Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction
Abstract
:1. Introduction
2. Results
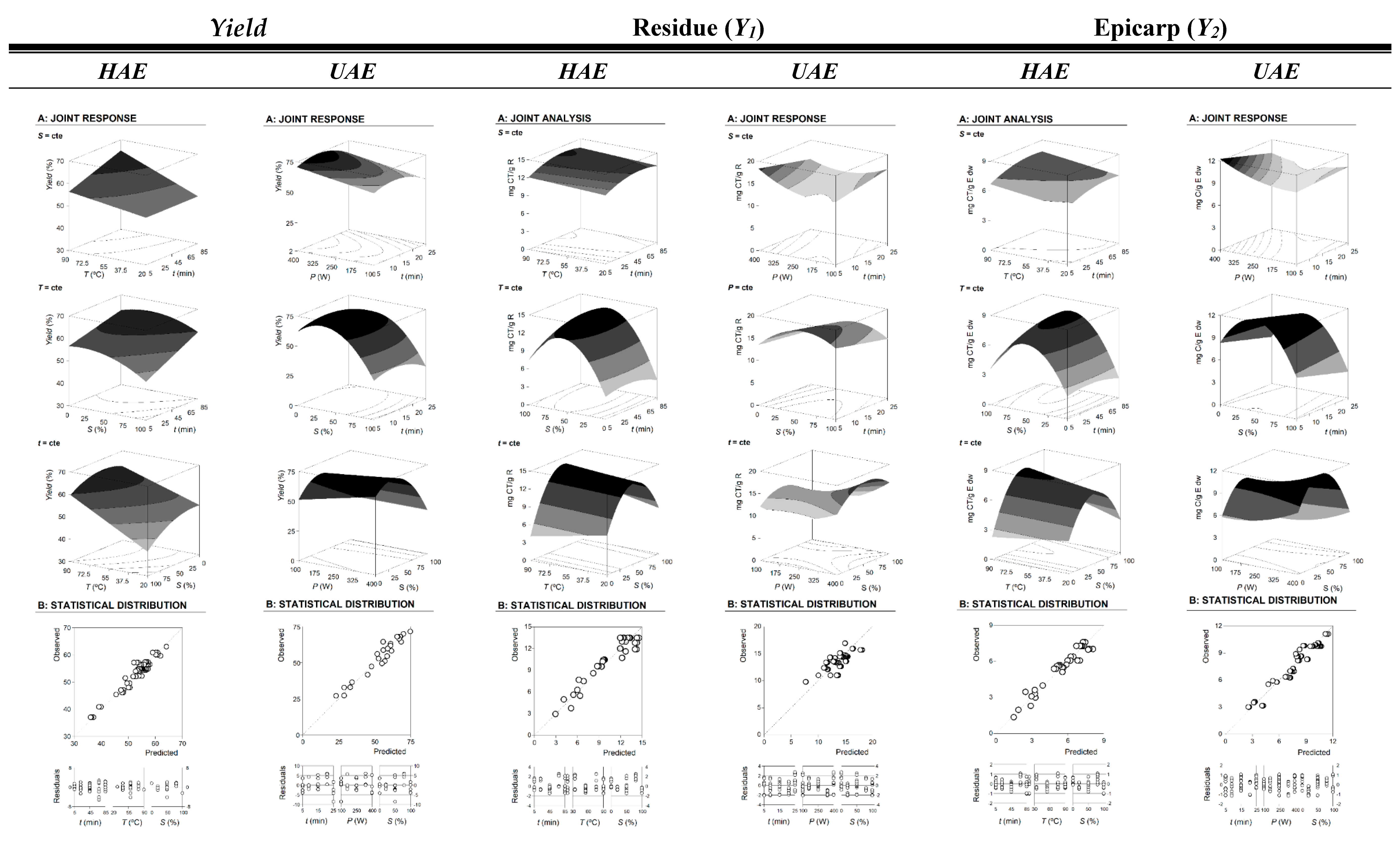
2.1. Development of RSM Models to Optimize Responses and Conditions
- For the HAE technique: In global terms, the significant parametric values within the linear effect (LE) group have a far more relevant contribution to the description of the responses than the interactive effect (IE), with the quadratic effect (QE) group being the less representative one (LE > IE >> QE). In the extraction Yield response, the three variables assessed (t, T, and S) showed similar contributions to its description. Regarding the response values of Y1 (mg CT/g R) and Y2 (mg CT/g E dw), the contribution of the variables is S >> T > t.
