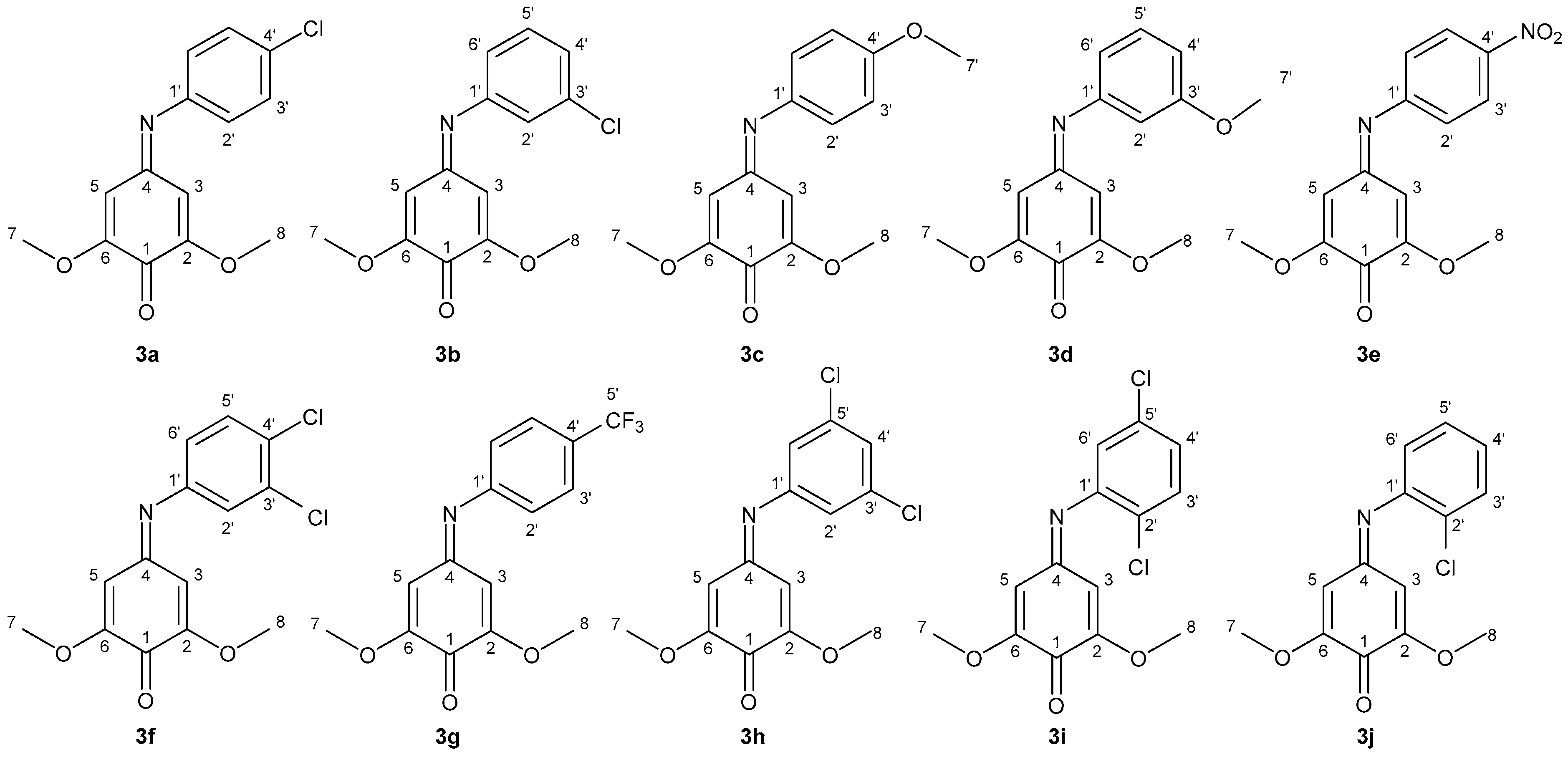
2.1. Laccase-Mediated Synthesis of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives
2.2. Antifungal Activity
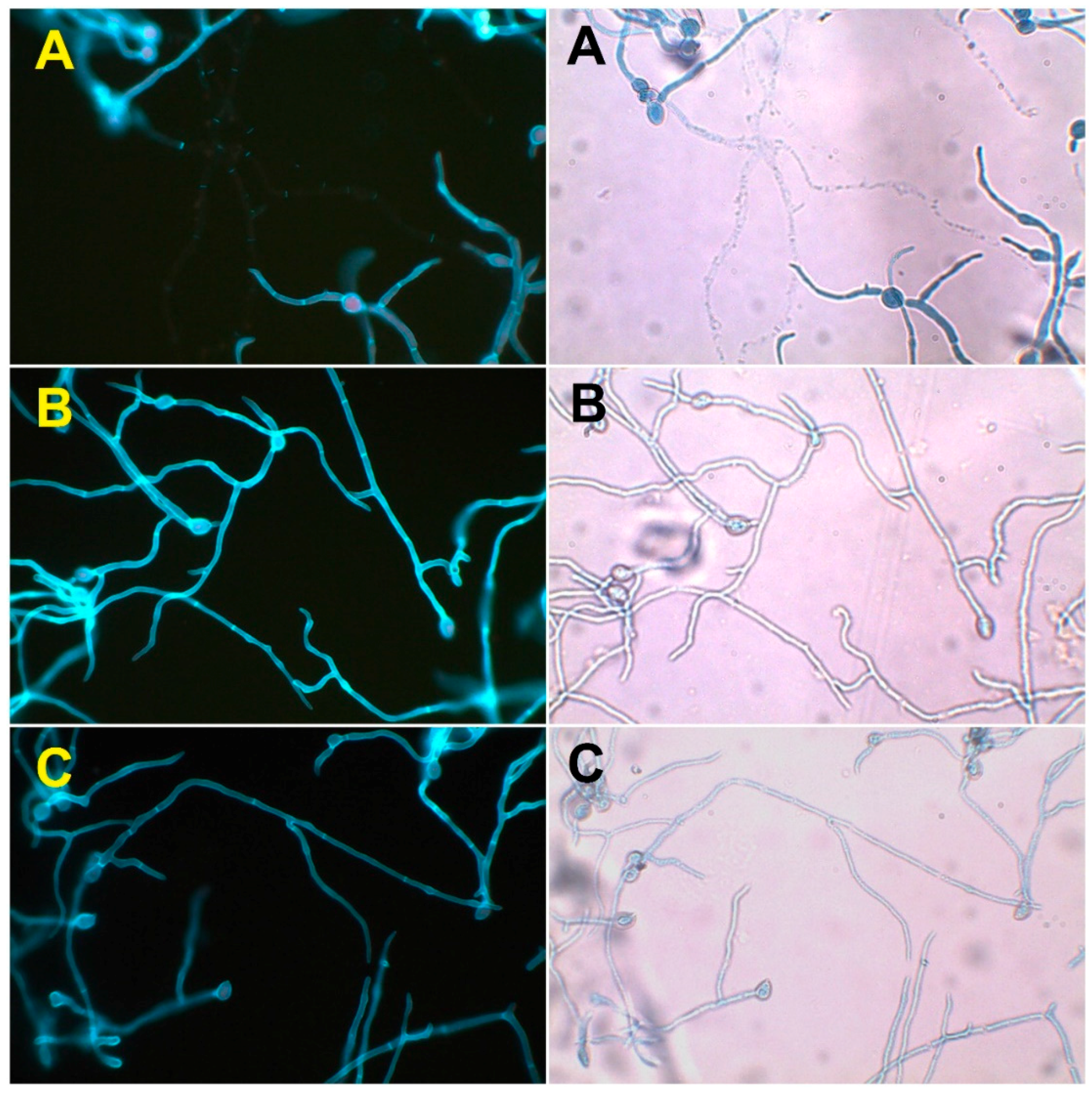
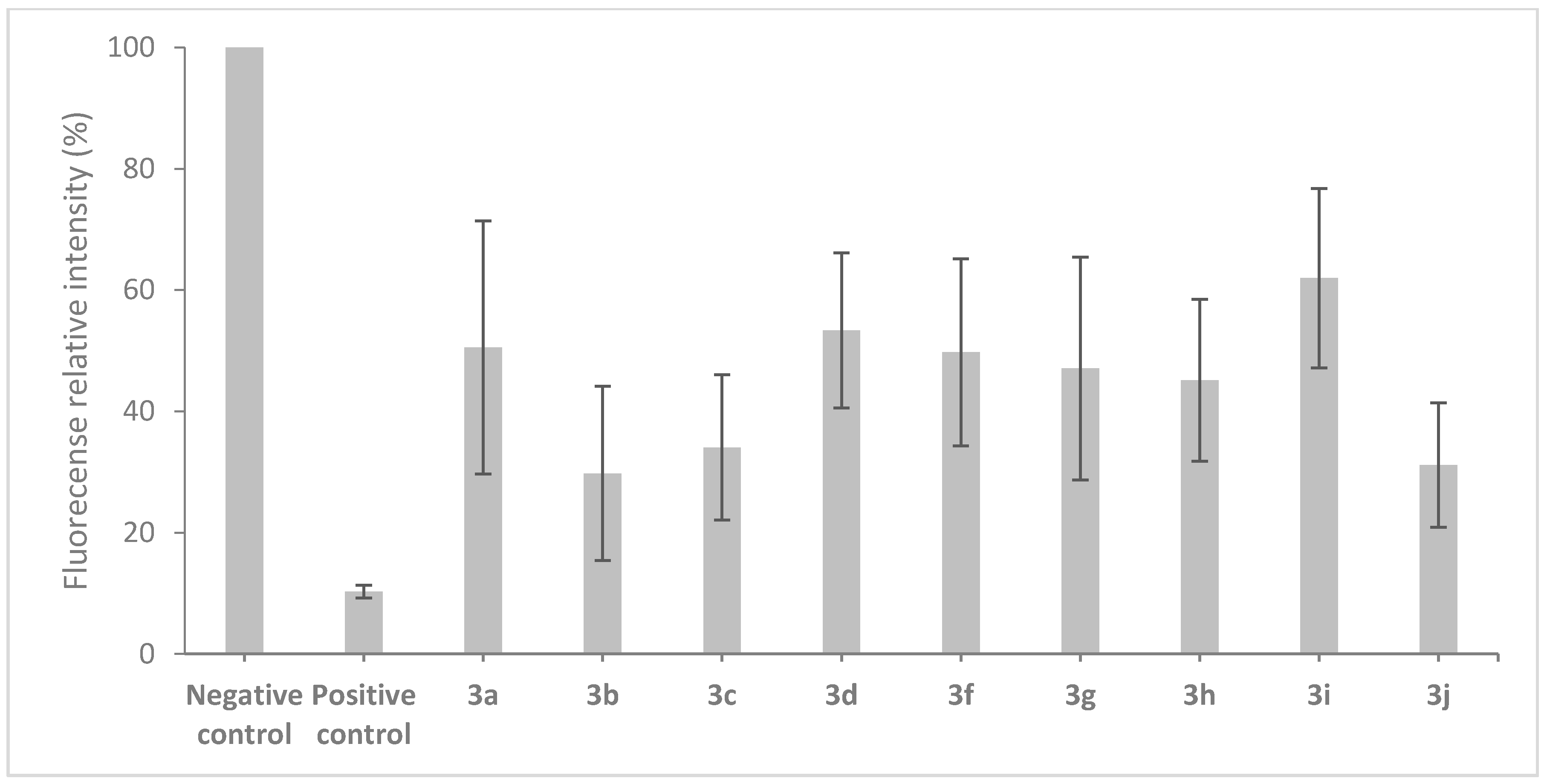
2.3. Effect on the Cell Wall Integrity of B. cinerea
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemical Reagents
3.3. Laccase-Mediated Synthesis of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives (Compounds 3a–j)
3.4. Spectroscopic Data
3.5. Fungal Strain and Culture Conditions
3.6. Antifungal Assay
Effect on Mycelial Growth
3.7. Effect on the Cell Wall Integrity of B. cinerea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: Cham, Switzerland, 2016; pp. 189–216. [Google Scholar]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of the Risk; Fungicide Resistance Action Committee: Brussels, Belgium, 2007; pp. 1–53. [Google Scholar]
- Pappas, A.C. Evolution of fungicide resistance in Botrytis cinerea in protected crops in Greece. Crop Prot. 1997, 16, 257–263. [Google Scholar] [CrossRef]
- Wilson, C.L.; Solar, J.M.; Ghaouth, A.E.; Wisniewski, M.E. Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 1997, 81, 204–210. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 195–217. [Google Scholar]
- Daoubi, M.; Durán-Patrón, R.; Hmamouchi, M.; Hernández-Galán, R.; Benharref, A.; Collado, I.G. Screening study for potential lead compounds for natural product-based fungicides: I. Synthesis and in vitro evaluation of coumarins against Botrytis cinerea. Pest Manag. Sci. 2004, 60, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Imperato, F. Phytochemistry: Advances in Research; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- Grayer, R.J.; Kokubun, T. Plant–fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Grayer, R.J.; Harborne, J.B. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar] [CrossRef]
- Osbourn, A.E. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 1996, 8, 1821–1831. [Google Scholar] [CrossRef]
