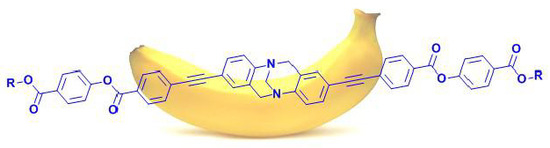
Design and Synthesis of a Novel Banana-Shaped Functional Molecule via Double Cross-Coupling
Abstract
:1. Introduction
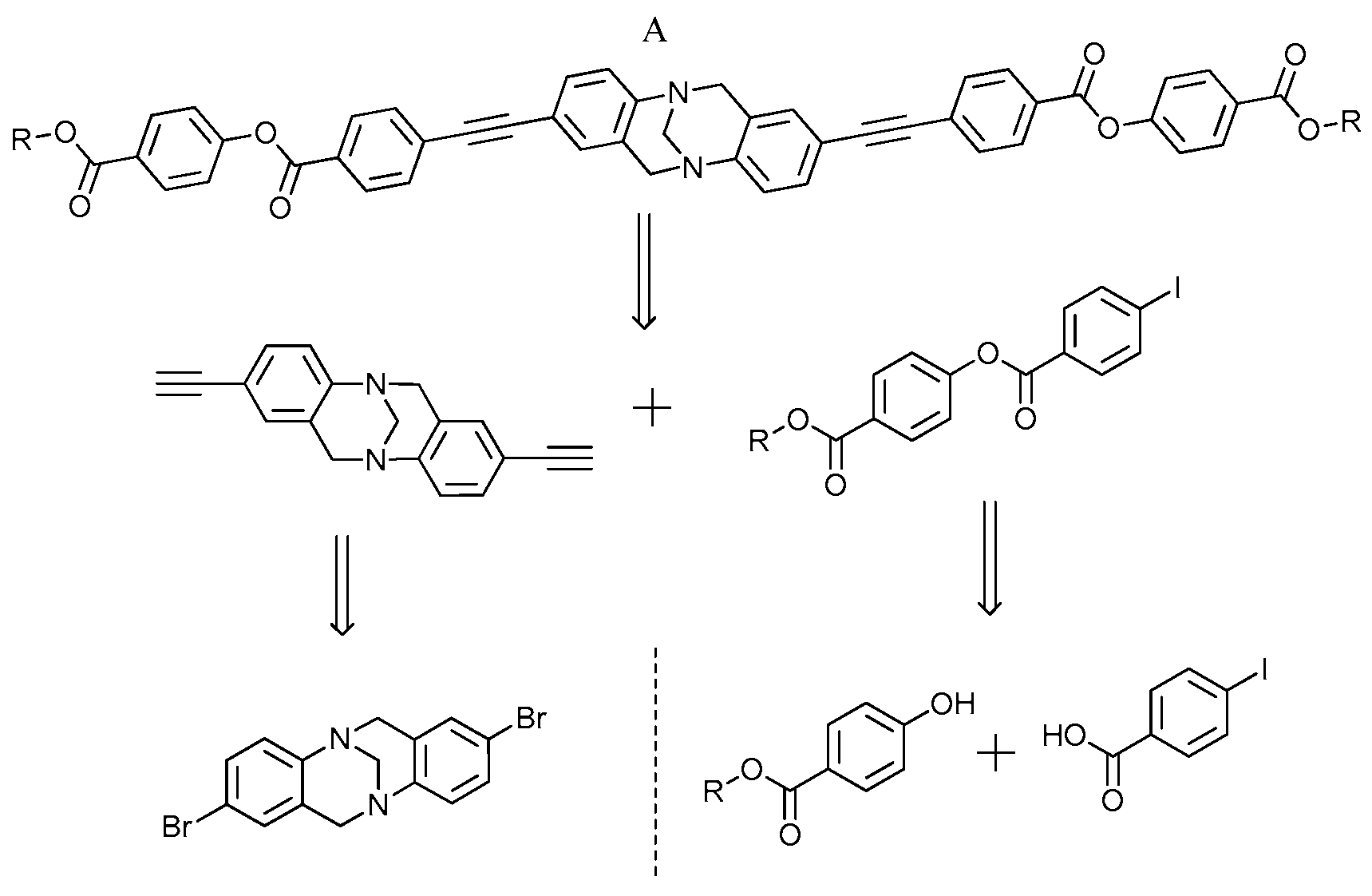
2. Experimental Section
2.1. General
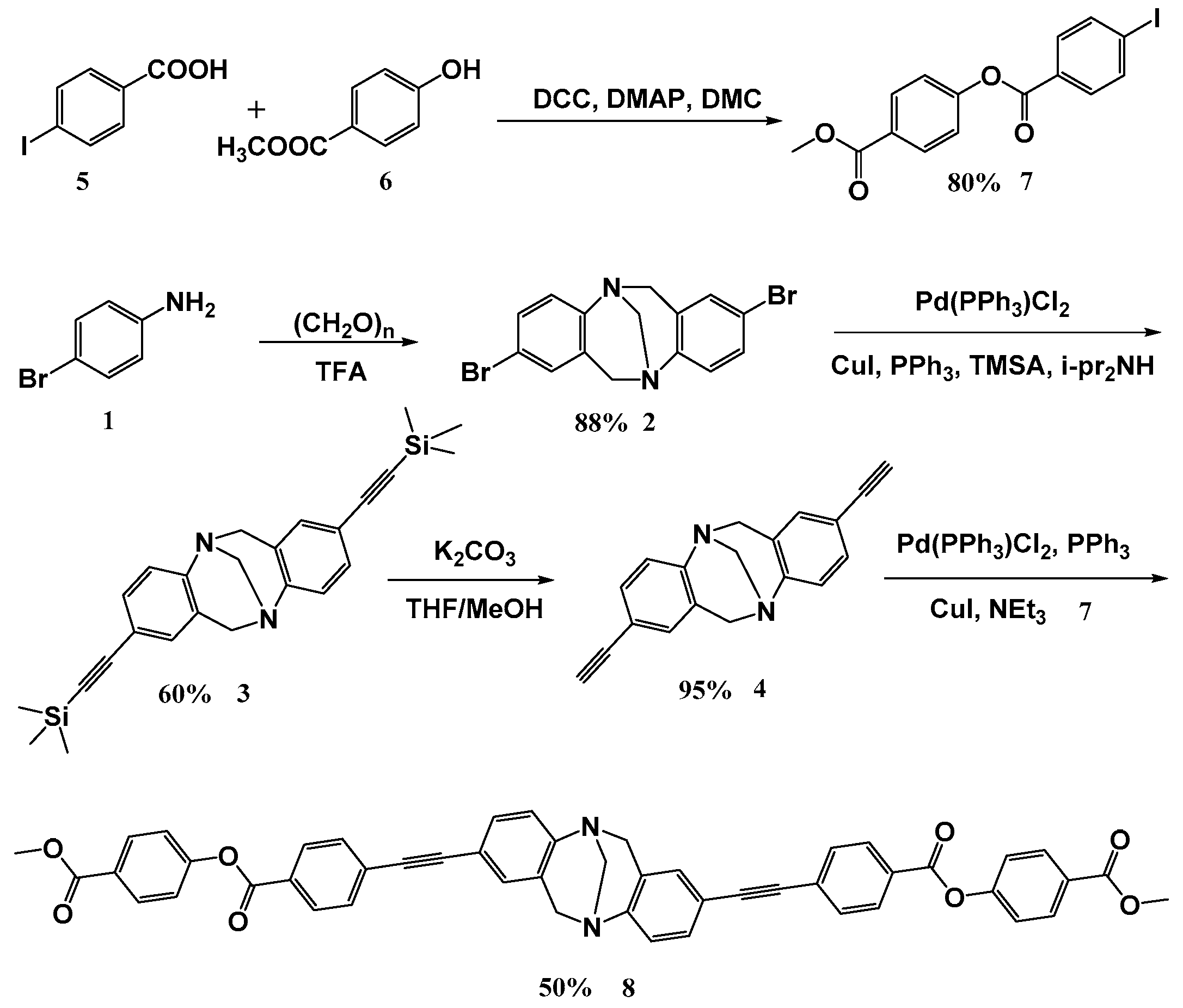
2.2. Experimental Procedure for the Preparation of 2,8-Dibromo-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (2)
2.3. Experimental Procedure for the Preparation of 2,8-Bis((trimethylsilyl)ethynyl)-6,12-dihydro-5,11-methanodiben-zo[b,f][1,5]diazocine (3)
2.4. Experimental Procedure for the Preparation of 2,8-Diethynyl-6,12-dihydro-5,11-methanodibenzo[b,f][1,5]diazoci-ne (4)
2.5. Experimental Procedure for the Preparation of 4-(Methoxycarbonyl)phenyl-4-iodobenzoate (7)
2.6. Experimental Procedure for the Preparation of Bis(4-(methoxycarbonyl)phenyl)4,4′-((6,12-dihydro-5,11-methano-dibenzo[b,f][1,5]diazocine-2,8-diyl)bis(ethyne-2,1-diyl))diben-zoate (8)
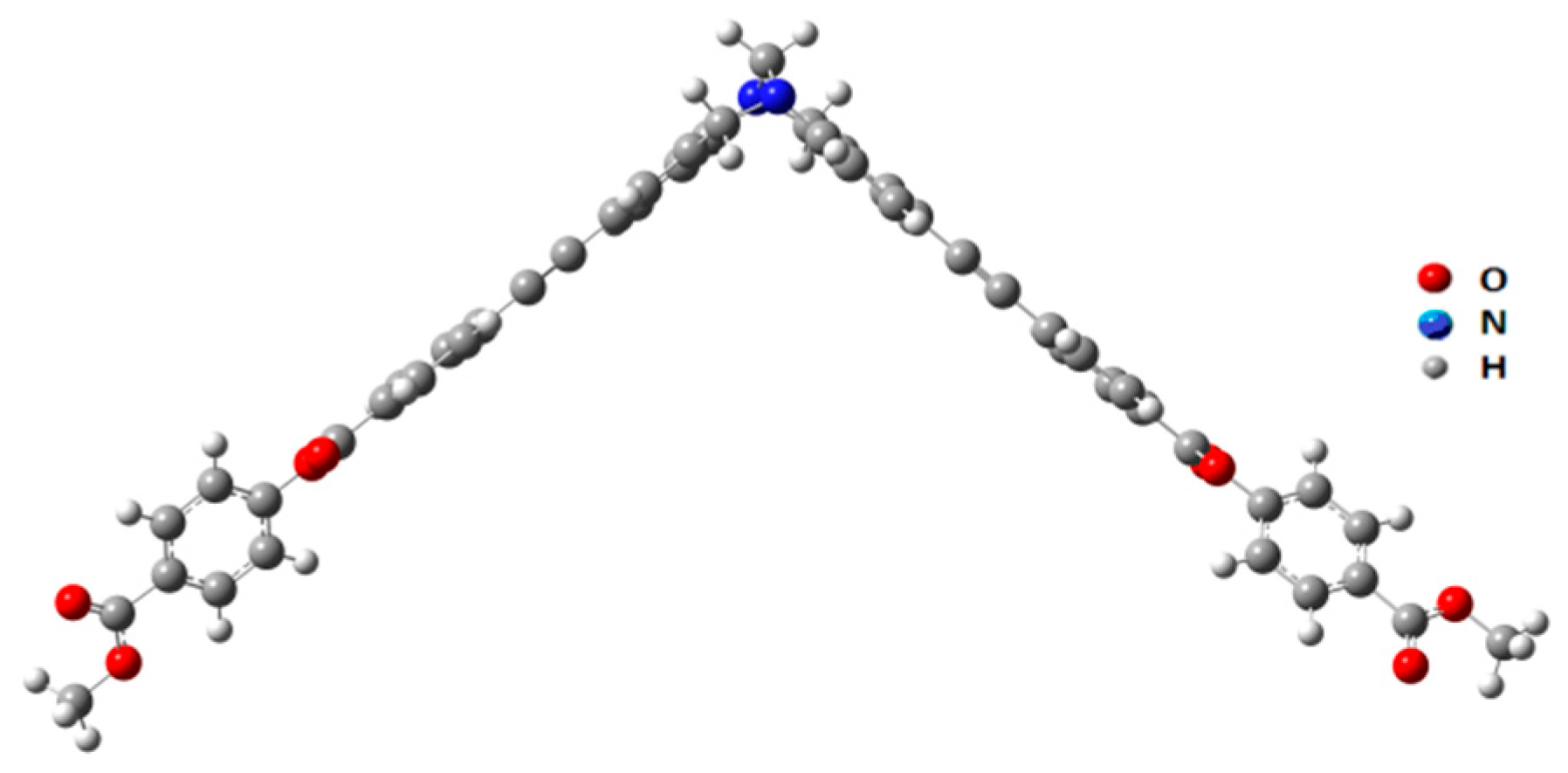
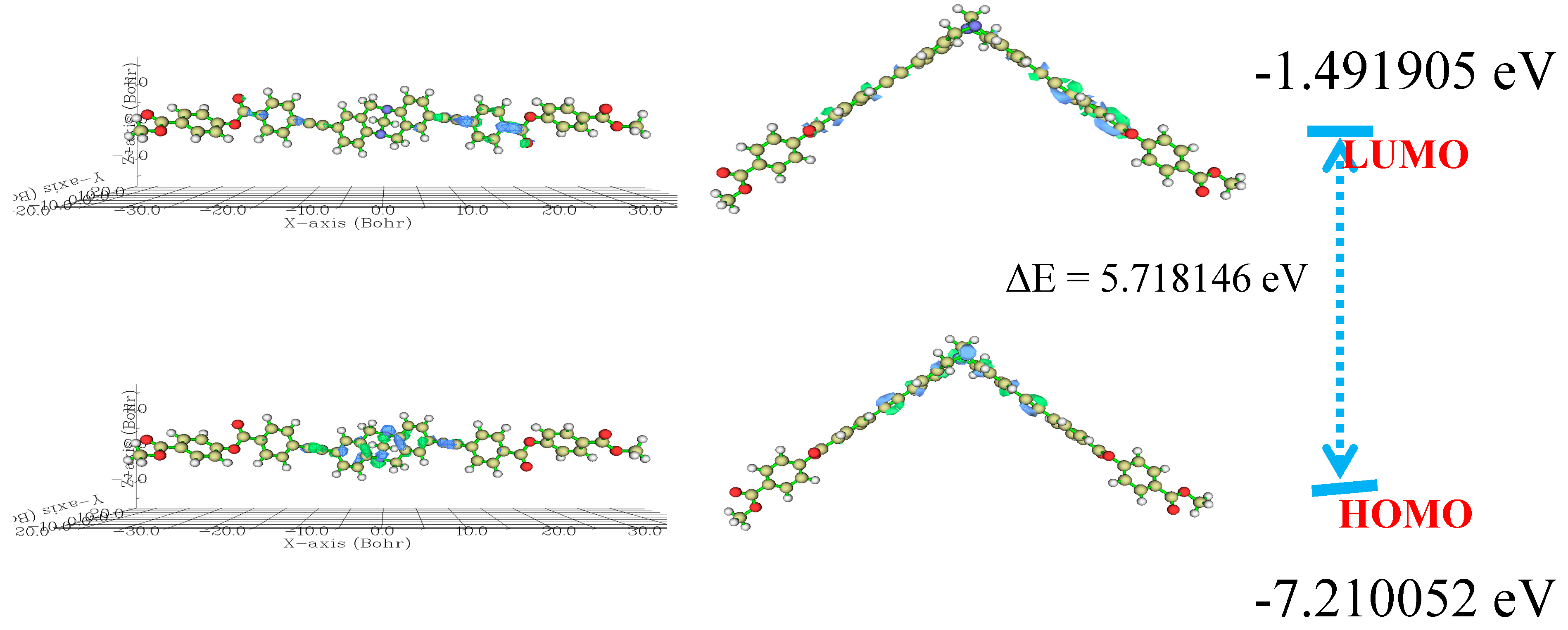
2.7. Theoretical Study
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Date Availability
References
- Harmata, M.; Kahraman, M. Congeners of Troeger’s base as chiral ligands. Tetrahedron Asymmetry 2000, 11, 2875. [Google Scholar] [CrossRef]
- Goldberg, Y.; Alper, H. Transition metal complexes of Tröger’s base and their catalytic activity for the hydrosilylation of alkynes. Tetrahedron Lett. 1995, 36, 369. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Cowart, M.D. New approaches to synthetic receptors. Synthesis and host properties of a water soluble macrocyclic analog of Tröger’s base. Tetrahedron Lett. 1986, 27, 5563. [Google Scholar] [CrossRef]
