Degradation of Proteins and Starch by Combined Immobilization of Protease, α-Amylase and β-Galactosidase on a Single Electrospun Nanofibrous Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Enzyme Assays
3. Experimental Section
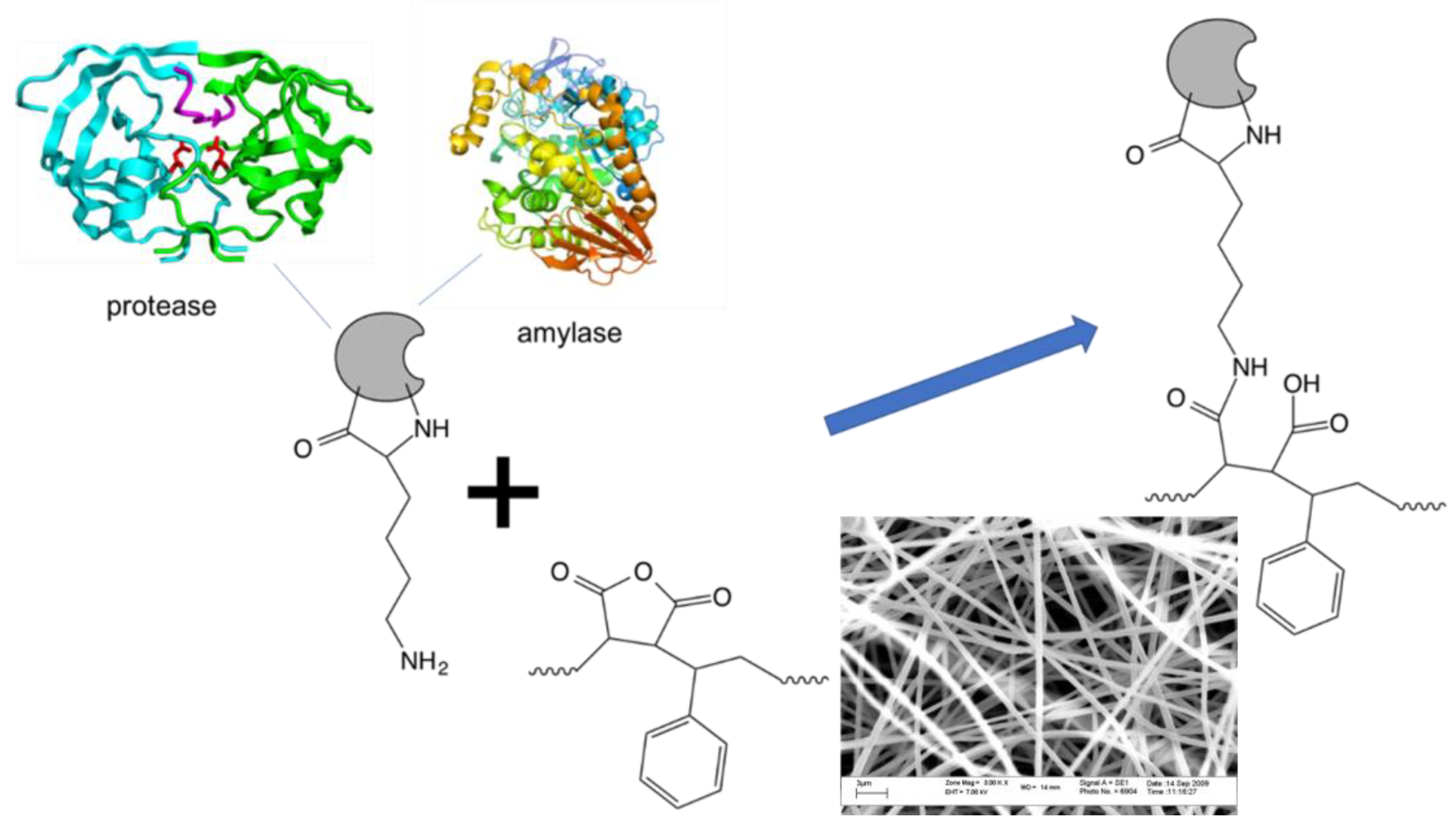
3.1. Electospinning of Poly(styrene-alt-MAnh) Nanofibrous Mats
3.2. Enzyme Immobilization
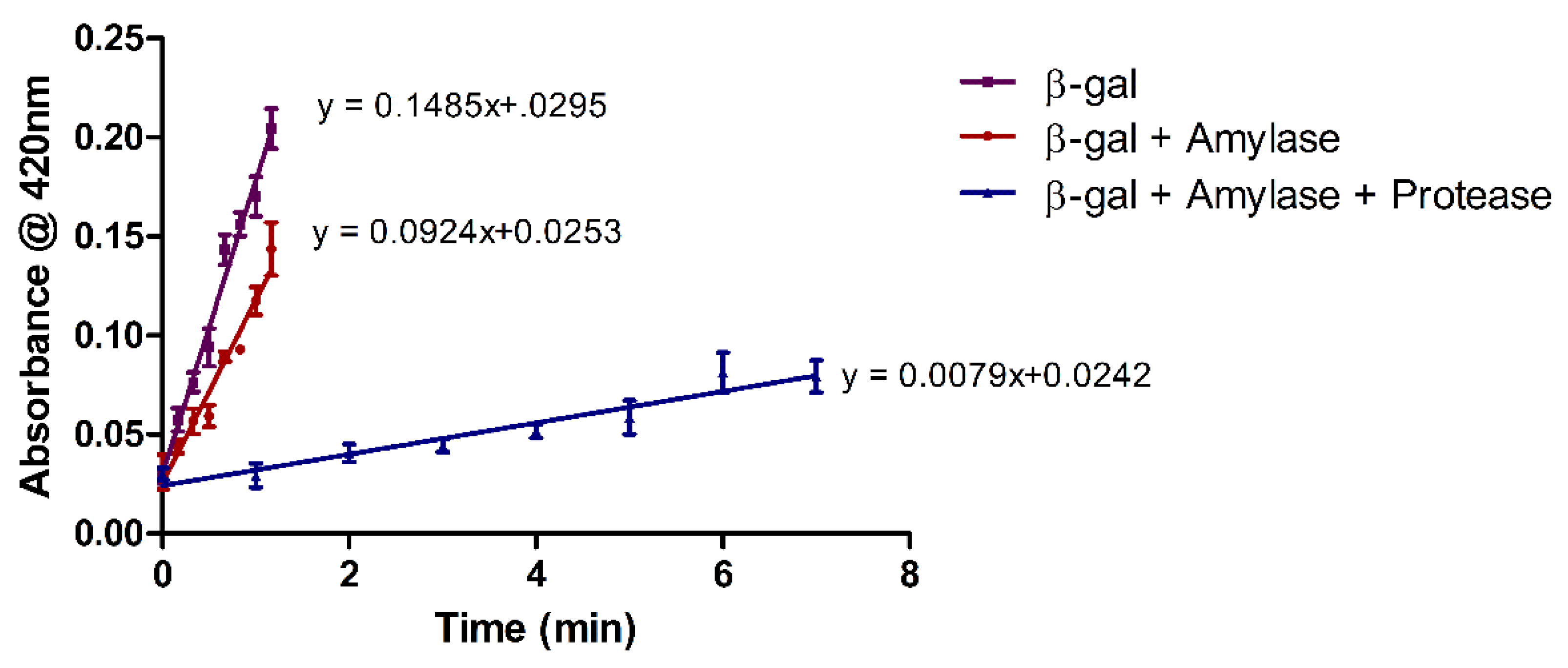
3.3. Enzyme Activity Assays
3.3.1. Protease Activity Assay
3.3.2. Amylase Activity Assay
3.3.3. Co-Immobilization of Multiple Enzymes on the Same Surface
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Huster, M.; Müller-Renno, C.; Ziegler, C.; Schlegel, C.; Ulber, R.; Muffler, K. Chloroperoxidase production by Caldariomyces fumago biofilms. Eng. Life Sci. 2016, 16, 88–92. [Google Scholar] [CrossRef]
- Haki, G.D.; Rakshit, S.K. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef]
- Olempska-Beer, Z.S.; Merker, R.I.; Ditto, M.D.; DiNovi, M.J. Food-processing enzymes from recombinant microorganisms—A review. Regul. Toxicol. Pharmacol. 2006, 45, 144–158. [Google Scholar] [CrossRef]
- Spohner, S.C.; Müller, H.; Quitmann, H.; Czermak, P. Expression of enzymes for the usage in food and feed industry with Pichia pastoris. J. Biotechnol. 2015, 202, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A Broader View: Microbial Enzymes and Their Relevance in Industries, Medicine, and Beyond. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lin, T.S.; Mou, C.Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Wong, D.E.; Senecal, K.J.; Goddard, J.M. Immobilization of chymotrypsin on hierarchical nylon 6,6 nanofiber improves enzyme performance. Colloids Surf. B Biointerfaces 2017, 154, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Talbert, J.N.; Goddard, J.M. Enzymes on material surfaces. Colloids Surf. B Biointerfaces 2012, 93, 8–19. [Google Scholar] [CrossRef]
- Li, S.-F.; Wu, W.-T. Lipase-immobilized electrospun PAN nanofibrous membranes for soybean oil hydrolysis. Biochem. Eng. J. 2009, 45, 48–53. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Yilmaz, M.; Arica, M.Y. Immobilization of a thermostable α-amylase onto reactive membranes: Kinetics characterization and application to continuous starch hydrolysis. Food Chem. 2004, 84, 591–599. [Google Scholar] [CrossRef]
- Saeki, D.; Nagao, S.; Sawada, I.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of antibacterial polyamide reverse osmosis membrane modified with a covalently immobilized enzyme. J. Memb. Sci. 2013, 428, 403–409. [Google Scholar] [CrossRef]
- Coetser, S.E.; Cloete, T.E. Biofouling and biocorrosion in industrial water systems. Crit. Rev. Microbiol. 2005, 31, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.L.; Werner, C. Enzymes for Antifouling Strategies. J. Adhes. Sci. Technol. 2012, 25, 2317–2344. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Hippius, C.; Werner, C. Immobilized enzymes affect biofilm formation. Biotechnol. Lett. 2011, 33, 1897–1904. [Google Scholar] [CrossRef]