- Harborne, J.B. The comparative biochemistry of phytoalexin induction in plants. Biochem. Syst. Ecol. 1999, 27, 335–367. [Google Scholar] [CrossRef]
- Caruso, F.; Mendoza, L.; Castro, P.; Cotoras, M.; Aguirre, M.; Matsuhiro, B.; Isaacs, M.; Rossi, M.; Viglianti, A.; Antonioletti, R. Antifungal activity of resveratrol against Botrytis cinerea is improved using 2-furyl derivatives. PLoS ONE 2011, 6, e25421. [Google Scholar] [CrossRef]
- Pinedo-Rivilla, C.; Bustillo, A.J.; Hernández-Galán, R.; Aleu, J.; Collado, I.G. Asymmetric preparation of antifungal 1-(4′-chlorophenyl)-1-cyclopropyl methanol and 1-(4′-chlorophenyl)-2-phenylethanol. Study of the detoxification mechanism by Botrytis cinerea. J. Mol. Catal. B Enzym. 2011, 70, 61–66. [Google Scholar] [CrossRef]
- Saiz-Urra, L.; Racero, J.C.; Macías-Sáchez, A.J.; Hernández-Galán, R.; Hanson, J.R.; Perez-Gonzalez, M.; Collado, I.G. Synthesis and quantitative structure-antifungal activity relationships of clovane derivatives against Botrytis cinerea. J. Agric. Food Chem. 2009, 57, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crops Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Mikolasch, A.; Schauer, F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl. Microbiol. Biotechnol. 2009, 82, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Mogharabi, M.; Faramarzi, M.A. Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Constantin, M.-A.; Conrad, J.; Merişor, E.; Koschorreck, K.; Urlacher, V.B.; Beifuss, U. Oxidative dimerization of (E)- and (Z)-2-propenylsesamol with O2 in the presence and absence of laccases and other catalysts: Selective formation of carpanones and benzopyrans under different reaction conditions. J. Org. Chem. 2012, 77, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Koschorreck, K.; Richter, S.M.; Ene, A.B.; Roduner, E.; Schmid, R.D.; Urlacher, V.B. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl. Microbiol. Biotechnol. 