- Adrian, J.C.; Wilcox, C.S. General effects of binding site water exclusion on hydrogen bond based molecular recognition systems: A closed binding sites is less affected by environmental changes than an open site. J. Am. Chem. Soc. 1992, 114, 1398. [Google Scholar] [CrossRef]
- Crossley, M.J.; Hambley, T.W.; Mackay, L.G. Porphyrin analogues of Tröger’s base: Large chiral cavities with a bimetallic binding site. J. Chem. Soc. Chem. Commun. 1995, 1077. [Google Scholar] [CrossRef]
- Hansson, A.P.; Norrby, P.O.; Wärnmark, K. A bis (crown-ether) analogue of Tröger’s base: Recognition of achiral and chiral primary bisammonium salts. Tetrahedron Lett. 1998, 39, 4565. [Google Scholar] [CrossRef]
- Paliwal, S.; Geib, S.; Wilcox, C.S. Molecular torsion balance for weak molecular recognition forces. effects of “Tilted-T” Edge-to-Face aromatic interactions on conformational selection and solid-state structure. J. Am. Chem. Soc. 1994, 116, 4497–4498. [Google Scholar] [CrossRef]
- Takezoe, H.; Takanishi, Y. Bent-core liquid crystals: Their mysterious and attractive world. Jpn. J. Appl. Phys. 2006, 45. [Google Scholar] [CrossRef]
- Reddy, R.A.; Tschierske, C. Bent-core liquid crystals: Polar order, superstructural chirality and spontaneous desymmetrisation in soft matter systems. J. Mater. Chem. 2006, 16, 907. [Google Scholar] [CrossRef]
- Niori, T.; Sekine, T.; Watanabe, J.; Furukawa, T.; Takezoe, H. Distinct ferroelectric smectic liquid crystals consisting of banana shaped achiral molecules. J. Mater. Chem. 1996, 6, 1231–1233. [Google Scholar] [CrossRef]