- For the UAE technique: The contribution to the description of effects of the responses by the significant parametric values is distributed as LE > QE >> IE. In all the responses assessed (extraction yield, Y1 and Y2 values), the contribution of the variables is S >> P > t.
2.2. Alternative Visual Illustration of the Effects of the Extraction Variables on the Target Responses Used
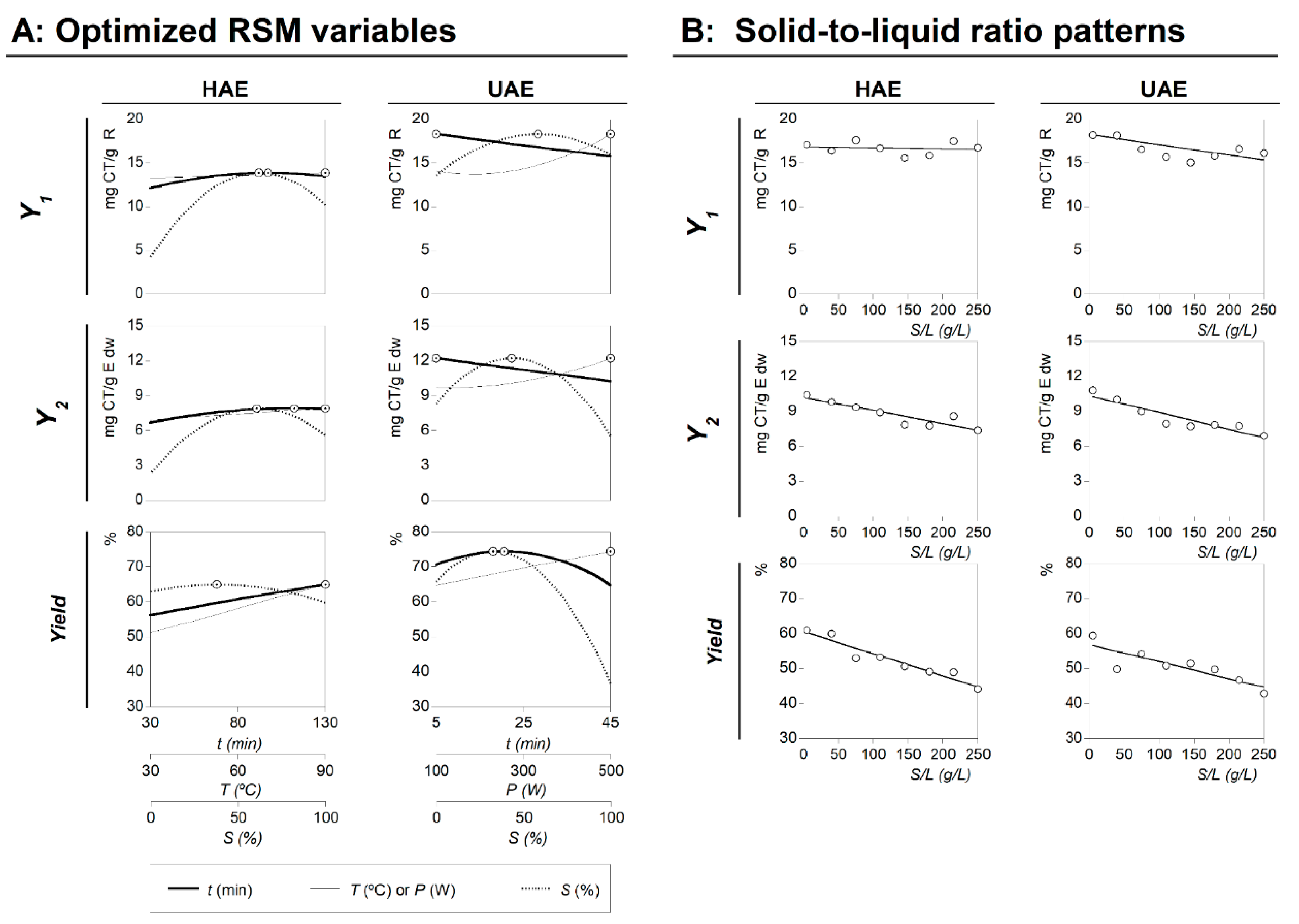
2.3. Conditions That Maximize the Anthocyanins Extraction and Experimental Verification
- For the HAE: the global optimal variable conditions were found at 49.02 ± 2.94 min, 90.00 ± 7.20 °C, and 50.00% ± 0.50% of ethanol, producing maximum response values of 13.93 ± 0.42 mg CT/g R (Y1), 7.93 ± 0.08 mg CT/g E dw (Y2), and 50.89% ± 3.05% (yield of the extracted residue).