2008, 79, 217–224. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Kudanga, T.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Enzymatic modification of 2,6-dimethoxyphenol for the synthesis of dimers with high antioxidant capacity. Process Biochem. 2012, 47, 1926–1932. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Kudanga, T.; Parker, A.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J. Mol. Catal. B Enzym. 2012, 74, 29–35. [Google Scholar] [CrossRef]
- Navarra, C.; Gavezzotti, P.; Monti, D.; Panzeri, W.; Riva, S. Biocatalyzed synthesis of enantiomerically enriched β-5-like dimer of 4-vinylphenol. J. Mol. Catal. B Enzym. 2012, 84, 115–120. [Google Scholar] [CrossRef]
- Mikolasch, A.; Niedermeyer, T.H.J.; Lalk, M.; Witt, S.; Seefeldt, S.; Hammer, E.; Schauer, F.; Gesell Salazar, M.; Hessel, S.; Jülich, W.-D.; et al. Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases II. Chem. Pharm. Bull. 2007, 55, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Mikolasch, A.; Hessel, S.; Salazar, M.G.; Neumann, H.; Manda, K.; Gordes, D.; Schmidt, E.; Thurow, K.; Hammer, E.; Lindequist, U.; et al. Synthesis of new N-analogous corollosporine derivatives with antibacterial activity by laccase-catalyzed amination. Chem. Pharm. Bull. 2008, 56, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Castro, P.; Melo, R.; Campos, A.M.; Zuñiga, G.; Guerrero, J.; Cotoras, M. Improvement of the antifungal activity against Botrytis cinerea of syringic acid, a phenolic acid from grape pomace. J. Chil. Chem. Soc. 2016, 61, 3039–3042. [Google Scholar] [CrossRef]
- Leroux, P. Recent developments in the mode of action of fungicides. Pestic. Sci. 1996, 47, 191–197. [Google Scholar] [CrossRef]