- Slattery, J.M.; Bruce, D.W.; Gao, Y.; Shimizu, K.; Slattery, J.M. Liquid-Crystalline Ionic Liquids as Ordered Reaction Media for the Diels–Alder Reaction. Chem. Eur. J. 2016, 1–12. [Google Scholar]
- Ube, T.; Kawasaki, K.; Ikeda, T. Photomobile Liquid-Crystalline Elastomers with Rearrangeable Networks. Adv. Mater. 2016, 28, 8212–8217. [Google Scholar] [CrossRef] [PubMed]
- Link, D.R.; Natale, G.; Shao, R.; Maclennan, J.E.; Clark, N.A.; Körblova, E.; Walba, D.M. Spontaneous formation of macroscopic chiral domains in a fluid smectic phase of achiral molecules. Science 1997, 278, 1924–1927. [Google Scholar] [CrossRef] [PubMed]
- Walba, D.M.; Körblova, E.; Shao, R.; Maclennan, J.E.; Link, D.R.; Glaser, M.A.; Clark, N.A. A ferroelectric liquid crystal conglomerate composed of racemic molecules. Science 2000, 288, 2181. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.A.; Fernsler, J.; Chattham, N. Polarization-modulated smectic liquid crystal phases. Science 2003, 301, 1204. [Google Scholar] [CrossRef] [PubMed]
- Bayón, R.; Coco, S.; Barcenilla, M.; Espinet, P.; Imbuluzqueta, G.; Hidalgo, J.; Rojas, E. Feasibility of Storing Latent Heat with Liquid Crystals. Proof of Concept at Lab Scale. Appl. Sci. 2016, 6, 121. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Srinivasa, H.T.; Kumar, S. Novel banana-discotic hybrid architectures. Beilstein J. Org. Chem. 2009, 5, 52. [Google Scholar] [CrossRef]
- Kumar, S. Self-organization of disc-like molecules: Chemical aspects. Chem. Soc. Rev. 2006, 35, 83. [Google Scholar] [CrossRef]
- Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; Tosoni, M. Discotic liquid crystals: From tailor-made synthesis to plastic electronics. Angew. Chem. Int. Ed. 2007, 46, 4832. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic liquid crystals: A new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902. [Google Scholar] [CrossRef]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev. 2007, 107, 718. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.P.; Chen, Y.M.; Zhou, Y.G.; Guo, W.; Wu, X.D.; Ma, C. An efficient one-pot synthesis of benzo [4, 5] imidazo [1, 2-a] quinoxalines via copper-catalyzed process. Org. Lett. 2013, 15, 5480–5483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Wu, Y.M.; Jia, J.; Zhang, D.J.; Ma, C. One-Pot Synthesis of Benzo [1, 4] thiazin-3 (4 H)-ones and a Theoretical Study of the S–N Type Smiles Rearrangement Mechanism. J. Org. Chem. 2012, 77, 8501–8506. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Niu, X.Y.; Huang, Z.X.; Zhao, C.H.; Liu, Y.; Ma, C. A novel kind of dimmer (excimer)-induced-AIE compound 2-phenylisothiazolo [5, 4-b] pyridin-3 (2H)-one as high selective bisulfite anion probe. Tetrahedron 2013, 69, 8250–8254. [Google Scholar] [CrossRef]
- Yang, B.C.; Tan, X.C.; Guo, R.Y.; Chen, S.W.; Zhang, Z.Y.; Chu, X.L.; Xie, C.X.; Zhang, D.J.; Ma, C. Transition Metal-Free One-Pot Synthesis of Fused 1, 4-Thiazepin-5 (4 H)-ones and Theoretical Study of the S–N Type Smiles Rearrangement Process. J. Org. Chem. 2014, 79, 8040–8048. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.C.; Du, Y.N.; Yang, B.C.; Ma, C. A novel type of AIE material as a highly selective fluorescent sensor for the detection of cysteine and glutathione. RSC Adv. 2015, 5, 55165. [Google Scholar] [CrossRef]
