The Role of Saccharomyces cerevisiae Yeast and Lactic Acid Bacteria in the Formation of 2-Propanol from Acetone during Fermentation of Rye Mashes Obtained Using Thermal-Pressure Method of Starch Liberation
Abstract
1. Introduction
2. Results and Discussion
2.1. Colorimetric and Chromatographic Evaluation of Sweet Mashes
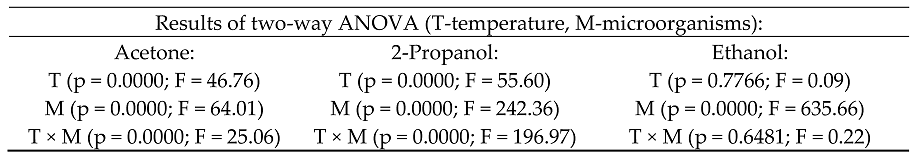
2.2. Fermentation Results of Sweet Mashes Using Yeast and Lactic Acid Bacteria at Different Temperatures
3. Materials and Methods
3.1. Industrial Scale Pressure-Thermal Treatment and Mashing Process
3.2. Laboratory Scale Pressure-Thermal Treatment, Mashing, and Fermentation Processes
- Ethanol Red yeast (Saccharomyces cerevisiae, Fermentis Division S.I. Lesaffre, Marcq-en-Barœul, France) (1.3×107 CFU/mL of mash), with the addition of 80 mg/L IsoStab® hop α-acid preparation (BetaTec GmbH, Nürnberg, Germany), to protect the process from bacterial contamination and prevent microbial infections;
- a mixture of lactic acid bacteria strains Lactococcus lactis ssp. lactis ŁOCK0877 and Lactobacillus casei ŁOCK0901, obtained from the ŁOCK Pure Culture Collection (final quantity 4.0×106 CFU/mL of mash), with the addition of nystatin, to prevent yeast growth.
3.3. Color Measurement
3.4. Determination of Total Sugars and Extract Content in the Mashes
3.5. HPLC Analysis of Fermented Mashes
3.6. Gas Chromatographic Analysis (HS-GC-MS) of Sweet and Fermented Mashes
- Temperature settings: oven temperature 50 °C, loop temperature 60 °C, transfer line temperature 70 °C.
- Timing settings: vial equilibration time 20 min, injection duration 0.7 min, GC cycle time 47 min.
- Vial and loop settings: vial shaking 136 shakes/min, fill pressure 15 psi, vial pressurization gas helium.
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Pielech-Przybylska, K.; Balcerek, M.; Nowak, A.; Wojtczak, M.; Czyżowska, A.; Dziekońska-Kubczak, U.; Patelski, P. The effect of different starch liberation and saccharification methods on the microbial contaminations of distillery mashes, fermentation efficiency, and spirits quality. Molecules 2017, 22, 1647. [Google Scholar] [CrossRef]
- Michalska, A.; Zieliński, H. Maillard reaction products in food. Food Sci. Technol. Quality 2007, 2, 5–16. (In Polish) [Google Scholar]
- Chuyen, N.V. Maillard reaction and food processing. Apllication aspects. In Process-Induced Chemical Changes in Food. Advances in Experimental Medicine and Biology; Shahidi, F., Ho, C.T., van Chuyen, N., Eds.; Springer: Boston, MA, USA, 1998; Volume 434, pp. 213–235. [Google Scholar]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2001, 11, 364–373. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. Changes during the extrusion of semolina in mixture with sugars. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Wong, K.H.; Aziz, S.A.; Mohamed, S. Sensory aroma from Maillard reaction of individual and combinations of amino acids with glucose in acidic conditions. Int. J. Food Sci. Technol. 2008, 43, 1512–1519. [Google Scholar] [CrossRef]
- Mu, K.; Wang, S.; Kitts, D.D. Evidence to indicate that Maillard reaction products can provide selective antimicrobial activity. Integr. Food Nutr. Metab. 2016, 3, 330–335. [Google Scholar] [CrossRef]
- Banerjee, N.; Bhatnagar, R.; Viswanathan, L. Inhibition of glycolysis by furfural in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1981, 11, 226–228. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Strąk, E.; Balcerek, M. Industrial technologies used for the production of ethanol. Acta Sci. Pol. Biotechnol. 2015, 14, 33–44. (In Polish). Available online: http://www.acta.media.pl/pl/action/getfull.php?id=4451 (accessed on 5 January 2019).