- Guo, Z.; Miyoshi, H.; Komyoji, T.; Haga, T.; Fujita, T. Uncoupling activity of a newly developed fungicide, fluazinam [3-chloro-N-(3-chloro-2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-2-pyridinamine]. BBA Bioenerg. 1991, 1056, 89–92. [Google Scholar] [CrossRef]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Bollag, J.M.; Minard, R.D.; Liu, S.Y. Cross-linkage between anilines and phenolic humus constituents. Environ. Sci. Technol. 1983, 17, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Freyer, A.; Minard, R.D. Enzymatic coupling of chloroanilines, syringic acid, vanillic acid and protocatechuic acid. Soil Biol. Biochem. 1994, 26, 135–142. [Google Scholar] [CrossRef]
- Hoff, T.; Liu, S.Y.; Bollag, J.M. Transformation of halogen-, alkyl-, and alkoxy-substituted anilines by a laccase of Trametes versicolor. Appl. Environ. Microbiol. 1985, 49, 1040–1045. [Google Scholar]
- Itoh, K.; Fujita, M.; Kumano, K.; Suyama, K.; Yamamoto, H. Phenolic acids affect transformations of chlorophenols by a Coriolus versicolor laccase. Soil Biol. Biochem. 2000, 32, 85–91. [Google Scholar] [CrossRef]
- Galletti, P.; Pori, M.; Funiciello, F.; Soldati, R.; Ballardini, A.; Giacomini, D. Laccase-mediator system for alcohol oxidation to carbonyls or carboxylic acids: Toward a sustainable synthesis of profens. ChemSusChem 2014, 7, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Urra, L.; Bustillo-Pérez, A.J.; Cruz-Monteagudo, M.; Pinedo-Rivilla, C.; Aleu, J.; Hernández-Galán, R.; Collado, I.G. Global antifungal profile optimization of chlorophenyl derivatives against Botrytis cinerea and Colletotrichum gloeosporioides. J. Agric. Food Chem. 2009, 57, 4838–4843. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, Z.; Wang, X.; Feng, H.; Wang, L.; Zhao, Y.; Wang, Y.; Tang, X. Synthesis and antifungal activity of aspirin derivatives. Asian J. Chem. 2014, 26, 7157–7159. [Google Scholar] [CrossRef]