- For the UAE: the global optimal variable conditions were found at 5.00 ± 0.15 min, 400.00 ± 32.00 W, and 47.98% ± 2.88% of ethanol, producing maximum response values of 18.17 ± 1.82 mg CT/g R (Y1), 11.76 ± 0.82 mg CT/g E dw (Y2), and 68.60% ± 2.06 % (yield of the extracted residue).
2.4. Dose-Response Analysis of the Solid-to-Liquid Ratio Effect at the Optimal Conditions
- For the Y1 value format, the response of the parametric m value in HAE and UAE presents a non-significant interval of confidence, which means that the changes in the response are not statistically supported and, therefore, the parameter must be considered equal to zero. In other words, the amount of anthocyanins in the extracted residue does not vary as a function of the S/L increase. The extraction values were defined numerically by the intercept parametric value (b) of the linear equation as 14.85 ± 2.29 and 18.25 ± 3.95 mg CT/g R for HAE (R2 = 0.9920) and UAE (R2 = 0.9817), respectively.
- For the Y2 value format, the parametric values for HAE were b = 9.21 ± 1.37 mg CT/g E dw and m = −0.0113 ± 0.0051, with R2 = 0.9566; while for UAE, b = 10.32 ± 1.48 mg CT/g E dw and m = −0.0143 ± 0.0038, with R2 = 0.9244. Negative m values show that the S/L increase leads to a decrease in the extraction ability, obtaining a maximum value of extraction at 5 g/L and a minimum at 250 g/L. However, the observed decrease is slight (less than −0.02), which means that the increase of 1 g/L implies the loss of 0.0113 ± 0.0051 mg CT/g E dw for the HAE process and 0.0143 ± 0.0038 mg CT/g E dw for UAE. Such values produce losses at the maximum tested experimental value (250 g/L) of ~15%, comparative with the one extracted at 5 g/L. Nevertheless, the economic advantages of working at 250 g/L are far more superior than the possible benefits of extracting at the optimal S/L value.
- For the Yield value format, the parametric values for HAE were b = 54.62% ± 4.87% and m = −0.0636 ± 0.0123, with R2 = 0.9516; whereas for UAE, b = 58.90% ± 7.77% and m = −0.0491 ± 0.0116, with R2 = 0.9618. Although, at the initial S/L values, the results obtained for HAE and UAE conducted to similar extraction yields, these values decreased as the S/L increased. The m parametric value is significantly lower for the UAE process, resulting in higher extraction yield values at 250 g/L. These results are in accordance with the conclusions highlighted in the literature, where UAE is reported as enhancing the extraction process by increasing the mass transfer between the plant material and the solvent [23]. The UAE leads to better cell disruption, facilitating the release of the extractable compounds by increasing the contact surface area between the solid and liquid phases [22,23].
2.5. Comparison with Other Studies Involving the Extraction of Anthocyanins
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Procedures for P. Spinosa Fruit Epicarps
4.2.1. Heat Assisted Extraction (HAE)
4.2.2. Ultrasound-Assisted Extraction (UAE)
4.2.3. Post-Extraction Sample Processing