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, A.; Kawai, T.; Yamamoto, Y.; Izawa, S.; Kawai, T.; Yamamoto, Y.; Izawa, S. Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A. Damages to naked and covered barley during harvester cropping. Agr. Eng. 2006, 13, 409–415. Available online: https://ir.ptir.org/artykuly/pl/88/IR(88)_454_pl.pdf (accessed on 5 January 2019).
- Karangwa, E.; Zhang, X.; Murekatete, N.; Masamba, K.; Raymond, L.V.; Shabbar, A.; Zhang, Y.; Duhoranimana, E.; Muhoza, B.; Song, S. Effect of substrate type on sensory characteristics and antioxidant capacity of sunflower Maillard reaction products. Eur. Food Res. Technol. 2015, 240, 939–960. [Google Scholar] [CrossRef]
- Kłosowski, G.; Błajet-Kosicka, A. Mechanisms of pyrazine compounds formation and validation of raw material thermal processing during technological process based on the presence of pyrazine in raw spirits. Biotechnologia 2010, 13, 147–160. (In Polish). Available online: http://pfb.info.pl/files/kwartalnik/1_2010/10.%20Klosowski.pdf (accessed on 5 January 2019).
- Cui, H.; Jia, C.; Hayat, K.; Yu, J.; Deng, S.; Karangwa, E.; Duhoranimana, E.; Xiaa, S.; Zhang, X. Controlled formation of flavor compounds by preparation and application of Maillard reaction intermediate (MRI) derived from xylose and phenylalanine. RSC Adv. 2017, 7, 45442–45451. [Google Scholar] [CrossRef]
- Singh, N.; Basu, S.; Vankelecom, I.F.J.; Balakrishnan, M. Covalently immobilized laccase for decolourization of glucose-glycine Maillard products as colourant of distillery wastewater. Appl. Biochem. Biotechnol. 2015, 177, 76–89. [Google Scholar] [CrossRef]
- Rooney, L.W.; Salem, A.; Johnson, J.A. Studies of the carbonyl compounds produced by sugar-amino acids reactions. I. Model systems. Cereal Chem. 1967, 44, 539–550. Available online: http://www.aaccnet.org/publications/cc/backissues/1967/documents/chem44_539.pdf (accessed on 5 January 2019).
- Mansour, A.F.; Pudil, F.; Janda, V.; Pokorný, J. Changes during the extrusion of semolina in mixture with sugars. Czech J. Food Sci. 2001, 19, 24–30. [Google Scholar] [CrossRef]
- Thorne, R.S.W. The assimilation of nitrogen from amino acids by yeast. J. Inst. Brew. 1937, 43, 288–293. [Google Scholar] [CrossRef]
- Sentheshanmuganathan, S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 1960, 74, 568–576. [Google Scholar] [CrossRef]
- Reazin, G.; Scales, H.; Andreasen, A. Mechanism of major congener formation in alcoholic grain fermentations. J. Agric. Food Chem. 1970, 18, 585–589. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Macko, D.; Miklaszewska, B.; Kotarska, K.; Czupryński, B. Influence of various yeast strains and selected starchy raw materials on production of higher alcohols during the alcoholic fermentation process. Eur. Food Res. Technol. 2015, 240, 233–242. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Jiang, R. Improving acetyl-CoA biosynthesis in Saccharomyces cerevisiae via the overexpression of pantothenate kinase and PDH bypass. Biotechnol. Biofuels 2017, 10, 41. [Google Scholar] [PubMed]