- Chen, C.-J.; Song, B.-A.; Yang, S.; Xu, G.-F.; Bhadury, P.S.; Jin, L.-H.; Hu, D.-Y.; Li, Q.-Z.; Liu, F.; Xue, W.; et al. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. 2007, 15, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Herth, W.; Schnepf, E. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Miwa, G.T.; Lu, A.Y.H.; Nelson, S.D. N-acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. USA 1984, 81, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- Pócsi, I.; Prade, R.A.; Penninckx, M.J. Glutathione, altruistic metabolite in fungi. Adv. Microb. Physiol. 2004, 49, 1–76. [Google Scholar] [PubMed]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Domínguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, J.C.; Ribas, J.C.; Devia, C.; Rodriguez, A.M.; et al. In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Muñoz, G.; Hinrichsen, P.; Brygoo, Y.; Giraud, T. Genetic characterisation of Botrytis cinerea populations in Chile. Mycol. Res. 2002, 106, 594–601. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–j are available from the authors. |

| Compounds | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 2a/3a | H | H | Cl | H |
| 2b/3b | H | Cl | H | H |
| 2c/3c | H | H | OCH3 | H |
| 2d/3d | H | OCH3 | H | H |
| 2e/3e | H | H | NO2 | H |
| 2f/3f | H | Cl | Cl | H |
| 2g/3g | H | H | CF3 | H |
| 2h/3h | H | Cl | H | Cl |
| 2i/3i | Cl | H | H | Cl |
| 2j/3j | Cl | H | H | H |
| Reaction | Product | Yield (%) Substrate Ratio (Syringic Acid: Aniline) | ||
|---|---|---|---|---|
| 1:2 | 1:1 | 2:1 | ||
| 1 | 3a | 10.4 | 36.9 | 55.5 |
| 2 | 3b | 38.1 | 72.0 | 24.1 |
| 3 | 3c | 15.5 | 26.7 | ND 1 |
| 4 | 3d | 8.2 | 24.2 | 4.1 |
| 5 | 3e | 22.6 | 56.8 | 36.4 |
| 6 | 3f | 41.8 | 50.4 | 44.8 |
| 7 | 3g | 12.7 | 10.2 | 8.2 |
| 8 | 3h | 15.8 | 74.0 | 29.0 |
| 9 | 3i | 6.9 | 38.3 | 23.9 |
| 10 | 3j | 39.7 | 13.6 | 44.9 |
| 13C | 1H | HMBC | HSQC | |
|---|---|---|---|---|
| 1 | 176,633 | 5, 3 | ||
| 4 | 157,816 | |||
| 2 | 155.741 | 8 | ||
| 6 | 154.881 | 7 | ||
| 1’ | 151.483 | 5’ | ||
| 3’ | 134.928 | 5’ | ||
| 5’ | 130.247 | 7.316 (t, J 8.0 Hz, 1H) | 5’ | |
| 4’ | 125.023 | 7.159 (d, J 8.0 Hz, 1H) | 6’, 2’ | 4’ |
| 2’ | 120.635 | 6.888 (s, 1H) | 6’, 4’ | 2’ |