4.3. Identification and Quantification of Anthocyanins by HPLC
4.4. Response Value Formats for Results Presentation
4.5. Experimental Design, Model Analysis, and Statistical Evaluation
4.5.1. RSM Experimental Design
4.5.2. Mathematical Model
4.5.3. Procedure to Optimize the Variables to a Maximum Response
4.5.4. Dose-Response Analysis of the Solid-to-Liquid Ratio
4.6. Mathematical Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Fernández-Ruiz, V.; Sánchez-Mata, M.S.O.S.C.C.; Cámara, M.; Morales, R.; Tardío, J. Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 2013, 115, 176–185. [Google Scholar] [CrossRef]
- Naziri, E.; Nenadis, N.; Mantzouridou, F.T.; Tsimidou, M.Z. Valorization of the major agrifood industrial by-products and waste from Central Macedonia (Greece) for the recovery of compounds for food applications. Food Res. Int. 2014, 65, 350–358. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Ieri, F.; Pinelli, P.; Romani, A. Simultaneous determination of anthocyanins, coumarins and phenolic acids in fruits, kernels and liqueur of Prunus mahaleb L. Food Chem. 2012, 135, 2157–2162. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Hernández-Herrero, J.A.; Frutos, M.J. Degradation kinetics of pigment, colour and stability of the antioxidant capacity in juice model systems from six anthocyanin sources. Int. J. Food Sci. Technol. 2011, 46, 2550–2557. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Dïaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Bals, O.; Grimi, N.; Vorobiev, E. Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innov. Food Sci. Emerg. Technol. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Morales, P.; Ferreira, I.C.F.R. Floral parts of Gomphrena globosa L. as a novel alternative source of betacyanins: Optimization of the extraction using response surface methodology. Food Chem. 2017, 229, 223–234. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Balasubramaniam, V.M. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. 2017, 237, 461–470. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. In Current Opinion in Food Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 7, pp. 20–26. ISBN 2214-7993. [Google Scholar]
- Jiménez, L.C.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. fruits. Food Chem. 2018, 264, 81–91. [Google Scholar]
- Montesano, D.; Fallarino, F.; Cossignani, L.; Simonetti, M.S.; Puccetti, P.; Damiani, P. Innovative extraction procedure for obtaining high pure lycopene from tomato. Eur. Food Res. Technol. 2008, 226, 327–335. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, E.A.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Modern extraction techniques optimized to extract betacyanins from Gomphrena globosa L. Ind. Crops Prod. 2017, 105, 29–40. [Google Scholar] [CrossRef]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I.C.F.R. Extraction of triterpenoids and phenolic compounds from: Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar]
