Microbial Asymmetric Functionalization of β-Cyclocitral-Derived Tetramethyl-Substituted γ-Lactone
Abstract
:1. Introduction
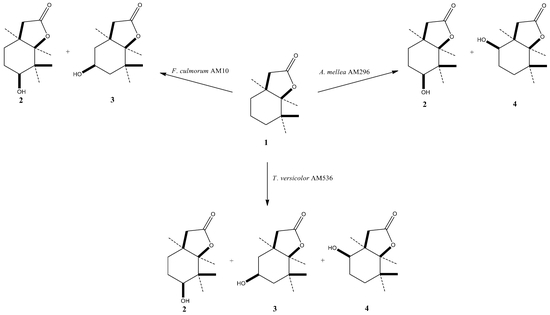
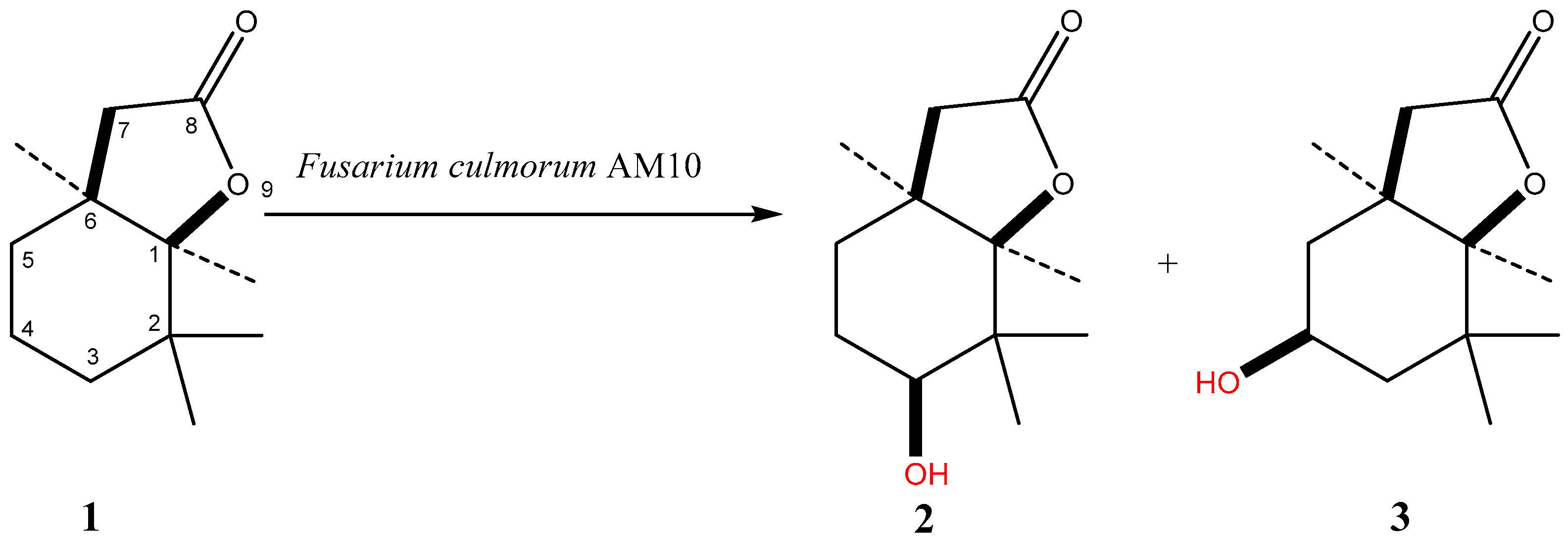
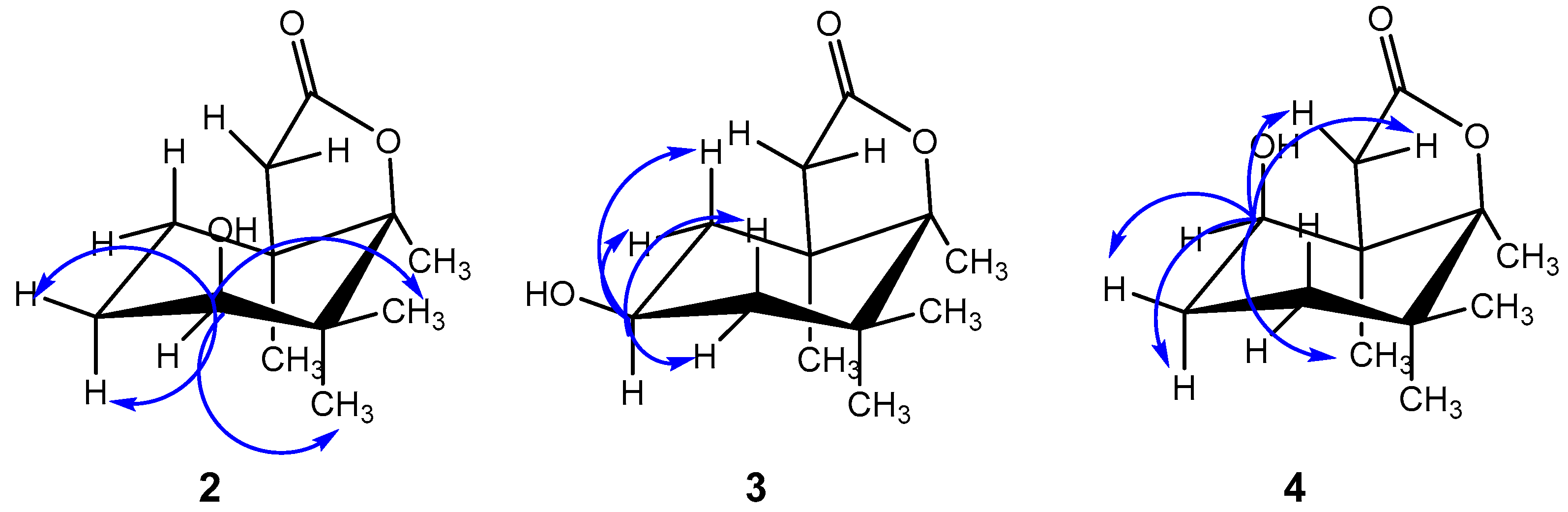
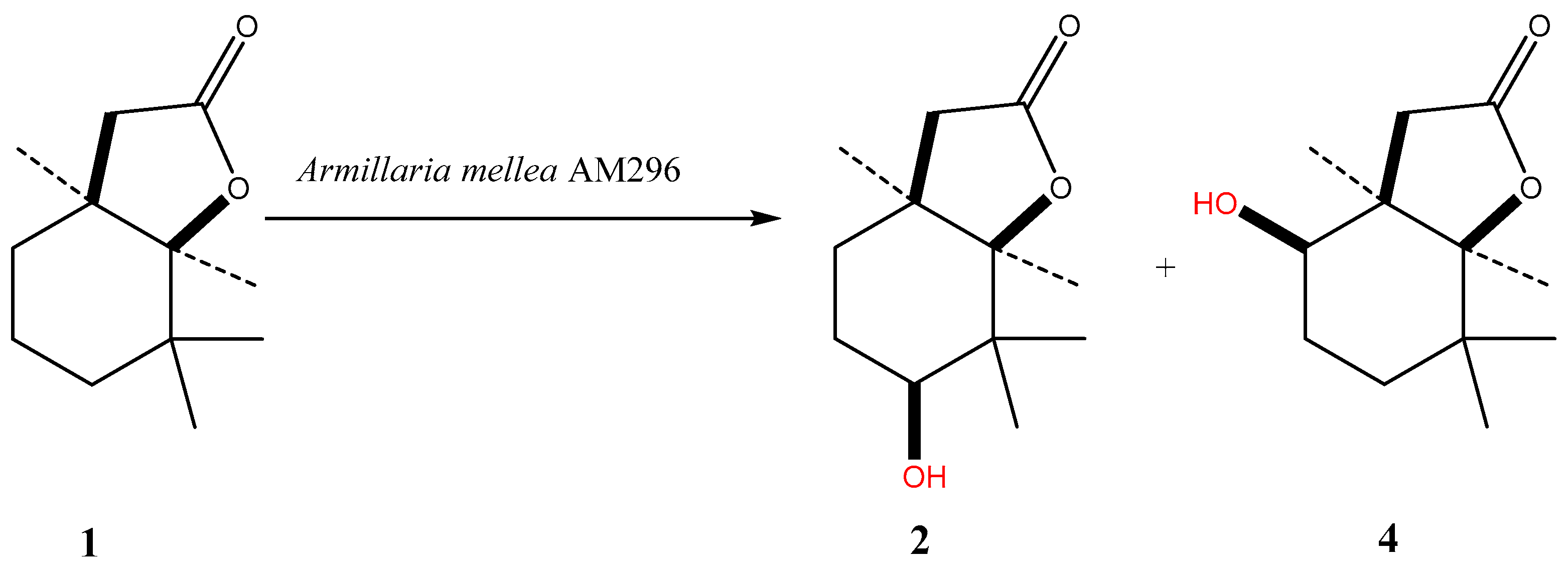
2. Results and Discussion
3. Material and Methods
3.1. Analysis
3.2. Substrates for Biotransformation
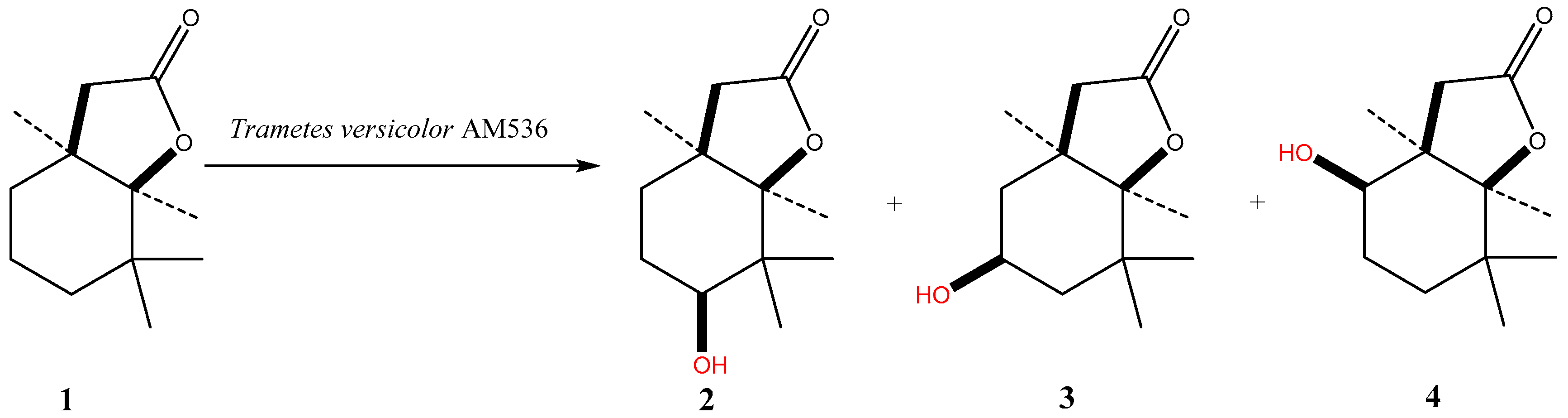
3.3. Microbial Transformations
3.4. Screening Procedure
3.5. Biotransformation
3.6. Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pawlak, A.; Obmińska-Mrukowicz, B.; Rapak, A. The dog as a model for comparative studies of lymphoma and leukemia in humans. Postep. Hig. Med. Dosw. 2013, 67, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Gładkowski, W.; Podkowik, M.; Bania, J.; Nawrot, J.; Białońska, A.; Wawrzeńczyk, C. Lactones 43. New biologically active lactones—β-cyclocitral derivatives. Pest Manag. Sci. 2014, 70, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Gładkowski, W.; Gaurina Srček, V.; Radošević, K.; Maciejewska, G.; Wawrzeńczyk, C. Regio- and enantioselective microbial hydroxylation and evaluation of cytotoxic activity of β-cyclocitral-derived halolactones. PLoS One 2017, 12, e0183429. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, M.C.; Lee, K.W.; Kang, S.M.; Lee, W.W.; Jeon, Y.J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. Coreanum. Algae 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Youn, U.J.; Park, E.J.; Kondratyuk, T.P.; Simmons, C.J.; Borris, R.P.; Tanamatayarat, P.; Wongwiwatthananukit, S.; Toyama, O.; Songsak, T.; Pezzuto, J.M.; et al. Anti-inflammatory sesquiterpene lactone from the flower of Vernonia cinerea. Bioorg. Med. Chem. Lett. 2012, 22, 5559–5562. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Hegazy, M.E.F.; Abd El Aty, A.A.; Ghabbour, H.A.; Alsaid, M.S.; Shahat, A.A.; Paré, P.W. Antimicrobial sesquiterpene lactones from Artemisia sieberi. J. Asian Nat. Prod. Res. 2017, 19, 1093–1101. [Google Scholar] [CrossRef]