- Tröger, J. Uber einige mittels nascierenden Formaldehyds entstehende Basen (About some bases formed via nascent formaldehyde). J. Prakt Chem. 1887, 36, 225. [Google Scholar] [CrossRef]
- Adrianm, J.C.; Wilcox, C.S. Orderly functional group dyads. Recognition of biotin and adenine derivatives by a new synthetic host. J. Am. Chem. Soc. 1989, 111, 8055. [Google Scholar] [CrossRef]
- Crossley, M.J.; Try, A.C.; Walton, R. Synthesis of accurate distance models of the primary donor-primary acceptor pair of bacterial photosynthetic reaction centres. Tetrahedron Lett. 1996, 37, 6807. [Google Scholar] [CrossRef]
- Try, A.C.; Painter, L.; Harding, M.M. Rigid chiral carbocyclic clefts as building blocks for the construction of new supramolecular hosts. Tetrahedron Lett. 1998, 39, 9809. [Google Scholar] [CrossRef]
- Tatibouët, A.; Demeunynck, M.; Andraud, C.; Collet, A.; Lhomme, J. Synthesis and study of an acridine substituted Tröger’s base: Preferential binding of the (–)-isomer to B-DNA. Chem. Commun. 1999, 23, 161. [Google Scholar] [CrossRef]
- Kubo, Y.; Ohno, T.; Yamanaka, J.; Tkita, S.; Iida, T.Y.; Ishimaru, J. Chirality-transfer control using a heterotopic zinc (II) porphyrin dimer. J. Am. Chem. Soc. 2001, 123, 12700. [Google Scholar] [CrossRef] [PubMed]
- Deprez, N.R.; McNitt, K.A.; Petersen, M.E.; Brown, R.G.; Lewis, D.E. Synthesis and fluorescence properties of naphthalimide-containing Tröger’s bases. Tetrahedron Lett. 2005, 46, 2149–2153. [Google Scholar] [CrossRef]