- Chen, M.H.; McClung, A.M.; Bergman, C.J. Phenolic content, anthocyanins and antiradical capacity of diverse purple bran rice genotypes as compared to other bran colors. J. Cereal Sci. 2017, 77, 110–119. [Google Scholar] [CrossRef]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef]
- Elisia, I.; Hu, C.; Popovich, D.G.; Kitts, D.D. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chem. 2006, 101, 1052–1058. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Kamal, A.; Filomena, M.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of ergosterol from Agaricus bisporus L. by-products using response surface methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barros, L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Saraiva, J.A.; Ferreira, I.C.F.R. Cold extraction of phenolic compounds from watercress by high hydrostatic pressure: Process modelling and optimization. Sep. Purif. Technol. 2018, 192, 501–512. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, O.; Ferreira, I.C.F.R. Optimization and comparison of maceration and microwave extraction systems for the production of phenolic compounds from Juglans regia L. for the valorization of walnut leaves. Ind. Crops Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Prieto, M.A.; Barreiro, F.M.F.; Oliveira, M.B.P.; Ferreira, I.C.F.R. Extraction of rosmarinic acid from Melissa officinalis L. by heat-, microwave- and ultrasound-assisted extraction techniques: A comparative study through response surface analysis. Sep. Purif. Technol. 2017, 186, 297–308. [Google Scholar] [CrossRef]
Sample Availability: Samples of the plant and extracts are available from the authors. |

| Experimental Design | HAE | UAE | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coded Values | HAE | UAE | Residue | Individual Content | Total Content | Residue | Individual Content | Total Content | |||||||||||||||
| X1 | X2 | X3 | X1: t | X2: T | X3: S | X1: t | X2: P | X3: S | Yield | Y1C1 | Y1C2 | Y2C1 | Y2C2 | Y1CT | Y2CT | Yield | Y1C1 | Y1C2 | Y2C1 | Y2C2 | Y1CT | Y2CT | |
| min | °C | % | min | W | % | % | mg/g R | mg/g R | mg/g E | mg/g E | mg/g R | mg/g E | % | mg/g R | mg/g R | mg/g E | mg/g E | mg/g R | mg/g E | ||||
| 1 | −1 | −1 | −1 | 21.2 | 34.2 | 20.3 | 9.1 | 160.8 | 20.3 | 53.75 | 6.65 | 3.04 | 3.58 | 1.64 | 9.73 | 5.21 | 61.24 | 9.26 | 3.65 | 5.67 | 2.24 | 12.91 | 7.91 |
| 2 | −1 | −1 | 1 | 21.2 | 34.2 | 79.7 | 9.1 | 160.8 | 79.7 | 45.43 | 7.54 | 4.65 | 3.42 | 2.11 | 12.22 | 5.54 | 52.87 | 9.45 | 4.44 | 5.00 | 2.35 | 13.89 | 7.34 |
| 3 | −1 | 1 | −1 | 21.2 | 75.8 | 20.3 | 9.1 | 339.2 | 20.3 | 57.26 | 6.08 | 2.81 | 3.48 | 1.61 | 8.78 | 5.09 | 70.17 | 8.96 | 3.44 | 6.29 | 2.41 | 12.40 | 8.70 |
| 4 | −1 | 1 | 1 | 21.2 | 75.8 | 79.7 | 9.1 | 339.2 | 79.7 | 48.85 | 8.35 | 4.44 | 4.08 | 2.17 | 12.74 | 6.25 | 58.80 | 9.36 | 4.51 | 5.50 | 2.65 | 13.87 | 8.15 |
| 5 | 1 | −1 | −1 | 68.8 | 34.2 | 20.3 | 20.9 | 160.8 | 20.3 | 55.45 | 6.88 | 2.90 | 3.82 | 1.61 | 9.66 | 5.42 | 59.12 | 10.66 | 3.69 | 6.30 | 2.18 | 14.35 | 8.48 |