- Walther, T.; François, J.M. Microbial production of propanol. Biotechnol. Adv. 2016, 34, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.; Lee, J.; Jang, Y.S.; Lee, S.Y. Metabolic engineering of microorganisms for the production of higher alcohols. MBio. 2014, 5. [Google Scholar] [CrossRef]
- Broda, M.; Leja, K. The microbiological situation of distilleries in Poland. Pol. J. Environ. Stud. 2010, 19, 901–906. [Google Scholar]
- Narendranath, N.V.; Hynes, S.H.; Thomas, K.C.; Ingledew, W.M. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl. Environ. Microbiol. 1997, 63, 4158–4163. [Google Scholar]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Expr. 2018, 8, 10. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Effect of lactobacilli on yeast growth, viability and batch and semi-continuous alcoholic fermentation of corn mash. J. Appl. Microbiol. 2001, 90, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Dongmo, S.N.; Procopio, S.; Sacher, B.; Becker, T. Flavor of lactic acid fermented malt based beverages: Current status and perspectives. Trends Food Sci. Technol. 2016, 54, 37–51. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Salmon, J.C.; Buchin, S.; De Gara, L.; Gobbetti, M. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices International. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ramachandriya, K.D.; Wilkins, M.R.; Delorme, M.J.M.; Zhu, X.; Kundiyana, D.K.; Atiyeh, H.K.; Huhnke, R.L. Reduction of acetone to isopropanol using producer gas fermenting microbes. Biotechnol. Bioeng. 2011, 108, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K. Studies on the microorganism producing isopropanol from acetone. Part 1. Certain facts concerning Lactobacillus brevis var. hofuensis nov. var. J. Gen. Appl. Microbiol. 1960, 6, 141–150. [Google Scholar] [CrossRef]
- Serafim, F.A.T.; Seixas, F.R.F.; Da Silva, A.A.; Galinaro, C.A.; Nascimento, E.S.P.; Buchviser, S.F.; Odello, L.; Franco, D.W. Correlation between chemical composition and sensory properties of Brazilian sugarcane spirits (cachaças). J. Braz. Chem. Soc. 2013, 24, 973–982. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The analysis of raw spirits—A review of methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Różański, M. Effect of starch liberation method and initial pH of sweet mashes on higher alcohols content in distillates obtained from different starchy raw materials. Process Biochem. 2018, 73, 29–37. [Google Scholar] [CrossRef]
- European Parliament, Council of the European Union. Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89. Off. J. Eur. Union 2008, L39, 16–54. [Google Scholar]
- Russian state standard GOST R 56389-2015, Rectified ethyl alcohol from food raw material “Classic”. Specifications. (In Russian). . Available online: http://internet-law.ru/gosts/gost/59786/, 2015 (accessed on 3 February 2019).