- Chung, C.Y.; Liu, C.H.; Burnouf, T.; Wang, G.H.; Chang, S.P.; Jassey, A.; Tai, C.J.; Tai, C.J.; Huang, C.J.; Richardson, C.D.; et al. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir. Res. 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Ito, T.; Aimaiti, S.; Win, N.N.; Kodama, T.; Morita, H. New sesquiterpene lactones, vernonilides A and B, from the seeds of Vernonia anthelmintica in Uyghur and their antiproliferative activities. Bioorg. Med. Chem. Lett. 2016, 26, 3608–3611. [Google Scholar] [CrossRef]
- Liu, Q.; Ahn, J.H.; Kim, S.B.; Lee, C.; Hwang, B.Y.; Lee, M.K. Sesquiterpene lactones from the roots of Lindera strychnifolia. Phytochemistry 2013, 87, 112–118. [Google Scholar] [CrossRef]
- Li, X.W.; Weng, L.; Gao, X.; Zhao, Y.; Pang, F.; Liu, J.H.; Zhang, H.F.; Hu, J.F. Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi. Bioorg. Med. Chem. Lett. 2011, 21, 366–372. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Żarowska, B.; Maciejewska, G.; Dancewicz, K.; Gabryś, B.; Anioł, M. Synthesis, biotransformation and biological activity of halolactones obtained from β-ionone. Tetrahedron 2016, 72, 637–644. [Google Scholar] [CrossRef]
- Wińska, K.; Grabarczyk, M.; Mączka, W.; Zarowska, B.; Maciejewska, G.; Dancewicz, K.; Gabrys, B.; Szumny, A.; Anioł, M. Biotransformation of bicyclic halolactones with a methyl group in the cyclohexane ring into hydroxylactones and their biological activity. Molecules 2016, 21, 1453. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Mączka, W.; Wińska, K.; Żarowska, B.; Anioł, M. Antimicrobial activity of hydroxylactone obtained by biotransformation of bromo- and iodolactone with gem-dimethylcyclohexane ring. J. Braz. Chem. Soc. 2013, 24, 1913–1919. [Google Scholar] [CrossRef]
- Nawrot, J.; Dams, I.; Wawrzeńczyk, C. Feeding deterrent activity of terpenoid lactones with a p-menthane system against stored-product pests. J. Stored Prod. Res. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Popsavin, V.; Srećo, B.; Krstić, I.; Popsavin, M.; Kojić, V.; Bogdanović, G. Synthesis and antitumour activity of new muricatacin and goniofufurone analogues. Eur. J. Med. Chem. 2006, 41, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Skrobiszewski, A.; Gładkowski, W.; Lis, M.; Gliszczyńska, A.; Maciejewska, G.; Klejdysz, T.; Obmińska-Mrukowicz, B.; Nawrot, J.; Wawrzeńczyk, C. Lactones. Part 45. Synthesis of hydroxylactones with aromatic ring and evaluation of their antifeedant and antiproliferative activity. Przem. Chem. 2014, 93, 1637–1643. [Google Scholar]
- Szczepanik, M.; Dams, I.; Wawrzenczyk, C. Terpenoid lactones with the p-menthane system as feeding deterrents to the lesser mealworm, Alphitobius diaperinus. Entomol. Exp. Appl. 2008, 128, 337–345. [Google Scholar] [CrossRef]
- Wińska, K.; Grabarczyk, M.; Mączka, W.; Żarowska, B.; Maciejewska, G.; Anioł, M. Antimicrobial activity of new bicyclic lactones withthree or four methyl groups obtained bothsynthetically. J. Saudi Chem. Soc. 2018, 22, 363–371. [Google Scholar] [CrossRef]
- Wu, H.B.; Wu, H.B.; Wang, W.S.; Liu, T.T.; Qi, M.G.; Feng, J.C.; Li, X.Y.; Liu, Y. Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium abrotanoides. Ind. Crops Prod. 2016, 92, 77–83. [Google Scholar] [CrossRef]
- Roh, C. Biotransformation for multiple regio-selective hydroxylation of isoflavonoid. Biocatal. Agric. Biotechnol. 2013, 2, 403–408. [Google Scholar] [CrossRef]
- Durairaj, P.; Jung, E.; Park, H.H.; Kim, B.G.; Yun, H. Comparative functional characterization of a novel benzoate hydroxylase cytochrome P450 of Fusarium oxysporum. Enzyme Microb. Technol. 2015, 70, 58–65. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, L.; Ge, Z.; Wang, X.; Li, Y.; Liu, X.; Liu, F.; Lu, F. Microbial hydroxylation of steroids by Penicillium decumbens. J. Mol. Catal. B Enzym. 2017, 133, S346–S351. [Google Scholar] [CrossRef]
- Peart, P.C.; Reynolds, W.F.; Reese, P.B. The facile bioconversion of testosterone by alginate-immobilised filamentous fungi. J. Mol. Catal. B Enzym. 2013, 95, 70–81. [Google Scholar] [CrossRef]