- Kim, B.C.; Nair, S.; Kim, J.; Kwak, J.H.; Grate, J.W.; Kim, S.H.; Gu, M.B. Preparation of biocatalytic nanofibres with high activity and stability via enzyme aggregate coating on polymer nanofibres. Nanotechnology 2005, 16, 382–388. [Google Scholar] [CrossRef]
- Zaharieva, E.I.; Georgiev, G.G.; Konstantinov, C.I. Coated reactive carriers based on copolymers of 1-vinyl-2-pyrrolidone and maleic anhydride for immobilization of enzymes. Biomaterials 1996, 17, 1609–1613. [Google Scholar] [CrossRef]
- Ye, P.; Xu, Z.; Wu, J.; Innocent, C.; Seta, P. Nanofibrous Membranes Containing Reactive Groups: Electrospinning from Poly (acrylonitrile-co-maleic acid) for Lipase Immobilization. Macromolecules 2006, 39, 1041–1045. [Google Scholar] [CrossRef]
- Pompe, T. Maleic anhydride copolymers—A versatile platform for molecular biosurface engineering. Biomacromolecules 2003, 4, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.L.; Lenk, T.; Werner, C. Immobilization of Bacillus licheniformis α-amylase onto reactive polymer films. J. Biotechnol. 2011, 154, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Cloete, W.J.; Adriaanse, C.; Swart, P.; Klumperman, B. Facile immobilization of enzymes on electrospun poly(styrene-alt-maleic anhydride) nanofibres. Polym. Chem. 2011, 2, 1479–1481. [Google Scholar] [CrossRef]
- Kinetic Analysis of β-Galactosidase Activity Using the PowerWaveTM HT and Gen5TM Data Analysis Software. Available online: https://www.biotek.com/resources/docs/B-Gal_Michaelis-Menten_App_Note.pdf (accessed on 12 November 2018).