- Davídek, T.; Devaud, S.; Robert, F.; Blank, I. Sugar fragmentation in the Maillard reaction cascade: Isotope labeling studies on the formation of acetic acid by a hydrolytic α-dicarbonyl cleavage mechanism. J. Agric. Food Chem. 2006, 54, 6667–6676. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Totsuka, H.; Ono, H. Browning of furfural and amino acids, and a novel yellow compound, furpipate, formed from lysine and furfural. Biosci. Biotechnol. Biochem. 2007, 71, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Arachchi, S.J.T.; Kim, Y.J.; Kim, D.W.; Oh, S.C.; Lee, Y.B. Optimization of Maillard reaction in model system of glucosamine and cysteine using response surface methodology. Prev. Nutr. Food Sci. 2017, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Kinetics of color development in glucose/amino acid model systems at different temperatures. Scientia Agropecuaria 2016, 7, 15–21. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Masoodi, F.A.; Baba, W.N.; Khan, A.A.; Ganie, B.A. Effect of different ripening stages on walnut kernel quality: Antioxidant activities, lipid characterization and antibacterial properties. J. Food Sci. Technol. 2017, 54, 3791–3801. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.B.; Ramaswamy, S.; Plapp, B.V. Yeast alcohol dehydrogenase structure and catalysis. Biochemistry 2014, 53, 5791–5803. [Google Scholar] [CrossRef]
- Green, D.W.; Suns, H.W.; Plapp, B.V. Inversion of the substrate specificity of yeast alcohol dehydrogenase. J. Biol. Chem. 1993, 268, 7792–7798. [Google Scholar]
- Ludwig, B.; Akundi, A.; Kendall, K. A long-chain secondary alcohol dehydrogenase from Rhodococcus erythropolis ATCC 4277. Appl. Environ. Microbiol. 1995, 61, 3729–3733. [Google Scholar]
- Hoshino, K. Studies on the microorganism producing isopropanol from acetone. Part 2. Enzyme investigation on the oxidation- reduction of Lactobacillus brevis var. hofuensis. J. Gen. Appl. Microbiol. 1960, 6, 151–164. Available online: https://www.jstage.jst.go.jp/article/jgam1955/6/3/6_3_151/_pdf (accessed on 5 January 2019). [CrossRef]
- Adamberg, K.; Kask, S.; Laht, T.M.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Garro, M.S.; Savoy de Giori, G. Effect of pH on Lactobacillus fermentum growth, raffinose removal, α-galactosidase activity and fermentation products. Appl. Microbiol. Biotechnol. 2004, 65, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, G.; De Vuyst, L.; Rimaux, T.; Allemeersch, J.; Weckx, S. Adaptation of Lactobacillus plantarum IMDO 130201, a wheat sourdough isolate, to growth in wheat sourdough simulation medium at different pH values through differential gene expression. Appl. Environ. Microbiol. 2011, 77, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.J.; Clydesdale, F.M. Food Colorimetry: Theory and Applications; AVI Publishing: Westport, CN, USA, 1975. [Google Scholar]