- Kollerov, V.V.; Monti, D.; Deshcherevskaya, N.O.; Lobastova, T.G.; Ferrandi, E.E.; Larovere, A.; Gulevskaya, S.A.; Riva, S.; Donova, M.V. Hydroxylation of lithocholic acid by selected actinobacteria and filamentous fungi. Steroids 2013, 78, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Wawrzeńczyk, C. Lactones 41. Synthesis and microbial hydroxylation of unsaturated terpenoid lactones with p-menthane ring systems. Molecules 2013, 18, 2778–2787. [Google Scholar] [CrossRef] [PubMed]
- Świzdor, A.; Kołek, T. Transformations of 4- and 17α-substituted testosterone analogues by Fusarium culmorum. Steroids 2005, 70, 817–824. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Gładkowski, W.; Dancewicz, K.; Gabryś, B.; Szczepanik, M. Transformation of β-damascone to (+)-(S)-4-hydroxy-β-damascone by fungal strains and its evaluation as a potential insecticide against aphids Myzus persicae and lesser mealworm Alphitobius diaperinus Panzer. Catal. Commun. 2016, 80, 39–43. [Google Scholar] [CrossRef]
- Furusawa, M.; Hashimoto, T.; Noma, Y.; Asakawa, Y. Biotransformation of citrus aromatics nootkatone and valencene by microorganisms. Chem. Pharm. Bull. 2005, 53, 1423–1429. [Google Scholar] [CrossRef]
- Draczyńska-Łusiak, B.; Siewiński, A. The ability of Armillariella mellea to transform cyclic monoterpenic alcohols and their acetates. J. Basic Microbiol. 1991, 31, 363–369. [Google Scholar] [CrossRef]
- Draczyńska, B.; Cagara, C.; Siewiński, A.; Rymkiewicz, A.; Zabża, A.; Leufven, A. Biotransformation of pinenes. J. Basic Microbiol. 1985, 25, 457–492. [Google Scholar]
- Wolfand, J.M.; LeFevre, G.H.; Luthy, R.G. Metabolization and degradation kinetics of the urban-use pesticide fipronil by white rot fungus Trametes versicolor. Environ. Sci. Process. Impacts 2016, 18, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-iodo-γ-lactones from cuminaldehyde, 2,5-dimethylbenzaldehyde and piperonal: Chemoenzymatic synthesis and antiproliferative activity. Tetrahedron-Asymmetr. 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Białońska, A.; Poradowski, D.; Drynda, A.; Urbaniak, M. Synthesis and anticancer activity of novel halolactones with β-aryl substituents from simple aromatic aldehydes. Tetrahedron 2013, 69, 10414–10423. [Google Scholar] [CrossRef]