| 6’ | 118.705 | 6.737 (d, J 7.9 Hz, 1H) | 4’, 2’ | 6’ |
| 5 | 111.717 | 6.368 (d, J 1.9 Hz, 1H) | 3 | 5 |
| 3 | 98.583 | 6.010 (d, J 1.9 Hz, 1H) | 5 | 3 |
| 7 | 56.321 | 3.874 (s, 3H) | 7 | |
| 8 | 56.212 | 3.670 (s, 3H) | 8 |
| Compound | EC50 (mM) |
|---|---|
| 3a | 0.065 ± 0.003 |
| 3b | 0.15 ± 0.01 |
| 3c | 0.54± 0.06 |
| 3d | 0.39 ± 0.02 |
| 3e | - * |
| 3f | 0.055 ± 0.004 |
| 3g | 0.101 ± 0.014 |
| 3h | 0.032 ± 0.003 |
| 3i | 0.065 ± 0.011 |
| 3j | 0.069 ± 0.004 |
| Compound | EC50 (mM) |
|---|---|
| 1 | >1.51 |
| 2a | 0.71 ± 0.08 |
| 2b | 0.59 ± 0.04 |
| 2c | >3.00 |
| 2d | >3.00 |
| 2e | 0.15 ± 0.01 |
| 2f | 0.047 ± 0.005 |
| 2g | 2.058 ± 0.434 |
| 2h | 0.12 ± 0.02 |
| 2i | 0.414 ± 0.031 |
| 2j | 1.09 ± 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, P.; Mendoza, L.; Vásquez, C.; Pereira, P.C.; Navarro, F.; Lizama, K.; Santander, R.; Cotoras, M. Antifungal Activity against Botrytis cinerea of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives. Molecules 2019, 24, 706. https://doi.org/10.3390/molecules24040706
Castro P, Mendoza L, Vásquez C, Pereira PC, Navarro F, Lizama K, Santander R, Cotoras M. Antifungal Activity against Botrytis cinerea of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives. Molecules. 2019; 24(4):706. https://doi.org/10.3390/molecules24040706
Chicago/Turabian StyleCastro, Paulo, Leonora Mendoza, Claudio Vásquez, Paz Cornejo Pereira, Freddy Navarro, Karin Lizama, Rocío Santander, and Milena Cotoras. 2019. "Antifungal Activity against Botrytis cinerea of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives" Molecules 24, no. 4: 706. https://doi.org/10.3390/molecules24040706
APA StyleCastro, P., Mendoza, L., Vásquez, C., Pereira, P. C., Navarro, F., Lizama, K., Santander, R., & Cotoras, M. (2019). Antifungal Activity against Botrytis cinerea of 2,6-Dimethoxy-4-(phenylimino)cyclohexa-2,5-dienone Derivatives. Molecules, 24(4), 706. https://doi.org/10.3390/molecules24040706