- Balcerek, M.; Pielech-Przybylska, K.; Strąk, E.; Patelski, P.; Dziekońska, U. Comparison of fermentation results and quality of the agricultural distillates obtained by application of commercial amylolytic preparations and cereal malts. Eur. Food Res. Technol. 2016, 242, 321–335. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Sample number | L* | a* | b* | YI | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 1 | 83.15ghijkl | 2.58 | 2.08j | 0.06 | 52.23hij | 1.62 | 89.93hi | 2.79 |
| 2 | 85.88ijklm | 2.05 | -0.53d | 0.01 | 40.20abc | 0.96 | 66.32abc | 1.58 |
| 3 | 84.48ghijklm | 2.27 | 1.37hi | 0.04 | 51.68ghi | 1.39 | 87.72gh | 2.35 |
| 4 | 82.70efghij | 1.91 | 2.53k | 0.06 | 54.24jkl | 1.25 | 93.89ij | 2.17 |
| 5 | 84.47ghijklm | 2.01 | 0.51f | 0.01 | 45.12e | 1.08 | 75.67ef | 1.80 |
| 6 | 85.20hijklm | 2.28 | 0.52f | 0.01 | 46.23e | 1.23 | 77.69f | 2.08 |
| 7 | 72.01ab | 1.66 | 14.06t | 0.32 | 78.30u | 1.81 | 155.66r | 3.59 |
| 8 | 87.59m | 2.34 | -1.21ab | 0.03 | 42.60cd | 1.14 | 69.64cd | 1.86 |
| 9 | 81.50efgh | 1.63 | 3.93m | 0.08 | 57.89n | 1.16 | 101.47k | 2.03 |
| 10 | 82.79fghij | 2.28 | 2.15j | 0.06 | 52.05hij | 1.43 | 89.96hi | 2.47 |
| 11 | 84.26ghijklm | 2.61 | 0.78g | 0.02 | 49.30fg | 1.53 | 83.75g | 2.60 |
| 12 | 83.66ghijklm | 1.59 | 1.14hi | 0.02 | 49.17f | 0.94 | 83.90g | 1.60 |
| 13 | 82.69efghij | 1.97 | 1.55i | 0.04 | 49.83fgh | 1.19 | 85.38g | 2.03 |
| 14 | 81.34efgh | 1.88 | 3.31l | 0.08 | 55.26klm | 1.27 | 96.92jk | 2.23 |
| 15 | 82.39efghi | 1.77 | 2.62k | 0.06 | 53.60ijk | 1.15 | 93.10ij | 2.00 |
| 16 | 70.90ab | 2.13 | 14.15t | 0.43 | 74.93t | 2.26 | 150.68p | 4.54 |
| 17 | 78.76def | 2.21 | 6.03n | 0.17 | 61.73o | 1.73 | 112.40l | 3.16 |
| 18 | 81.61efgh | 2.25 | 3.74m | 0.10 | 57.48mn | 1.58 | 100.86k | 2.77 |
| 19 | 78.71de | 2.17 | 5.95n | 0.16 | 62.70o | 1.72 | 114.06l | 3.14 |
| 20 | 76.10cd | 2.04 | 9.10r | 0.24 | 68.88r | 1.85 | 129.79n | 3.48 |
| 21 | 81.73efgh | 2.13 | 3.27l | 0.09 | 56.08lmn | 1.46 | 98.33k | 2.56 |
| 22 | 77.10cd | 1.71 | 7.83o | 0.17 | 65.81p | 1.46 | 121.96m | 2.71 |
| 23 | 81.54efgh | 1.88 | 3.43l | 0.08 | 56.81mn | 1.31 | 99.73k | 2.30 |
| 24 | 80.95efg | 1.77 | 3.82m | 0.08 | 57.02mn | 1.25 | 100.60k | 2.20 |
| 25 | 86.73jklm | 2.37 | -0.76de | 0.02 | 41.59bc | 1.14 | 68.81bcd | 1.88 |
| 26 | 83.02ghijk | 2.22 | 0.75g | 0.02 | 41.90bc | 1.12 | 72.26de | 1.93 |
| 27 | 74.37bc | 1.99 | 11.02s | 0.29 | 71.33s | 1.91 | 137.31o | 3.67 |
| 28 | 87.55m | 2.10 | -1.24ab | 0.03 | 38.32a | 0.92 | 62.43a | 1.50 |
| 29 | 87.01klm | 2.40 | -1.10b | 0.03 | 40.61abc | 1.12 | 66.86abc | 1.84 |
| 30 | 87.18lm | 2.44 | -1.25ab | 0.03 | 39.60ab | 1.11 | 65.00ab | 1.82 |
| 31 | 84.09ghijklm | 1.87 | -0.16e | 0.00 | 44.56de | 0.99 | 75.65ef | 1.68 |
| 32 | 86.69jklm | 1.99 | -0.85c | 0.02 | 39.56ab | 0.91 | 65.26abc | 1.50 |
| 33 | 87.58m | 2.27 | -1.43ab | 0.04 | 38.14a | 0.99 | 62.44a | 1.62 |
| 34 | 70.33a | 1.39 | 14.82u | 0.29 | 76.95tu | 1.52 | 156.02r | 3.09 |