- Pawlak, A.; Gładkowski, W.; Mazur, M.; Henklewska, M.; Obmińska-Mrukowicz, B.; Rapak, A. Optically active stereoisomers of 5-(1-iodoethyl)-4-(4′-isopropylphenyl)dihydrofuran-2-one: The effect of the configuration of stereocenters on apoptosis induction in canine cancer cell lines. Chem. Biol. Interact. 2017, 5, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Gładkowski, W.; Kutkowska, J.; Mazur, M.; Obmińska-Mrukowicz, B.; Rapak, A. Enantiomeric trans β-aryl-δ-iodo-γ-lactones derived from 2,5-dimethylbenzaldehyde induce apoptosis in canine lymphoma cell lines by downregulation of anti-apoptotic Bcl-2 family members Bcl-xL and Bcl-2. Bioorg. Med. Chem. Lett. 2018, 28, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.C.G.; da Silva Melo, P.; Aoyama, H.; Haun, M.; Duran, N.; Ferreira, C.V. Cytotoxic effect of the diterpene lactone Dehydrocrotonin from Croton cajucara on Human Promyelocytic leukemia cells. Planta Med. 2003, 69, 67–69. [Google Scholar] [CrossRef]
- Bouhenna, M.M.; Orlikova, B.; Talhi, O.; Schram, B.; Pinto, D.C.G.A.; Taibi, N.; Bachari, K.; Diederich, M.; Silva, A.M.S.; Mameri, N. Anti-proliferative, cytotoxic and NF-κB inhibitory properties of spiro(lactone-cyclohexanone) compounds in human leukemia. Anticancer Res. 2017, 37, 5225–5233. [Google Scholar]
- Ruengeler, P.; Castro, V.; Mora, G.; Goeren, N.; Vichnewski, W.; Pahl, H.L.; Merfort, I.; Schmidt, T.J. Inhibition of transcription factor NF-κB by sesquiterpene lactones: A proposed molecular mechanism of action. Bioorg. Med. Chem. 1999, 7, 2343–2352. [Google Scholar] [CrossRef]
- Nakaichi, M.; Taura, Y.; Kanki, M.; Mamba, K.; Tsujimoto, H.; Nakama, S. Establishment and characterization of a new canine B-cell leukemia cell Line. J. Vet. Med. Sci. 1996, 58, 469–471. [Google Scholar] [CrossRef]
- Pawlak, A.; Zioło, E.; Kutkowska, J.; Błażejczyk, A.; Wietrzyk, J.; Krupa, A.; Hildebrand, W.; Dzięgiel, P.; Dzimira, S.; Obmińska Mrukowicz, B.; et al. A novel canine B-cell leukemia cell line. Establishment, characterization and sensitivity to chemotherapeutics. Vet. Comp. Oncol. 2017, 15, 1218–1231. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Strain | Time of Incubation (days) | Lactone 1 | Biotransformation Products | ||