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Elnashar, M.M.M. Biotechnology of Biopolymers; IntechOpen: London, UK, 2011; pp. 3–32. [Google Scholar]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.-F.; Khajeh, K.; Ghasempur, S.; Ghaemi, N.; Siadat, S.-O.R. Covalent attachment of cholesterol oxidase and horseradish peroxidase on perlite through silanization: Activity, stability and co-immobilization. J. Biotechnol. 2007, 131, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y. Coimmobilization of glucoamylase and glucose isomerase by molecular deposition technique for one-step conversion of dextrin to fructose. J. Biotechnol. 1999, 67, 33–40. [Google Scholar] [CrossRef]
- Hilton, S.; McCubbin, W.D.; Kay, C.M.; Buckley, J.T. Purification and spectral study of a microbial fatty acyltransferase: Activation by limited proteolysis. Am. Chem. Soc. 1990, 29, 9072–9078. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux nanofiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofibrous scaffolds. J. Memb. Sci. 2009, 326, 484–492. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Enzyme | Enzyme Loading a | Activity b | Free Enzyme Activity b | % Retention c |
|---|---|---|---|---|
| protease | 7.54 ± 0.98 | 7.78 × 10−4 ± 1.32 × 10−5 | 8.87 × 10−4 ± 8.58 × 10−6 | 87.0% |
| α-amylase | 39.7 ± 0.42 | 0.808 × 100 ± 2.78 × 10−3 | 8.81 × 100 ± 0.175 × 100 | 9.0% |
| protease + α-amylase | N/A | 1.54 × 10−5 ± 4.40 × 10−7 | 8.87 × 10−4 ± 8.58 × 10−6 | 1.7% (protease) |
| N/A | 2.37 × 100 ± 1.17 × 10−1 | 8.81 × 100 ± 0.175 × 100 | 27.0 % (α-amylase) |
| Enzyme | Total Enzyme Loading (mg) | Amylase Activity (µmol·min·mg·cm−2) |
|---|---|---|
| α-amylase | 35.2 ± 0.47 | 16 × 10−3 ± 2.40 × 10−4 |
| α-amylase + protease | 10.5 ± 3.81 | 36 × 10−3 ± 2.60 × 10−4 |
| α-amylase + protease + β-galactosidase | 18.3 ± 1.58 | 5 × 10−3 ± 1.11 × 10−5 |
| BSA | 1.35 ± 0.63 | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cloete, W.J.; Hayward, S.; Swart, P.; Klumperman, B. Degradation of Proteins and Starch by Combined Immobilization of Protease, α-Amylase and β-Galactosidase on a Single Electrospun Nanofibrous Membrane. Molecules 2019, 24, 508. https://doi.org/10.3390/molecules24030508
Cloete WJ, Hayward S, Swart P, Klumperman B. Degradation of Proteins and Starch by Combined Immobilization of Protease, α-Amylase and β-Galactosidase on a Single Electrospun Nanofibrous Membrane. Molecules. 2019; 24(3):508. https://doi.org/10.3390/molecules24030508
Chicago/Turabian StyleCloete, William J., Stefan Hayward, Pieter Swart, and Bert Klumperman. 2019. "Degradation of Proteins and Starch by Combined Immobilization of Protease, α-Amylase and β-Galactosidase on a Single Electrospun Nanofibrous Membrane" Molecules 24, no. 3: 508. https://doi.org/10.3390/molecules24030508
APA StyleCloete, W. J., Hayward, S., Swart, P., & Klumperman, B. (2019). Degradation of Proteins and Starch by Combined Immobilization of Protease, α-Amylase and β-Galactosidase on a Single Electrospun Nanofibrous Membrane. Molecules, 24(3), 508. https://doi.org/10.3390/molecules24030508