- Valík, M.; Malina, J.; Palivec, L.; Foltýnová, J.; Tkadlecová, M.; Urbanová, M.; Brabec, V.; Král, V. Tröger’s base scaffold in racemic and chiral fashion as a spacer for bisdistamycin formation. Synthesis and DNA binding study. Tetrahedron 2006, 62, 8591. [Google Scholar] [CrossRef]
- Hof, F.; Schär, M.; Scofield, D.M.; Fischer, F.; Diederich, F.; Sergeyev, S. Preparation of Tröger Base Derivatives by Cross-Coupling Methodologies. Helv. Chim. Acta 2005, 88, 2333–2344. [Google Scholar] [CrossRef]
- Kiehne, U.; Weilandt, T.; Lützen, A. Diastereoselective Self-Assembly of Double-Stranded Helicates from Tröger’s Base Derivatives. Org. Lett. 2007, 9, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Šturala, J.; Cibulka, R. Synthesis of Symmetrical Dinitro- and Diamino- Substituted Tröger’s Base Analogues. Eur. J. Org. Chem. 2012, 36, 7066–7074. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F.S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef]
- Jensen, J.; Tejler, J.; Wärnmark, K. General Protocols for the Synthesis of C 2-Symmetric and Asymmetric 2, 8-Disubstituted Analogues of Tröger’s Base via Efficient Bromine−Lithium Exchanges of 2, 8-Dibromo-6H, 12 H-5, 11-methanodibenzo [b, f][1, 5] diazocine. J. Org. Chem. 2002, 67, 6008–6014. [Google Scholar] [CrossRef]
- Faroughi, M.; Try, A.C.; Klepetko, J.; Turner, P. Changing the shape of Tröger’s base. Tetrahedron Lett. 2007, 48, 6548–6551. [Google Scholar] [CrossRef]