| 35 | 83.75ghijklm | 2.24 | 1.58i | 0.04 | 50.55fgh | 1.35 | 86.42gh | 2.31 |
| 36 | 76.56cd | 2.04 | 8.47p | 0.23 | 66.02p | 1.76 | 123.48m | 3.30 |
| 37 | 86.79jklm | 2.32 | -0.71cd | 0.02 | 40.48abc | 1.08 | 66.78abc | 1.78 |
| Sample number | Furfural (mg/L) | Acetone (mg/L) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| 1 | 3.07g | 0.10 | 1.35l | 0.04 |
| 2 | 2.01de | 0.05 | 0.78cde | 0.02 |
| 3 | 4.09i | 0.11 | 1.06h | 0.03 |
| 4 | 4.30ij | 0.10 | 1.07h | 0.02 |
| 5 | 2.19ef | 0.05 | 0.81de | 0.02 |
| 6 | 0.32a | 0.01 | 1.03gh | 0.03 |
| 7 | 22.73t | 0.52 | 1.91p | 0.04 |
| 8 | 4.00i | 0.11 | 1.08h | 0.03 |
| 9 | 6.47l | 0.18 | 1.24jk | 0.03 |
| 10 | 4.58j | 0.13 | 1.18ij | 0.03 |
| 11 | 4.03i | 0.11 | 1.24ijk | 0.03 |
| 12 | 2.87g | 0.08 | 1.27k | 0.04 |
| 13 | 3.67h | 0.10 | 1.18i | 0.03 |
| 14 | 2.29ef | 0.06 | 1.22ijk | 0.03 |
| 15 | 2.51f | 0.07 | 0.99g | 0.03 |
| 16 | 18.80s | 0.52 | 1.91p | 0.05 |
| 17 | 7.68m | 0.21 | 1.18ij | 0.03 |
| 18 | 6.15k | 0.17 | 1.02gh | 0.03 |
| 19 | 7.46m | 0.21 | 1.46m | 0.04 |
| 20 | 10.23n | 0.28 | 1.66n | 0.05 |
| 21 | 4.30ij | 0.12 | 1.46m | 0.04 |
| 22 | 6.67l | 0.18 | 1.84o | 0.05 |
| 23 | 3.20g | 0.09 | 1.22ijk | 0.03 |
| 24 | 2.50f | 0.07 | 1.02gh | 0.03 |
| 25 | 1.85d | 0.05 | 0.74bc | 0.02 |
| 26 | 0.45ab | 0.01 | 0.76cd | 0.02 |
| 27 | 11.67p | 0.32 | 2.20r | 0.06 |
| 28 | 2.19ef | 0.06 | 0.83e | 0.02 |
| 29 | 2.35ef | 0.06 | 0.69b | 0.02 |
| 30 | 0.58ab | 0.02 | 0.77cd | 0.02 |
| 31 | 1.18c | 0.03 | 0.91f | 0.03 |
| 32 | 0.69b | 0.02 | 0.59a | 0.02 |
| 33 | 0.47ab | 0.01 | 0.61a | 0.02 |
| 34 | 15.49r | 0.43 | 2.40s | 0.07 |
| 35 | 3.17g | 0.09 | 1.05h | 0.03 |
| 36 | 11.11o | 0.31 | 1.23ijk | 0.03 |
| 37 | 3.02g | 0.08 | 0.75bc | 0.02 |
| Compound | L* | a* | b* | YI | ||||
|---|---|---|---|---|---|---|---|---|
| R | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Furfural | −0.8955 | 0.0000 | 0.9297 | 0.0000 | 0.8832 | 0.0000 | 0.9105 | 0.0000 |
| Acetone | −0.8963 | 0.0000 | 0.9018 | 0.0000 | 0.9043 | 0.0000 | 0.9117 | 0.0000 |
| Pairs of Relationships | r | R2 | F | p-Value | Regression Equation |
|---|---|---|---|---|---|
| Furfural vs. L* | −0.8955 | 0.8020 | 141.7559 | 0.0000 | Furfural = 84.9769 − 0.9738 * L* |
| Furfural vs. a* | 0.9297 | 0.8644 | 223.0314 | 0.0000 | Furfural = 1.7217 + 1.0442 * a* |
| Furfural vs. b* | 0.8832 | 0.7800 | 124.0878 | 0.0000 | Furfural = -15.8436 + 0.3944 * b* |
| Furfural vs. YI | 0.9105 | 0.8291 | 169.7743 | 0.0000 | Furfural = −11.3539 + 0.175 * YI |
| Acetone vs. L* | −0.8963 | 0.8034 | 143.0305 | 0.0000 | Acetone = 7.9397 − 0.0824 * L* |
| Acetone vs. a* | 0.9018 | 0.8132 | 152.3914 | 0.0000 | Acetone = 0.9003 + 0.0857 * a* |
| Acetone vs. b* | 0.9043 | 0.8178 | 157.0876 | 0.0000 | Acetone = −0.6367 + 0.0342 * b* |
| Acetone vs. YI | 0.9117 | 0.8311 | 172.2824 | 0.0000 | Acetone = −0.2161 + 0.0148 * YI |
| Sample | Temperature (°C) | Microorganism Added to Sweet Mash (Before Fermentation) | Compound Name | Concentration | |