|---|---|---|---|---|---|
| 2 | 3 | 4 | |||
| Fusarium culmorum AM10 | 2 | 35 | 28 | 37 | - |
| 4 | 19 | 32 | 49 | - | |
| 7 | 7 | 41 | 52 | - | |
| 10 | 1 | 45 | 53 | - | |
| 12 | 0 | 46 | 53 | - | |
| Armillaria mellea AM296 | 2 | 41 | 53 | - | 6 |
| 4 | 13 | 70 | - | 17 | |
| 7 | 0 | 75 | - | 25 | |
| Trametes versicolor AM536 | 2 | 55 | 31 | 3 | 11 |
| 4 | 20 | 59 | 6 | 15 | |
| 7 | 9 | 68 | 6 | 17 | |
| 10 | 3 | 71 | 7 | 19 | |
| 12 | 2 | 71 | 8 | 19 | |
| 14 | 2 | 72 | 8 | 18 | |
| Cell Line | Compound (IC50 µg/mL) | |||
|---|---|---|---|---|
| 2 | 3 | 4 | Etoposide | |
| CLB 70 | 33.21 ± 2.14 a1 | 32.43 ± 1.72 a | 33.97 ± 3.66 a | 14.31 ± 2.83 |
| GL-1 | 28.67 ± 1.91 a | 26.76 ± 5.65 a | 22.75 ± 1.27 a | 4.4 ± 1.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, M.; Gładkowski, W.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Wawrzeńczyk, C. Microbial Asymmetric Functionalization of β-Cyclocitral-Derived Tetramethyl-Substituted γ-Lactone. Molecules 2019, 24, 666. https://doi.org/10.3390/molecules24040666
Mazur M, Gładkowski W, Pawlak A, Obmińska-Mrukowicz B, Maciejewska G, Wawrzeńczyk C. Microbial Asymmetric Functionalization of β-Cyclocitral-Derived Tetramethyl-Substituted γ-Lactone. Molecules. 2019; 24(4):666. https://doi.org/10.3390/molecules24040666
Chicago/Turabian StyleMazur, Marcelina, Witold Gładkowski, Aleksandra Pawlak, Bożena Obmińska-Mrukowicz, Gabriela Maciejewska, and Czesław Wawrzeńczyk. 2019. "Microbial Asymmetric Functionalization of β-Cyclocitral-Derived Tetramethyl-Substituted γ-Lactone" Molecules 24, no. 4: 666. https://doi.org/10.3390/molecules24040666
APA StyleMazur, M., Gładkowski, W., Pawlak, A., Obmińska-Mrukowicz, B., Maciejewska, G., & Wawrzeńczyk, C. (2019). Microbial Asymmetric Functionalization of β-Cyclocitral-Derived Tetramethyl-Substituted γ-Lactone. Molecules, 24(4), 666. https://doi.org/10.3390/molecules24040666