- Vande Velde, C.M.; Didier, D.; Blockhuys, F.; Sergeyev, S. Racemic. 1, 2, 3, 4, 7, 8, 9, 10-octafluoro-6H, 12H-5, 11- methanodibenzo[b, f][1, 5] diazocine: An octafluorinated analogue of Tröger’s base. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, 538. [Google Scholar] [CrossRef] [PubMed]
- Demeunynck, M.; Tatibouët, A. Progress in Heterocyclic Chemistry; Gribble, G.W., Gilchrist, T.L., Eds.; Pergamon: Oxford, UK, 1999; Volume 11, pp. 1–20. [Google Scholar]
- Dolenský, B.; Elguero, J.; Kral, V.; Pardo, C.; Valik, M. Current Tröger’s base chemistry. Adv. Heterocycl. Chem. 2007, 93, 1–56. [Google Scholar]
- Valik, M.; Strongin, R.M.; Král, V. Tröger’s Base Derivatives-New Life for Old Compounds. Supramol. Chem. 2005, 17, 347–367. [Google Scholar] [CrossRef]
- Pardo, C.; Sesmilo, E.; Gutiérrez-Puebla, E.; Monge, A.; Elguero, J.; Fruchier, A. New chiral molecular tweezers with a bis-Tröger’s base skeleton. J. Org. Chem. 2001, 66, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Wilen, S.H.; Qi, J.Z.; Williard, P.G. Resolution, asymmetric transformation, and configuration of Troeger’s base. Application of Troeger’s base as a chiral solvating agent. J. Org. Chem. 1991, 56, 485. [Google Scholar] [CrossRef]
- Boyle, E.M.; Comby, S.; Molloy, J.K.; Gunnlaugsson, T. Thiourea Derived Tröger’s Bases as Molecular Cleft Receptors and Colorimetric Sensors for Anions. J. Org. Chem. 2013, 78, 8312–8319. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.U.; Jalett, H.P.; Lottenbach, W.; Studer, M. Heterogeneous enantioselective hydrogenation of ethyl pyruvate catalyzed by cinchona-modified Pt catalysts: Effect of modifier structure. J. Am. Chem. Soc. 2000, 122, 12675. [Google Scholar] [CrossRef]