| 6 | 1 | −1 | 1 | 68.8 | 34.2 | 79.7 | 20.9 | 160.8 | 79.7 | 50.00 | 10.05 | 4.05 | 5.03 | 2.02 | 14.03 | 7.05 | 55.22 | 8.99 | 4.14 | 4.97 | 2.29 | 13.14 | 7.25 |
| 7 | 1 | 1 | −1 | 68.8 | 75.8 | 20.3 | 20.9 | 339.2 | 20.3 | 60.18 | 6.72 | 2.81 | 4.04 | 1.69 | 9.37 | 5.73 | 67.05 | 8.79 | 2.86 | 5.90 | 1.92 | 11.65 | 7.82 |
| 8 | 1 | 1 | 1 | 68.8 | 75.8 | 79.7 | 20.9 | 339.2 | 79.7 | 53.09 | 11.01 | 4.39 | 5.84 | 2.33 | 15.26 | 8.17 | 57.19 | 8.69 | 4.24 | 4.97 | 2.42 | 12.92 | 7.39 |
| 9 | −1.68 | 0 | 0 | 5 | 55 | 50 | 5 | 250 | 50 | 53.88 | 8.15 | 3.80 | 4.39 | 2.05 | 12.01 | 6.44 | 68.58 | 10.53 | 4.77 | 7.22 | 3.27 | 15.30 | 10.49 |
| 10 | 1.68 | 0 | 0 | 85 | 55 | 50 | 25 | 250 | 50 | 56.51 | 9.09 | 3.70 | 5.14 | 2.09 | 12.74 | 7.23 | 56.13 | 10.14 | 4.56 | 5.69 | 2.56 | 14.71 | 8.25 |
| 11 | 0 | −1.68 | 0 | 45 | 20 | 50 | 15 | 100 | 50 | 49.49 | 11.09 | 4.70 | 5.49 | 2.33 | 15.59 | 7.82 | 55.99 | 12.41 | 5.45 | 6.95 | 3.05 | 17.86 | 10.00 |
| 12 | 0 | 1.68 | 0 | 45 | 90 | 50 | 15 | 400 | 50 | 60.78 | 8.68 | 3.42 | 5.27 | 2.08 | 11.96 | 7.36 | 76.95 | 10.60 | 4.40 | 8.16 | 3.38 | 15.00 | 11.54 |
| 13 | 0 | 0 | −1.68 | 45 | 55 | 0 | 15 | 250 | 0 | 54.73 | 3.81 | 1.63 | 2.09 | 0.89 | 5.46 | 2.98 | 50.18 | 8.22 | 1.92 | 4.12 | 1.19 | 10.14 | 5.31 |
| 14 | 0 | 0 | 1.68 | 45 | 55 | 100 | 15 | 250 | 100 | 47.62 | 5.68 | 2.69 | 2.70 | 1.28 | 8.23 | 3.99 | 34.40 | 11.06 | 1.15 | 3.81 | 0.39 | 12.21 | 4.20 |
| 15 | −1.68 | −1.68 | −1.68 | 5 | 20 | 0 | 5 | 100 | 0 | 54.39 | 4.24 | 1.85 | 2.30 | 1.01 | 6.01 | 3.31 | 47.94 | 8.15 | 3.73 | 3.91 | 1.79 | 11.88 | 5.69 |
| 16 | −1.68 | −1.68 | 1.68 | 5 | 20 | 100 | 5 | 100 | 100 | 36.34 | 2.45 | 1.65 | 0.89 | 0.60 | 4.12 | 1.49 | 33.12 | 6.25 | 1.50 | 2.07 | 0.50 | 7.75 | 2.57 |
| 17 | −1.68 | 1.68 | −1.68 | 5 | 90 | 0 | 5 | 400 | 0 | 56.79 | 3.53 | 1.59 | 2.00 | 0.90 | 5.13 | 2.90 | 61.16 | 10.89 | 4.46 | 6.66 | 2.73 | 15.36 | 9.39 |
| 18 | −1.68 | 1.68 | 1.68 | 5 | 90 | 100 | 5 | 400 | 100 | 47.24 | 4.51 | 2.39 | 2.13 | 1.13 | 6.88 | 3.26 | 28.92 | 11.57 | 4.64 | 3.34 | 1.34 | 16.21 | 4.69 |
| 19 | 1.68 | −1.68 | −1.68 | 85 | 20 | 0 | 25 | 100 | 0 | 51.72 | 4.45 | 2.00 | 2.30 | 1.03 | 6.40 | 3.33 | 45.30 | 9.99 | 4.55 | 4.53 | 2.06 | 14.54 | 6.59 |
| 20 | 1.68 | −1.68 | 1.68 | 85 | 20 | 100 | 25 | 100 | 100 | 39.88 | 4.46 | 1.75 | 1.78 | 0.70 | 6.21 | 2.48 | 28.91 | 9.60 | 1.71 | 2.78 | 0.50 | 11.31 | 3.27 |
| 21 | 1.68 | 1.68 | −1.68 | 85 | 90 | 0 | 25 | 400 | 0 | 64.04 | 2.14 | 0.78 | 1.37 | 0.50 | 2.95 | 1.87 | 51.96 | 9.85 | 3.88 | 5.12 | 2.02 | 13.73 | 7.13 |
| 22 | 1.68 | 1.68 | 1.68 | 85 | 90 | 100 | 25 | 400 | 100 | 61.84 | 6.75 | 2.82 | 4.17 | 1.74 | 9.54 | 5.91 | 23.34 | 10.08 | 3.72 | 2.35 | 0.87 | 13.80 | 3.22 |
| 23 | 0 | 0 | 0 | 45 | 55 | 50 | 15 | 250 | 50 | 56.07 | 8.65 | 3.78 | 4.85 | 2.12 | 12.53 | 6.97 | 65.72 | 10.85 | 4.50 | 7.13 | 2.96 | 15.35 | 10.08 |
| 24 | 0 | 0 | 0 | 45 | 55 | 50 | 15 | 250 | 50 | 56.55 | 8.79 | 4.13 | 4.97 | 2.34 | 13.00 | 7.31 | 65.90 | 10.93 | 4.34 | 7.20 | 2.86 | 15.27 | 10.06 |