|---|---|---|---|---|---|
| Mean (n=3) | SD | ||||
| Sweet mash* | - | - | Acetone (mg/L) | 1.11c | 0.04 |
| 2-Propanol (mg/L) | nd | - | |||
| Fermented mash** | 27 | Yeast S. cerevisiae (with addition of α-hop acids) | Acetone (mg/L) | 0.86b | 0.04 |
| 2-Propanol (mg/L) | 0.23b | 0.01 | |||
| Ethanol (g/L) | 54.5B | 4.2 | |||
| Lactic acid (g/L) | nd | - | |||
| Lactic acid bacteria (with addition of nystatin) | Acetone (mg/L) | 0.89b | 0.04 | ||
| 2-Propanol (mg/L) | 0.07a | 0.01 | |||
| Ethanol (g/L) | 0.8A | 0.0 | |||
| Lactic acid (g/L) | 3.1A | 0.4 | |||
| Fermented mash*** | 35 | Yeast S. cerevisiae (with addition of α-hop acids) | Acetone (mg/L) | 0.80b | 0.04 |
| 2-Propanol (mg/L) | 0.27b | 0.01 | |||
| Ethanol (g/L) | 52.9B | 4.4 | |||
| Lactic acid (g/L) | nd | - | |||
| Lactic acid bacteria (with addition of nystatin) | Acetone (mg/L) | 0.23a | 0.02 | ||
| 2-Propanol (mg/L) | 0.79c | 0.04 | |||
| Ethanol (g/L) | 1.2A | 0.1 | |||
| Lactic acid (g/L) | 5.2B | 0.6 | |||
| Cyle of Yeast Inoculation | Compound Name | Concentration | |
|---|---|---|---|
| Mean (n = 3) | SD | ||
| 1st | Lactic acid (g/L) | 1.1a | 0.2 |
| Acetic acid (g/L) | 0.4A | 0.1 | |
| 10th | Lactic acid (g/L) | 2.1b | 0.2 |
| Acetic acid (g/L) | 0.7B | 0.1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Pacholczyk-Sienicka, B.; Ciepielowski, G.; Albrecht, Ł.; Patelski, P. The Role of Saccharomyces cerevisiae Yeast and Lactic Acid Bacteria in the Formation of 2-Propanol from Acetone during Fermentation of Rye Mashes Obtained Using Thermal-Pressure Method of Starch Liberation. Molecules 2019, 24, 610. https://doi.org/10.3390/molecules24030610
Pielech-Przybylska K, Balcerek M, Dziekońska-Kubczak U, Pacholczyk-Sienicka B, Ciepielowski G, Albrecht Ł, Patelski P. The Role of Saccharomyces cerevisiae Yeast and Lactic Acid Bacteria in the Formation of 2-Propanol from Acetone during Fermentation of Rye Mashes Obtained Using Thermal-Pressure Method of Starch Liberation. Molecules. 2019; 24(3):610. https://doi.org/10.3390/molecules24030610
Chicago/Turabian StylePielech-Przybylska, Katarzyna, Maria Balcerek, Urszula Dziekońska-Kubczak, Barbara Pacholczyk-Sienicka, Grzegorz Ciepielowski, Łukasz Albrecht, and Piotr Patelski. 2019. "The Role of Saccharomyces cerevisiae Yeast and Lactic Acid Bacteria in the Formation of 2-Propanol from Acetone during Fermentation of Rye Mashes Obtained Using Thermal-Pressure Method of Starch Liberation" Molecules 24, no. 3: 610. https://doi.org/10.3390/molecules24030610
APA StylePielech-Przybylska, K., Balcerek, M., Dziekońska-Kubczak, U., Pacholczyk-Sienicka, B., Ciepielowski, G., Albrecht, Ł., & Patelski, P. (2019). The Role of Saccharomyces cerevisiae Yeast and Lactic Acid Bacteria in the Formation of 2-Propanol from Acetone during Fermentation of Rye Mashes Obtained Using Thermal-Pressure Method of Starch Liberation. Molecules, 24(3), 610. https://doi.org/10.3390/molecules24030610