- Yuan, C.X.; Tao, X.T.; Ren, Y.; Li, Y.; Yang, J.X.; Yu, W.T.; Wang, L.; Jiang, M.H. Synthesis, structure, and aggregation-induced emission of a novel lambda (Λ)-shaped pyridinium salt based on tröger’s base. J. Phys. Chem. C 2007, 111, 12811. [Google Scholar] [CrossRef]
- Xin, Q.; Tao, X.T.; Wang, F.Z.; Sun, J.L.; Zou, D.C.; Wang, F.J.; Liu, H.J.; Liu, Z.; Ren, Y.; Jiang, M.H. Fluorene-based Tröger’s base analogues: Potential electroluminescent materials. Org. Electron. 2008, 9, 1076–1086. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03 Citation. 2004. Available online: http://gaussian.com/g03citation/ (accessed on 1 November 2016).
Sample Availability: Samples of the compounds 3–8 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Shen, G.; Huang, X.; Liu, R. Design and Synthesis of a Novel Banana-Shaped Functional Molecule via Double Cross-Coupling. Molecules 2019, 24, 698. https://doi.org/10.3390/molecules24040698
Yang B, Shen G, Huang X, Liu R. Design and Synthesis of a Novel Banana-Shaped Functional Molecule via Double Cross-Coupling. Molecules. 2019; 24(4):698. https://doi.org/10.3390/molecules24040698
Chicago/Turabian StyleYang, Bingchuan, Guodong Shen, Xianqiang Huang, and Rutao Liu. 2019. "Design and Synthesis of a Novel Banana-Shaped Functional Molecule via Double Cross-Coupling" Molecules 24, no. 4: 698. https://doi.org/10.3390/molecules24040698
APA StyleYang, B., Shen, G., Huang, X., & Liu, R. (2019). Design and Synthesis of a Novel Banana-Shaped Functional Molecule via Double Cross-Coupling. Molecules, 24(4), 698. https://doi.org/10.3390/molecules24040698