| 25 | 0 | 0 | 0 | 45 | 55 | 50 | 15 | 250 | 50 | 54.57 | 8.99 | 4.09 | 4.91 | 2.23 | 13.22 | 7.14 | 66.06 | 10.44 | 4.04 | 6.89 | 2.67 | 14.47 | 9.56 |
| 26 | 0 | 0 | 0 | 45 | 55 | 50 | 15 | 250 | 50 | 54.35 | 8.65 | 3.78 | 4.70 | 2.05 | 12.36 | 6.76 | 67.94 | 11.08 | 4.35 | 7.53 | 2.96 | 15.43 | 10.48 |
| 27 | 0 | 0 | 0 | 45 | 55 | 50 | 15 | 250 | 50 | 54.57 | 9.26 | 4.20 | 5.02 | 2.27 | 13.33 | 7.14 | 67.80 | 10.27 | 4.09 | 6.96 | 2.77 | 14.36 | 9.74 |
| 28 | 0 | 0 | 0 | 45 | 55 | 50 | 15 | 250 | 50 | 54.35 | 9.12 | 4.18 | 5.02 | 2.30 | 13.37 | 6.76 | 68.10 | 10.26 | 3.92 | 6.99 | 2.67 | 14.18 | 9.66 |
| Parameters | Residue | Individual Content | Total Content | |||||
|---|---|---|---|---|---|---|---|---|
| Yield | Y1C1 | Y1C2 | Y2C1 | Y2C2 | Y1CT | Y2CT | ||
| HAE | ||||||||
| Intercept | b0 | 54.86 ± 0.72 | 9.35 ± 0.38 | 4.15 ± 0.19 | 5.07 ± 0.17 | 2.26 ± 0.08 | 13.48 ± 0.55 | 7.29 ± 0.23 |
| Linear effect | b1 | 1.54 ± 0.43 | 0.33 ± 0.21 | ns | 0.25 ± 0.09 | ns | 0.28 ± 0.21 | 0.26 ± 0.13 |
| b2 | 3.12 ± 0.43 | ns | ns | 0.15 ± 0.09 | 0.05 ± 0.02 | ns | 0.19 ± 0.13 | |
| b3 | −3.07 ± 0.43 | 0.56 ± 0.21 | 0.33 ± 0.11 | 0.17 ± 0.09 | 0.11 ± 0.05 | 0.88 ± 0.31 | 0.27 ± 0.13 | |
| Quadratic effect | b11 | ns | −0.26 ± 0.21 | −0.09 ± 0.07 | −0.10 ± 0.05 | −0.05 ± 0.03 | −0.35 ± 0.26 | −0.16 ± 0.15 |
| b22 | ns | ns | ns | ns | ns | ns | ns | |
| b33 | −1.24 ± 0.42 | −1.60 ± 0.26 | −0.69 ± 0.13 | −0.94 ± 0.11 | −0.40 ± 0.06 | −2.26 ± 0.37 | −1.33 ± 0.15 | |
| Interactive effect | b12 | 0.78 ± 0.31 | 0.00 ± 0.00 | ns | ns | ns | ns | ns |
| b13 | 0.54 ± 0.31 | 0.26 ± 0.15 | 0.04 ± 0.02 | 0.18 ± 0.07 | 0.04 ± 0.03 | 0.31 ± 0.22 | 0.22 ± 0.09 | |
| b23 | 0.69 ± 0.31 | 0.31 ± 0.15 | 0.14 ± 0.08 | 0.21 ± 0.07 | 0.09 ± 0.03 | 0.45 ± 0.22 | 0.29 ± 0.09 | |
| Statistics (R²) | 0.9375 | 0.9100 | 0.8755 | 0.9443 | 0.9272 | 0.9046 | 0.9489 | |
| UAE | ||||||||
| Intercept | b0 | 68.11 ± 1.70 | 10.42 ± 0.47 | 4.10 ± 0.28 | 6.98 ± 0.22 | 2.83 ± 0.13 | 14.46 ± 0.62 | 9.75 ± 0.31 |
| Linear effect | b1 | −1.70 ± 0.96 | ns | ns | −0.13 ± 0.12 | −0.10 ± 0.07 | ns | −0.22 ± 0.17 |
| b2 | 2.12 ± 0.96 | 0.23 ± 0.21 | 0.18 ± 0.15 | 0.25 ± 0.12 | 0.14 ± 0.07 | 0.37 ± 0.35 | 0.36 ± 0.17 | |
| b3 | −6.46 ± 0.96 | ns | −0.16 ± 0.15 | −0.55 ± 0.12 | −0.26 ± 0.07 | ns | −0.82 ± 0.17 | |
| Quadratic effect | b11 | −2.29 ± 1.16 | ns | 0.26 ± 0.20 | −0.13 ± 0.11 | ns | ns | ns |
| b22 | ns | ns | 0.35 ± 0.20 | ns | 0.19 ± 0.09 | 0.66 ± 0.42 | 0.27 ± 0.21 | |
| b33 | −7.33 ± 1.16 | −0.36 ± 0.27 | −0.84 ± 0.20 | −1.02 ± 0.15 | −0.68 ± 0.09 | −1.22 ± 0.42 | −1.84 ± 0.21 | |
| Interactive effect | b12 | ns | −0.34 ± 0.20 | −0.11 ± 0.11 | −0.18 ± 0.09 | −0.07 ± 0.05 | −0.46 ± 0.25 | −0.23 ± 0.12 |
| b13 | ns | ns | ns | ns | ns | ns | ns | |
| b23 | −1.29 ± 0.69 | 0.17 ± 0.10 | 0.22 ± 0.11 | −0.08 ± 0.05 | ns | 0.41 ± 0.25 | ns | |
| Statistics (R²) | 0.9431 | 0.7825 | 0.9032 | 0.9316 | 0.9035 | 0.8986 | 0.9380 | |
| Criteria | Optimal Variable Conditions | Optimum Response | |||||
|---|---|---|---|---|---|---|---|
| X1: t (min) | X2: T (°C) or P(W) | X3: S (%) | |||||
| (A) Individual optimal variable conditions | |||||||
| HAE | Yield | 85.00 ± 8.50 | 90.00 ± 4.50 | 38.01 ± 3.04 | 65.10 ± 3.91 | % | |
| Y1 | C1 | 64.89 ± 5.19 | 90.00 ± 8.10 | 62.01 ± 3.10 | 9.71 ± 0.49 | mg C1/g R | |
| C2 | 47.18 ± 3.30 | 90.00 ± 7.20 | 62.22 ± 6.22 | 4.27 ± 0.34 | mg C2/g R | ||
| CT | 58.85 ± 5.89 | 90.00 ± 9.00 | 61.97 ± 6.20 | 13.89 ± 0.14 | mg CT/g R | ||
| Y2 | C1 | 84.27 ± 0.84 | 90.00 ± 3.60 | 63.11 ± 3.79 | 5.64 ± 0.51 | mg C1/g E dw | |
| C2 | 48.06 ± 1.92 | 90.00 ± 0.90 | 59.98 ± 3.60 | 2.38 ± 0.21 | mg C2/g E dw | ||
| CT | 70.76 ± 5.66 | 90.00 ± 4.50 | 60.82 ± 3.04 | 7.89 ± 0.55 | mg CT/g E dw | ||
| UAE | Yield | 12.79 ± 0.51 | 400.00 ± 32.00 | 32.51 ± 1.95 | 74.53 ± 2.24 | % | |
| Y1 | C1 | 5.00 ± 0.10 | 400.00 ± 28.00 | 61.80 ± 1.85 | 11.82 ± 0.71 | mg C1/g R | |
| C2 | 5.00 ± 0.10 | 400.00 ± 28.00 | 53.58 ± 1.07 | 6.45 ± 0.45 | mg C2/g R | ||
| CT | 5.00 ± 0.10 | 400.00 ± 20.00 | 58.39 ± 4.09 | 18.32 ± 1.47 | mg CT/g R | ||
| Y2 | C1 | 5.00 ± 0.50 | 400.00 ± 4.00 | 40.11 ± 2.81 | 7.88 ± 0.16 | mg C1/g E dw | |
| C2 | 5.00 ± 0.40 | 400.00 ± 28.00 | 44.35 ± 4.43 | 3.96 ± 0.28 | mg C2/g E dw | ||
| CT | 5.00 ± 0.35 | 400.00 ± 20.00 | 43.37 ± 4.34 | 12.23 ± 0.86 | mg CT/g E dw | ||
| (B) Global optimal variable conditions | |||||||
| HAE | Yield | 49.02 ± 2.94 | 90.00 ± 7.20 | 50.00 ± 0.50 | 50.89 ± 3.05 | % | |
| Y1 | C1 | 9.71 ± 0.29 | mg C1/g R | ||||
| C2 | 4.22 ± 0.13 | mg C2/g R | |||||
| CT | 13.93 ± 0.42 | mg CT/g R | |||||
| Y2 | C1 | 5.57 ± 0.11 | mg C1/g E dw | ||||
| C2 | 2.36 ± 0.05 | mg C2/g E dw | |||||
| CT | 7.93 ± 0.08 | mg CT/g E dw | |||||
| UAE | Yield | 5.00 ± 0.15 | 400.00 ± 32.00 | 47.98 ± 2.88 | 68.60 ± 2.06 | % | |
| Y1 | C1 | 11.74 ± 0.23 | mg C1/g R | ||||
| C2 | 6.43 ± 0.32 | mg C2/g R | |||||
| CT | 18.17 ± 1.82 | mg CT/g R | |||||
| Y2 | C1 | 7.81 ± 0.47 | mg C1/g E dw | ||||
| C2 | 3.95 ± 0.24 | mg C2/g E dw | |||||
| CT | 11.76 ± 0.82 | mg CT/g E dw | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leichtweis, M.G.; Pereira, C.; Prieto, M.A.; Barreiro, M.F.; Baraldi, I.J.; Barros, L.; Ferreira, I.C.F.R. Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction. Molecules 2019, 24, 573. https://doi.org/10.3390/molecules24030573
Leichtweis MG, Pereira C, Prieto MA, Barreiro MF, Baraldi IJ, Barros L, Ferreira ICFR. Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction. Molecules. 2019; 24(3):573. https://doi.org/10.3390/molecules24030573
Chicago/Turabian StyleLeichtweis, Maria G., Carla Pereira, M.A. Prieto, Maria Filomena Barreiro, Ilton José Baraldi, Lillian Barros, and Isabel C.F.R. Ferreira. 2019. "Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction" Molecules 24, no. 3: 573. https://doi.org/10.3390/molecules24030573
APA StyleLeichtweis, M. G., Pereira, C., Prieto, M. A., Barreiro, M. F., Baraldi, I. J., Barros, L., & Ferreira, I. C. F. R. (2019). Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction. Molecules, 24(3), 573. https://doi.org/10.3390/molecules24030573